Abstract
Although Streptococcus suis has attracted public attention as a major swine and human pathogen, this bacterium has also been isolated from other animals, including ruminants. However, recent taxonomic studies revealed the existence of other species that were previously identified as S. suis, and some of these isolates were reclassified as the novel species Streptococcus ruminantium. In Japan, biochemically identified S. suis is frequently isolated from diseased ruminants; however, such isolates have not yet been identified accurately, and their aetiological importance in ruminants is unclear. Therefore, to understand the importance of S. suis and S. suis-like bacteria in ruminants, we reclassified S. suis isolates from ruminants according to the updated classification and investigated their genetic diversity. Although both S. suis and S. ruminantium were isolated from healthy and diseased ruminants, most of the isolates from diseased animals were S. ruminantium, implying that S. ruminantium is more likely to be associated with ruminant disease than S. suis. However, the ruminant S. suis and S. ruminantium isolates from diseased animals were classified into diverse genotypes rather than belonging to certain clonal groups. Genome sequence analysis of 20 S. ruminantium isolates provided information about the antibiotic resistance, potential virulence, and serological diversity of this species. We further developed an S. ruminantium-specific PCR assay to aid in the identification of this bacterium. The information obtained and the method established in this study will contribute to the accurate diagnosis of ruminant streptococcal infections.
Similar content being viewed by others

Introduction
Streptococcus suis is a Gram-positive bacterium that is isolated from the upper respiratory tract of pigs [1]. S. suis is recognized as an emerging zoonotic pathogen that has been linked to various diseases, including septicaemia, meningitis, and endocarditis, in both swine and humans [2,3,4]. Multilocus sequence typing (MLST) has been the most widely used genotyping tool for S. suis [3, 5]. As of March 2019, 1170 sequence types (STs) had been described [6], and the zoonotic isolates were grouped into several clonal complexes (CCs) [3, 7]. S. suis has traditionally been classified into 35 serotypes on the basis of antigenic differences in its capsular polysaccharides [7]. Among these serotypes, serotype 2 is most commonly associated with systemic infections in pigs and humans [3]. These genotypic and serological data suggest that S. suis human infections are mainly caused by the strains present in pigs. However, S. suis infections also occur in other animals, including ruminants [2, 8, 9]. Due to the limited genotypic and serological data available for the isolates from these animals, the epidemiological relationship between zoonotic isolates and isolates from other animals remains unclear.
Although S. suis is considered to be a species with genetically and serologically diverse strains, recent taxonomic analysis has led to the reclassification of several S. suis serotype reference strains [7, 10,11,12]. Currently, S. suis reference strains of serotypes 32 and 34 are regarded as Streptococcus orisratti [10], and those of serotypes 20, 22 and 26 are regarded as Streptococcus parasuis [11]. In 2017, Tohya et al. [12] proposed the reclassification of the serotype 33 reference strain as the new species Streptococcus ruminantium. Additionally, several S. suis-like isolates that were previously identified as S. suis but are not taxonomically considered S. suis have been found [7]. These S. suis-like strains are difficult to distinguish from S. suis by biochemical tests [7]; however, they can be detected by PCR amplification of the glutamate dehydrogenase gene (gdh-PCR) [13], which is widely used as a detection method for S. suis [7, 14] since the gdh-PCR approach was designed to detect 35 serotype reference strains, including six reference strains (serotypes 20, 22, 26, and 32–34) that are currently regarded as different species [13]. As such, in many clinical cases, S. suis-like strains may be misidentified as S. suis in diagnostic laboratories. To accurately discriminate between S. suis and S. suis-like bacteria, Ishida et al. [14] developed a PCR strategy targeting a DNA repair protein gene (recN-PCR), in which the primers designed to discriminate the serotype reference strains of authentic S. suis from the six reference strains reclassified as other species. This recN-PCR method is currently used in diagnostic laboratories for S. suis identification [15, 16].
Among the S. suis-like strains, S. ruminantium has been isolated from cattle with endocarditis and a lamb with arthritis [12, 17], suggesting that S. ruminantium is associated with ruminant disease. Isolation of S. suis from diseased ruminants has also been reported [18,19,20,21,22]; however, many of these reports were published prior to the proposal of S. ruminantium as a species. Given that some of these isolates may have been misidentified, the aetiological importance of S. suis and S. suis-like bacteria, including S. ruminantium, in ruminants remains unknown. Correct identification of these streptococcal isolates is crucial for the precise diagnosis of streptococcal disease and an accurate understanding of the role of these bacteria in ruminants.
In the diagnostic service of the National Institute of Animal Health in Japan (NIAH-Japan), we have encountered many “S. suis” isolates from ruminants that were identified by biochemical tests and/or gdh-PCR. In this study, to understand the aetiological importance of S. suis and S. suis-like bacteria in ruminants, these “S. suis” isolates were characterized on the basis of the recent classification; a PCR strategy for detecting S. ruminantium was developed; and the presence of these bacteria in the tonsils of healthy cattle was investigated. Reidentified and newly isolated S. suis and S. ruminantium strains were further genotyped by MLST or pulsed-field gel electrophoresis (PFGE) analysis as well as typing of the capsular polysaccharide synthesis genes (cps), where the cps types were numbered according to the expected serotypes (e.g., serotype 3 for cps type 3); however, serotypes 1 and 1/2 cannot be distinguished from serotypes 14 and 2, respectively [23]. Furthermore, 20 of the S. ruminantium isolates were subjected to genome sequence analysis to gain insights into the pathogenicity and antibiotic resistance of S. ruminantium strains.
Materials and methods
Bacterial strains
Sixty-four streptococcal isolates were collected between 1992 and 2017 in our laboratory as part of the diagnostic services provided by NIAH-Japan (Table 1 and Additional file 1). Fifty-six of the strains were isolated from cattle (n = 53), sheep (n = 2), and a goat (n = 1) that were either diseased or dead, although 18 of the strains were isolated from sites that were likely unrelated to the disease or where information on clinical signs was lacking. The remaining isolates came from healthy cattle (n = 7) and a milk-feeding robot for calves (n = 1). All of the ruminant isolates were collected from different animals, although 12 of them and one environmental isolate came from the same farm (Additional file 1). These 64 isolates had been previously identified as S. suis by gdh-PCR [13] and the API 20 Strep system (BioMérieux, France) or the Rapid ID 32 Strep system (BioMérieux).
An additional 55 streptococcal isolates were collected from the tonsils of 110 cattle during a meat inspection at the Yamagata Nairiku Meat Inspection Center in Japan in 2012 (Additional file 2). The strains were isolated from aseptically dissected tonsillar samples, from which the surface stamps were streaked on Colistin-oxolinic acid blood agar [Columbia agar (Becton–Dickinson, Sparks, MD, USA) supplemented with 5% defibrinated sheep blood and Streptococcus-selective supplement (10 mg/L of colistin sulphate and 5 mg/L of oxolinic acid; Oxoid, UK)] [24]. After incubation at 37 °C for 1–2 days in a candle jar, up to 8 α-haemolytic colonies from each tonsillar sample were selected for Gram staining and catalase and oxidase tests. Gram-positive, catalase-negative, and oxidase-negative cocci were further characterized using the API 20 Strep system and gdh-PCR [13]. The isolates identified as S. suis by both the API 20 Strep system and gdh-PCR were used in this study.
DNA extraction, PCR, and amplicon sequencing
All the isolates were cultured overnight on Todd-Hewitt (TH) agar (Becton–Dickinson) at 37 °C under aerobic conditions with 5% CO2. InstaGene Matrix (Bio-Rad, Hercules, CA, USA) was used to extract bacterial DNA according to the manufacturer’s instructions. All of the PCR assays were performed using TaKaRa Ex Taq polymerase (Takara Bio Inc., Kusatsu, Japan) and QIAGEN Multiplex Master PCR Mix (Qiagen, Hilden, Germany), according to the manufacturers’ instructions. For sequencing, the amplified PCR products were purified using the QIAquick PCR purification kit (Qiagen) following the manufacturer’s instructions and sequenced with a 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) using a BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems). Sequence assembly was performed using SEQUENCHER 5.4 (Gene Codes Corp., Ann Arbor, MI, USA).
PCR for S. suis identification and typing
All the isolates (n = 119) were subjected to gdh-PCR as previously described [13]. For the identification of authentic S. suis, recN-PCR was performed as previously described [14]. The 119 isolates were also analysed by cps-typing, which is a two-step multiplex PCR method used for the typing of the cps gene cluster of S. suis, as previously described [23]. Moreover, we performed MLST according to published protocols using clinical isolates of authentic S. suis [5]. The STs were determined using the S. suis MLST database. Novel alleles and STs were assigned through the submission of the data to the database.
Sequence analysis of 16S rRNA and superoxide dismutase (sodA) genes
The 16S rRNA gene fragments (> 1.5 kbp) of all isolates were amplified and sequenced as previously described [25]. Pairwise similarities between the 16S rRNA gene sequences of the isolates used in this study and the type strain of each species were calculated using the EzBioCloud server [26] and the web Basic Local Alignment Search Tool (BLAST) of the National Center for Biotechnology Information (NCBI). In this study, 98.65 % sequence similarity was used as the threshold for differentiating two species [27].
With regard to the isolates identified as S. ruminantium by 16S rRNA gene sequencing, a partial sequencing analysis of the superoxide dismutase gene (sodA) was conducted as an additional assay for species identification to identify more precisely, referring to a previous report [12]. The amplification and sequencing of sodA were performed as previously described [28]. BLAST analysis was conducted to calculate the similarities between the tested isolates and the S. ruminantium type strain GUT-187T (GenBank accession no. LC195049).
PCR for the discrimination of S. ruminantium from S. suis
Primers were designed as follows: the 16S rRNA gene sequences from 35 S. suis serotype reference strains (serotypes 1–34 and 1/2) and S. ruminantium GUT-187T and DAT741 were aligned by MAFFT FFT-NS-i v7.215 [29] with the default parameters. The alignment profile was then manually searched for the distinctive regions of S. ruminantium GUT-187T, DAT741 and EA1832.92 (serotype 33). The specificity of the potential primer regions was confirmed using the Ribosomal Database Project’s (RDP) Probe Match algorithm (Release 11, Update 5) [30]. Accordingly, the sequences of the forward and reverse primers were 5′-GCAAGTGGAACGCAACTTTTCA-3′ and 5′-CTATGTATCGTTGCCTTGGTAG-3′, respectively. The 16S rRNA gene sequences of the S. suis strains used for this analysis were obtained from their draft genome sequences [31]. PCR was carried out using TaKaRa Ex Taq polymerase (Takara Bio Inc.) under the following conditions: 95 °C for 2 min, followed by 30 cycles of 95 °C for 20 s, 60 °C for 10 s, and 72 °C for 20 s.
To evaluate primer specificity, the PCR assay was tested with 12 type strains of Streptococcus spp., three ruminant-derived S. suis serotype reference strains, two S. orisratti strains (S. suis serotype 32 and 34 reference strains), ten streptococcal field isolates from ruminants (Additional file 3), and the 119 isolates analysed in this study. The analytical sensitivity of the PCR assay, corresponding to the minimum number of bacterial cells that are detected by the assay, was determined using S. ruminantium DAT741. In brief, the strain was grown to an optical density at 600 nm of 0.1 in TH broth, at which point the genomic DNA was extracted from 100 µL of a bacterial suspension with 100 μL of InstaGene Matrix (Bio-Rad). Ten-fold serial dilutions of the extracted DNA were used for the PCR assay. The number of colony-forming units (CFUs) of the above bacterial suspensions was also determined by plating serial dilutions on TH agar, followed by incubation overnight at 37 °C under aerobic conditions with 5% CO2.
PFGE
PFGE was performed according to the procedures described previously [32] with slight modifications. Briefly, bacterial cells were harvested from brain heart infusion (BHI) agar (Becton–Dickinson), washed with Tris-saline buffer (10 mM Tris–HCl [pH 8.0], 1 M NaCl), and suspended in EDTA–sarcosine buffer (6 mM Tris–HCl, 1 mM NaCl, 100 mM EDTA, 1% sodium N-lauroylsarcosine; pH 7.6). The suspension was mixed with an equal volume of 1.0% SeaKem Gold agarose (Lonza Rockland, Rockland, ME, USA) in EDTA–sarcosine buffer, and the mixture was solidified in a 0.7-mm sample plug caster (Bio-Rad). The sample plugs were then incubated in lysis buffer (0.5 M EDTA [pH 8.0], 2.5 mg/mL lysozyme, 10 U/mL mutanolysin) for 3 h at 37 °C, followed by proteinase K solution (0.5 M EDTA [pH 8.0], 1% sodium N-lauroylsarcosine, 1 mg/mL proteinase K) for 18 h at 50 °C. The samples were next treated twice with 1 mM Pefabloc SC (Roche Applied Science, Basel, Switzerland) in Tris–EDTA (TE) buffer (10 mM Tris–HCl, 1 mM EDTA: pH 8.0) for 30 min at 50 °C and washed three times with TE at room temperature. The genomic DNA in each plug was then digested with 20 U of SmaI (Takara Bio) for 18 h at 30 °C and separated on a 1.0% SeaKem Gold agarose gel in Tris–borate–EDTA buffer (44.5 mM Tris, 44.5 mM boric acid, 1 mM EDTA: pH 8.0) supplemented with 50 µM thiourea using a CHEF-DR II System (Bio-Rad Laboratories). The electrophoresis conditions were as follows: 5.5 V/cm with pulse times of 2–10 s for 13 h and 20–25 s for 6 h at 14 °C. The PFGE patterns present from 48.5 to 630.5 kbp were analysed on the basis of the Dice-predicted similarity of two patterns, and the unweighted pair group method with average linkage clustering was used to construct the corresponding dendrogram at a setting of 1.0% position tolerance using GelJ v2.0 [33].
Whole-genome sequencing, annotation, and pangenome analysis
Among the 76 isolates, 10 isolates from diseased sites (three from the heart, one from a liver abscess, one from a pulmonary abscess, five from the lungs) and 10 isolates from the tonsils or oral cavities were selected for whole-genome sequencing in this study (Additional file 4). For these analyses, the DNeasy Blood and Tissue Kit (QIAGEN) was used for the extraction of genomic DNA; genomic libraries were constructed using the Nextera XT DNA Library Preparation kit (Illumina, Inc., San Diego, CA, USA); and genome sequence data were obtained using an Illumina HiSeq 2500 sequencer (Illumina). Adapter and low-quality sequences were trimmed from the generated read sequences using FastQC and Trimmomatic [34]; the resulting sequences were assembled using Velvet v1.2.08 [35]. S. ruminantium DAT741 from a diseased cow with endocarditis was also sequenced on the PacBio RSII platform (Pacific BioSciences Inc., Menlo Park, CA, USA) at Macrogen Japan Corp. (Kyoto, Japan) to determine its complete genome sequence. De novo genome assembly was performed via the Hierarchical Genome Assembly Process (HGAP3) with SMRT Portal v2.3.0, and annotation of the genome assemblies was executed with Prokka v1.12 (default setting) [36]. The circular map of the S. ruminantium DAT741 chromosome was visualized using in silico MolecularCloning software (In Silico Biology, Inc., Japan). Thereafter, the pangenome was analysed using Roary v3.11.2 (with default parameters) [37]. Genes related to antibiotic resistance were searched using the Resistance Gene Identifier in the Comprehensive Antibiotic Resistance Database (CARD) [38]. Contigs including antibiotic resistance genes were compared to all integrative conjugative element (ICE) sequences in ICEberg v2.0 [39] using web nucleotide BLAST.
Antibiotic susceptibility testing
The minimal inhibitory concentrations (MICs) of tetracycline, erythromycin, chloramphenicol, kanamycin, streptomycin, amoxicillin, ampicillin, and benzylpenicillin were measured on Mueller–Hinton agar (Oxoid) containing defibrinated 5% sheep blood using Etest strips (BioMérieux) according to the manufacturers’ instructions.
Identification and typing of the putative cps gene clusters of 21 S. ruminantium strains
To identify putative cps gene clusters, contigs that contained genes similar to those of the cps gene cluster and its flanking genes orfX, encoding a hypothetical protein, and glf, encoding the UDP-galactopyranose mutase, of EA1832.92 (GenBank accession no. AB737837) were isolated from the pangenome data using Roary. Gaps between contigs were amplified by PCR, and their sizes were compared to unigenes with no gaps. The 21 putative cps gene clusters were classified according to their genetic organization based on the pangenome data using Roary. The Artemis Comparison Tool (ACT) [40] was used to visualize the comparison data between two sequences using BLASTN (bit-scores above 50 and E-values lower than 1e–8 were displayed).
Primers were then designed against the characteristic regions of each identified cps gene cluster type to classify the cps types of the S. ruminantium strains. The target cps genes, primer sequences, and predicted size(s) of each PCR product are shown in Additional file 5. All of the PCR assays were carried out using TaKaRa Ex Taq polymerase (Takara Bio Inc.) under the following conditions: 95 °C for 2 min followed by 30 cycles of 95 °C for 20 s, 60 °C for 10 s, and 72 °C for 40 s.
Data availability
All of the sequences determined in this study were deposited in the DDBJ/ENA/GenBank databases under accession numbers LC316845-LC316868, LC316870-LC3169000, LC316903-LC316941, LC377185-LC377187, LC337291-LC337338, LC337341-LC337368, BCFA01000001-BCFA01000063, BCFD01000001-BCFD01000049, BCFE01000001-BCFE01000040, BCFF01000001-BCFF01000047, BCFB01000001-BCFB01000063, BCEZ01000001-BCEZ01000068, BCFG01000001-BCFG01000058, BCFH01000001-BCFH01000058, BCFI01000001-BCFI01000050, BCFC01000001-BCFC01000061, BCES01000001-BCES01000040, BCEY01000001-BCEY01000040, BCET01000001-BCET01000041, BCEU01000001-BCEU01000040, BCEP01000001-BCEP01000043, BCEV01000001-BCEV01000045, BCEQ 01000001-BCEQ 01000156, BCEW01000001-BCEW01000044, BCER01000001-BCER01000040, BCEX01000001-BCEX01000031, and CP019557 (Additional files 1, 2, 3 and 4).
Results
Ruminant “S. suis” isolates were frequently reclassified as S. ruminantium
The 16S rRNA gene sequence analysis of the ruminant “S. suis” isolates collected through the diagnostic service of NIAH-Japan indicated that most of the isolates (55/64) were not S. suis, as the pairwise sequence similarities between their respective 16S rRNA gene sequences and that of S. suis S735T were less than 98% (Table 1 and Additional file 1). Accordingly, 54 of these isolates were reclassified as S. ruminantium based on the pairwise sequence similarity of the 16S rRNA (99.08–100%) and sodA (99.77–100%) genes between S. ruminantium GUT-187T and the respective isolates. The 16S rRNA gene sequence of the remaining isolate was identical to that of S. parasuis STU-286T (Additional file 1).
The 64 analysed S. ruminantium isolates were isolated from both healthy cattle and cattle with diseases including pneumonia, respiratory diseases, endocarditis, arthritis, and torticollis (Table 1 and Additional file 1). Similarly, S. suis was isolated from four calves with meningitis and astasia and five healthy cattle. Furthermore, these isolates were identified as S. suis by recN-PCR (Additional file 1).
PCR to discriminate between S. ruminantium and S. suis
Next, a PCR strategy was developed to discriminate S. ruminantium from S. suis and S. suis-like bacteria using S. ruminantium-specific 16S rRNA primers. The primer design was based on the alignment of the 16S rRNA gene sequences of 35 S. suis serotype reference strains and two S. ruminantium strains (GUT-187T and DAT741) (Additional file 6). The PCR approach detected the 54 isolates identified in this study as S. ruminantium and the serotype 33 reference strain EA1832.92. Furthermore, 37 non-S. ruminantium isolates, including 13 S. suis, S. parasuis and S. orisratti strains, were not detected (Table 1 and Additional files 1 and 3). Using the described method with S. ruminantium DAT741, the detection limit of this PCR strategy was 2.7 × 103 CFU/mL (Additional file 7).
Cattle carried S. suis and/or S. ruminantium in their tonsils
The carrier rates for these bacteria in the tonsils of cattle were investigated. Accordingly, gdh-PCR-positive streptococci were isolated from the tonsils of 48 (43.6%) of the tested cattle (n = 110; Additional file 2). Further identification by recN-PCR and the developed S. ruminantium-specific PCR assay with the gdh-PCR-positive isolates revealed that 32 and 22 strains belonged to the species S. suis and S. ruminantium, respectively (Additional file 2). The S. ruminantium strains were further confirmed by the analysis of the 16S rRNA and sodA genes (Additional file 2).
The sequence and cps types of the cattle S. suis clinical isolates were different from those of human and porcine clinical isolates
Given the finding that S. suis can cause severe disease in calves, the S. suis isolates were also typed according to their cps gene clusters. Notably, the four S. suis isolates from diseased calves were not classified as cps type 2. Instead, two of the isolates were typed as cps type 8, one as type 10, and one was untypable (Table 2 and Additional file 8). Furthermore, cps typing of the remaining S. suis isolates from the oral cavities and tonsils of cattle indicated that cps type 8 was the most common (10/37) and that a significant proportion were untypable (23/37; Table 2). MLST classified the four S. suis isolates as novel STs (ST999, ST1000, ST1002, and ST1003); these STs did not share at least five alleles each with any STs in the MLST database of S. suis (Additional file 8).
Classification of S. ruminantium isolates by pulsotype
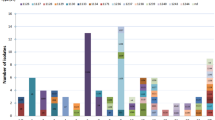
PFGE was performed to evaluate the degree of genetic diversity and clonality of S. ruminantium isolates from healthy and diseased ruminants. The PFGE profiles of eight of the tested isolates were not analysed because they showed smearing due to DNA degradation or indigestion. As such, the PFGE patterns of 68 ruminant isolates and EA1832.92 were distinguished, producing 66 different pulsotypes (Figure 1). The 66 pulsotypes were clustered into 43 groups with a 75% similarity cutoff level, meaning that less than three fragment differences existed between the profiles. Many of the clusters (26/43) were composed of a single isolate, whereas 17 clusters contained two to seven isolates. Isolates collected from the same farm were all classified into different groups with the exception of two isolates from group 20 and five from group 43, which were all isolated from Farm A (Figure 1).
SmaI-digested PFGE patterns of S. ruminantium. The left panel presents the dendrogram based on the PFGE profiles. Some strains, including MO411, MO494, MO535, MO675, and DTK371, seemed to exhibit slightly affected PFGE profiles, probably due to the nuclease degradation of DNA. The right panel presents the strain information (highlighting with a red background corresponds to isolates from the lesions of diseased animals; blue, goat isolate; red, sheep isolates; green, environmental isolate; see Additional files 1 and 2 for more details).
Genome analysis revealed genes present in disease-associated isolates
To investigate whether S. ruminantium isolates associated with diseases in ruminants exhibit specific genetic markers, a comparative analysis was performed using the genome sequences of 20 S. ruminantium isolates, ten isolates from diseased tissue sites and ten from the tonsils or oral cavity of cattle. The genome sizes of the isolates ranged from 1.98 to 2.29 Mb, and there were 1873–2217 predicted coding sequences (Additional file 4).
Pangenome analysis with Roary identified 4163 clusters of orthologous groups (COGs) in the genes of the 20 S. ruminantium isolates and EA1832.92 (Additional file 9). Although there were no COGs that were exclusively conserved in all ten clinical isolates and exclusively absent in seven or more of the other isolates, eleven COGs were specifically present in the clinical isolates from lesions of the heart, abscesses and a joint (Table 3). Furthermore, each of these genes was present in the genome of S. ruminantium GUT187T, which was isolated from the heart of a cow with endocarditis [41].
Variations were discovered in cps gene clusters of S. ruminantium
In this study, cps type 33 isolates accounted for less than 25% of the tested isolates (Table 4 and Additional files 1 and 2). The genomes of all 20 S. ruminantium isolates exhibited putative cps genes between orfX and glf (Figure 2A and Additional file 10), and these genes were regarded as cps clusters of S. ruminantium. These cps clusters were divided into three main types (Type I–III) according to their genetic organization (Figure 2A and Additional file 10). Types I and II were further divided into four and two subtypes (Type IA–ID and IIA–IIB) based on the differences in one or two genes (Figure 2A). Among the sub-types, types IA and IC were classified into cps type 33 when analysed using the cps-typing scheme for S. suis [23]. In total, seven cps clusters (cpsIA-D, cpsIIA-B, and cpsIII) were identified in the 20 isolates.
Classification scheme for S. ruminantium cps gene clusters. A Each coloured arrow represents a coding sequence, the colour of which indicates the predicted function. The target regions of the respective PCR assays are appended to the corresponding arrows. Light blue blocks indicate conserved regions according to pairwise BLASTN comparison data. B The cps-type classification scheme based on the PCR profiles.
To classify the seven identified cps clusters of S. ruminantium and investigate the distribution of the cps types in our isolates, we designed a PCR-based cps-typing scheme for S. ruminantium using eight primer pairs (Figure 2). Among the 76 S. ruminantium isolates used in this study, 61 isolates (80.3%) were classified into one of the seven types using this typing scheme, while 15 isolates (19.7%) were untypable (Table 4). The distribution of the cps types from the lesion-associated isolates (n = 34) was comparable to that of the isolates from healthy animals and the environment (Table 4), although isolates classified as Type III were only isolated from the lesions of diseased cattle (n = 2).
Antibiotic resistance genes were located in ICE-like elements in S. ruminantium
Our search of the 20 S. ruminantium genomes using CARD identified genes that putatively confer resistance to tetracyclines (tetO and tetM), aminoglycosides (aph (3′)-IIIa), streptomycin (aad(6), aadK and ant(6)-Ib), phenicols (catD and cat-TC), macrolides (ermB), and streptothricin (sat-4) in 13 of the isolates (Table 5). Antibiotic susceptibility tests indicated remarkably high MIC values of tetracycline (≥ 16 μg/mL) in the isolates carrying tetO or tetM, with the exception of the tetO-positive strain DTK397 (MIC, 1.5 μg/mL) (Additional file 11). Furthermore, high MICs were noted for streptomycin (≥ 96 μg/mL) in the isolates carrying aad(6), aadK or ant(6)-Ib; erythromycin (≥ 12 μg/mL) in the isolates carrying ermB; kanamycin (≥ 256 μg/mL) in the isolates carrying aph (3′)-IIIa; and chloramphenicol (24 μg/mL) in the isolate carrying catD and cat-TC (Additional file 11). Comparatively, the MICs were low for DAT741, which carried none of these genes (Additional file 11). Among the identified antibiotic resistance genes, tetO, ermB and ant(6)-Ib were present in more than 40% of the isolates, and ten isolates possessed multiple antibiotic resistance genes (Table 5). Further comparative analysis indicated that almost all of the resistance genes were located in genomic islands (GIs) at four different chromosomal loci (1–4; Figure 3 and Additional file 12). Notably, tetO, sat-4, aad(6), ermB, aph (3′)-IIIa and cat-TC of DAT837 and tetO, ant(6)-Ib and ermB of DTK377 were the exceptions, as their chromosomal locations were not identified. The nucleotide sequences of these GIs showed similarities to those of ICEs present in the ICEberg database, particularly the ICESa2603 family (Figure 3A and Additional file 13).
S. ruminantium GIs that carry antibiotic resistance genes (A) and their chromosomal locations (B). A The GIs were compared with the representative ICEs Tn916, Tn1806, ICESa2603, Ctn2, Tn2008, and 2096-RD.2. Coloured arrows outlined by blue lines indicate antibiotic resistance genes; red blocks, conserved regions based on pairwise TBLASTX comparison data; brackets, core genes of ICESa2603a family ICEs. B Chromosomal locations of the GIs in the DAT741 genome. GC content, GC skew and deduced ORFs are represented in the inner map.
Discussion
In the present study, many of the isolates biochemically identified as S. suis from diseased ruminants in Japan were re-identified as S. ruminantium according to the most recent classification. S. suis was also found to be involved in clinical cases in cattle with severe diseases or clinical signs such as astasia and meningitis. The isolation of S. suis from diseased ruminants has been reported not only in Japan but also in North America and Belgium [18,19,20,21], although it is uncertain whether these isolates were accurately speciated, as S. ruminantium had not yet been proposed. Notably, discrimination between S. ruminantium and S. suis through biochemical tests has traditionally been difficult. Indeed, at the onset of this study, there was no differentiating test available for diagnostic laboratories. Therefore, in the present study, we developed an S. ruminantium-specific PCR assay; this novel PCR assay, together with recN-PCR for S. suis detection, will be key to the accurate diagnosis of streptococcal diseases in ruminants.
This study also revealed that both S. suis and S. ruminantium can be isolated from the tonsils and oral cavities of healthy cattle. Notably, in a previous Belgian study, S. suis was isolated from the tonsils of healthy cattle in 40% of the investigated population [42]. Although these isolates were identified on the basis of biochemical tests, the prevalence was comparable to the isolation of S. ruminantium and S. suis reported in this study (42.7%). Thus, these findings suggest that S. ruminantium and S. suis can naturally inhabit the tonsils and oral cavity of cattle.
According to the MLST analysis, the S. suis clinical isolates from cattle were apparently different from the swine isolates and were typed as four novel STs. Considering that S. suis can be isolated from healthy cattle, S. suis is likely an opportunistic pathogen in cattle. In this study, no bovine S. suis isolates were grouped into CCs or serotype 2, which are frequently found in the isolates from patients with severe systemic diseases. However, a previous Japanese study reported a case of serotype 2 S. suis infection in a dairy farmer who had no history of contact with pigs [43]. Although serotype 2 S. suis was not detected in the cattle from this farm, serotype 2 S. suis has been isolated from diseased cattle in Canada and Belgium [18, 19]; therefore, bovine S. suis may cause human disease. Serotype 8 strains were most frequently isolated from healthy and diseased cattle in this study. Although it remains unclear why serotype 8 was isolated most frequently due to the limited data on ruminant isolates, our data suggest that the serotype 8 capsule may be one of the types of S. suis that could adapt to cattle in Japan. Further investigation by MLST and serotyping of S. suis isolates from diseased ruminants from geographically diverse regions will elucidate the presence or absence of CCs and serotypes strongly associated with ruminant disease and the risk of human disease posed by ruminant isolates.
The present study highlights that S. ruminantium may be associated with respiratory diseases, pneumonia, and torticollis in ruminants as well as endocarditis and arthritis, as previously reported [12, 17]. An earlier study also reported the association of S. ruminantium with various diseases in cattle [21], although the isolates were simultaneously identified as S. suis, showing the best 16S rRNA sequence matches with the S. suis serotype 33 type strain (99–100%). Collectively, the data suggest that S. ruminantium can be associated with various diseases in ruminants, although the associations observed in some cases, especially with respiratory diseases and pneumonia cases, are questionable due to the isolation of these bacteria from lesions together with other pathogens in the present and previous reports [21]. In our study, S. suis was more frequently isolated from the tonsils of healthy cattle than S. ruminantium, whereas S. ruminantium was more frequently found in the lesions of diseased ruminants than S. suis (Table 1). Therefore, S. ruminantium may be more likely to be associated with disease in ruminants than S. suis. However, PFGE analyses from this and previous studies [21] suggest that strains with various genetic backgrounds can be associated with diseases in ruminants. Furthermore, our PFGE data indicated that S. ruminantium genetic diversity was detectable at the farm level and that the pulsotype was not associated with health or disease status. Given that healthy cattle also carry S. ruminantium, S. ruminantium may contribute to the development of disease as a primary or secondary pathogen or may be a commensal or an opportunistic pathogen. Additionally, among the 11 COGs distinctively present in the clinical isolates identified by our analysis, two showed over 80% similarity to salR and salK, a two-component signal transduction system that contributes to the virulence of highly invasive S. suis 05ZYH33 in pigs [44]. Further analysis of the function of each distinctive COG, including these two, is necessary to elucidate whether these gene products contribute to virulence and disease severity.
In a previous study by Tohya et al. [12], all the S. ruminantium isolates obtained from cattle with endocarditis were classified as serotype 33. However, this study revealed variation in the cps gene clusters of S. ruminantium isolates. In addition, approximately 20% of the tested isolates were untypable by cps-typing PCR, possibly due to the existence of novel cps types that cannot be classified by PCR or have lost of some part of the cps gene cluster, including the target genes for PCR. Furthermore, the cps-typing PCR strategy for S. ruminantium described in this study only targets two genes, and thus, the possibility of the existence of more variations in the cps gene clusters cannot be ruled out. These data suggest that this species is serologically diverse, although additional data from the serological analysis of each cps type of isolate will be required. Our data implied no clear associations between cps type and ruminant disease. However, some isolates seemed to show high hydrophobicity when cultured in broth, implying that these isolates may be unencapsulated or produce low amounts of capsule. In S. suis, the presence of several unserotypable isolates that were typable by cps typing PCR has been reported [7, 23]; thus, further confirmation of capsule expression in isolates of each cps type will be necessary to clarify whether certain serological types of S. ruminant are associated with ruminant diseases.
A previous study [45] indicated that the cps33 gene cluster shared relatively high amino acid sequence similarity with the cps9 gene cluster. In fact, two-way cross-reactions between serotypes 9 and 33 have been reported [17]. According to our data, approximately 50% of S. ruminantium isolates were typed as Type IA–ID, which are identical (IA) or similar (IB–ID) to the cps33 gene cluster. Furthermore, several serotype 9 S. suis isolates were found in cattle in previous studies [19, 20]. Although further serological analysis will be needed, this type of antigenicity may be preferable for adaptation to cattle. None of the cps gene clusters of known serotypes in S. suis showed amino acid sequence similarity in most of the region with the cps IIA–B and III gene clusters (data not shown).
In addition to variations in cps gene clusters, our genome analysis of 20 S. ruminantium isolates identified genes that putatively confer resistance to streptothricin, tetracyclines, macrolides, aminoglycosides, and phenicols, particularly in the isolates from the lungs, tonsils, and oral cavity, which are microenvironments that other bacterial species also inhabit. In S. suis, many antibiotic resistance genes have been shown to be located in ICEs [31, 46]. In S. ruminantium, many of the antibiotic resistance genes identified in this study were also located in ICESa2603 family ICEs, which are putative mobile genetic elements (MGEs) found in several S. suis and other streptococcal isolates from pigs and cattle in China [46, 47] (Figure 3 and Additional file 13). The transfer of some of the ICESa2603 family ICEs to other Streptococcus spp. has been experimentally verified [46, 47], and many of the ICE-like elements identified in this study included almost all of the core genes of this ICE family [46], as well as genes encoding the Type IV secretion system, integrase, and excisionase (Figure 3 and Additional file 12). Although further study of the transfer of the identified ICE-like elements is required, S. ruminantium may be a reservoir of antibiotic resistance genes that can transfer resistance genes to other streptococci via ICEs.
By contrast, beta-lactam resistance genes were not found in S. ruminantium in this study, and the MICs were low for these antibiotics (MIC range of amoxicillin, 0.012–0.08 µg/mL; ampicillin, ≤ 0.016 µg/mL; and benzylpenicillin, 0.016–0.023 µg/mL). Other groups have also reported high susceptibility to beta-lactams in S. suis and S. ruminantium [21, 48]. Although continuous studies are necessary to monitor antibiotic resistance in ruminant S. suis and S. ruminantium, these data suggest that penicillins are effective for the treatment of these infections in ruminants.
In conclusion, this study revealed that both S. suis and S. ruminantium can be associated with ruminant disease, and the differentiation between these two species is now aided by our novel PCR strategy, which will be key to the accurate diagnosis of streptococcal disease in ruminants. The information and tools established through this work will also contribute to the accumulation of S. suis and S. ruminantium data, resulting in an increased understanding of the epidemiology, pathogenicity, virulence, and zoonotic potential of these two species.
References
Gottschalk M (2012) Streptococcosis. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW (eds) In diseases of swine, 10th edn. Wiley-Blackwell, Ames, pp 841–855
Staats JJ, Feder I, Okwumabua O, Chengappa MM (1997) Streptococcus suis: past and present. Vet Res Commun 21:381–407
Goyette-Desjardins G, Auger JP, Xu J, Segura M, Gottschalk M (2014) Streptococcus suis, an important pig pathogen and emerging zoonotic agent—an update on the worldwide distribution based on serotyping and sequence typing. Emerg Microbes Infect 3:e45
Gottschalk M, Xu J, Calzas C, Segura M (2010) Streptococcus suis: a new emerging or an old neglected zoonotic pathogen? Future Microbiol 5:371–391
King SJ, Leigh JA, Heath PJ, Luque I, Tarradas C, Dowson CG, Whatmore AM (2002) Development of a multilocus sequence typing scheme for the pig pathogen Streptococcus suis: identification of virulent clones and potential capsular serotype exchange. J Clin Microbiol 40:3671–3680
PubMLST. Streptococcus suis MLST Databases. http://pubmlst.org/ssuis/. Accessed 25 Mar 2013
Okura M, Osaki M, Nomoto R, Arai S, Osawa R, Sekizaki T, Takamatsu D (2016) Current taxonomical situation of Streptococcus suis. Pathogens 5:E45
Devriese LA, Haesebrouck F, de Herdt P, Dom P, Ducatelle R, Desmidt M, Messier S, Higgins R (1994) Streptococcus suis infections in birds. Avian Pathol 23:721–724
Risco D, Fernández-Llario P, Cuesta JM, García–Jiménez WL, Gonçalves P, Martínez R, García A, Rosales R, Gómez L, de Mendoza JH (2015) Fatal case of Streptococcus suis infection in a young wild boar (Sus. scrofa) from southwestern Spain. J Zoo Wildl Med 46:3
Hill JE, Gottschalk M, Brousseau R, Harel J, Hemmingsen SM, Goh SH (2005) Biochemical analysis, cpn60 and 16S rDNA sequence data indicate that Streptococcus suis serotypes 32 and 34, isolated from pigs, are Streptococcus orisratti. Vet Microbiol 107:63–66
Nomoto R, Maruyama F, Ishida S, Tohya M, Sekizaki T, Osawa R (2015) Reappraisal of the taxonomy of Streptococcus suis serotypes 20, 22 and 26: Streptococcus parasuis sp. nov. Int J Syst Evol Microbiol 65:438–443
Tohya M, Arai S, Tomida J, Watanabe T, Kawamura Y, Katsumi M, Ushimizu M, Ishida-Kuroki K, Yoshizumi M, Uzawa Y, Iguchi S, Yoshida A, Kikuchi K, Sekizaki T (2017) Defining the taxonomic status of Streptococcus suis serotype 33: the proposal for Streptococcus ruminantium sp. nov. Int J Syst Evol Microbiol 67:3660–3665
Okwumabua O, O’Connor M, Shull E (2003) A polymerase chain reaction (PCR) assay specific for Streptococcus suis based on the gene encoding the glutamate dehydrogenase. FEMS Microbiol Lett 218:79–84
Ishida S, le Tien HT, Osawa R, Tohya M, Nomoto R, Kawamura Y, Takahashi T, Kikuchi N, Kikuchi K, Sekizaki T (2014) Development of an appropriate PCR system for the reclassification of Streptococcus suis. J Microbiol Methods 107:66–70
Meekhanon N, Kaewmongkol S, Phimpraphai W, Okura M, Osaki M, Sekizaki T, Takamatsu D (2017) Potentially hazardous Streptococcus suis strains latent in asymptomatic pigs in a major swine production area of Thailand. J Med Microbiol 66:662–669
Meekhanon N, Kaewmongkol S, Jirawattanapong P, Kaminsonsakul T, Kongsoi S, Chumsing S, Okura M, Ueno Y, Sekizaki T, Takamatsu D (2019) High rate misidentification of biochemically determined Streptococcus isolates from swine clinical specimens. J Vet Med Sci 81:567–572
Higgins R, Gottschalk M, Boudreau M, Lebrun A, Henrichsen J (1995) Description of six new capsular types (29–34) of Streptococcus suis. J Vet Diagn Invest 7:405–406
Hommez J, Wullepit J, Cassimon P, Castryck F, Ceyssens K, Devriese LA (1988) Streptococcus suis and other streptococcal species as a cause of extramammary infection in ruminants. Vet Rec 123:626–627
Higgins R, Gottschalk M, Fecteau G, Sauvageau R, De Guise S, Du Tremblay D (1990) Quebec. Isolation of Streptococcus suis from cattle. Can Vet J 31:529
Kataoka Y, Sugimoto C, Nakazawa M, Morozumi T, Kashiwazaki M (1993) The epidemiological studies of Streptococcus suis infections in Japan from 1987 to 1991. J Vet Med Sci 55:623–626
Okwumabua O, Peterson H, Hsu HM, Bochsler P, Behr M (2017) Isolation and partial characterization of Streptococcus suis from clinical cases in cattle. J Vet Diagn Invest 29:160–168
Komatsu T, Watando E, Inaba N, Sugie K, Okura M, Shibahara T (2018) Bovine vegetative endocarditis caused by Streptococcus suis. J Vet Med Sci 80:1567–1571
Okura M, Lachance C, Osaki M, Sekizaki T, Maruyama F, Nozawa T, Nakagawa I, Hamada S, Rossignol C, Gottschalk M, Takamatsu D (2014) Development of a two-step multiplex PCR assay for typing of capsular polysaccharide synthesis gene clusters of Streptococcus suis. J Clin Microbiol 52:1714–1719
Petts DN (1984) Colistin-oxolinic acid-blood agar: a new selective medium for streptococci. J Clin Microbiol 19:4–7
Dorsch M, Stackebrandt E (1992) Some modifications in the procedure of direct sequencing of PCR amplified 16S rDNA. J Microbiol Methods 16:271–279
Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J (2017) Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol 67:1613–1617
Kim M, Oh HS, Park SC, Chun J (2014) Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int J Syst Evol Microbiol 64:346–351
Poyart C, Quesne G, Coulon S, Berche P, Trieu-Cuot P (1998) Identification of streptococci to species level by sequencing the gene encoding the manganese-dependent superoxide dismutase. J Clin Microbiol 36:41–47
Katoh K, Rozewicki J, Yamada KD (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20:1160–1166
Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, Brown CT, Porras-Alfaro A, Kuske CR, Tiedje JM (2014) Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res 42:D633–D642
Okura M, Nozawa T, Watanabe T, Murase K, Nakagawa I, Takamatsu D, Osaki M, Sekizaki T, Gottschalk M, Hamada S, Maruyama F (2017) A locus encoding variable defence systems against invading DNA identified in Streptococcus suis. Genome Biol Evol 9:1000–1012
Arai R, Tominaga K, Wu M, Okura M, Ito K, Okamura N, Onishi H, Osaki M, Sugimura Y, Yoshiyama M, Takamatsu D (2012) Diversity of Melissococcus plutonius from honeybee larvae in Japan and experimental reproduction of European foulbrood with cultured atypical isolates. PLoS One 7:e33708
Heras J, Domínguez C, Mata E, Pascual V, Lozano C, Torres C, Zarazaga M (2015) GelJ—a tool for analyzing DNA fingerprint gel images. BMC Bioinformatics 16:270
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120
Zerbino DR, Birney E (2008) Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res 18:821–829
Seemann T (2014) Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069
Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MT, Fookes M, Falush D, Keane JA, Parkhill J (2015) Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 31:3691–3693
Jia B, Raphenya AR, Alcock B, Waglechner N, Guo P, Tsang KK, Lago BA, Dave BM, Pereira S, Sharma AN, Doshi S, Courtot M, Lo R, Williams LE, Frye JG, Elsayegh T, Sardar D, Westman EL, Pawlowski AC, Johnson TA, Brinkman FS, Wright GD, McArthur AG (2017) CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res 45:D566–D573
Bi D, Xu Z, Harrison EM, Tai C, Wei Y, He X, Jia S, Deng Z, Rajakumar K, Ou HY (2013) ICEberg: a web-based resource for integrative and conjugative elements found in Bacteria. Nucleic Acids Res 57:3348–3357
Carver TJ, Rutherford KM, Berriman M, Rajandream MA, Barrell BG, Parkhill J (2005) ACT: the Artemis Comparison Tool. Bioinformatics 21:3422–3423
Tohya M, Sekizaki T, Miyoshi-Akiyama T (2018) Complete genome sequence of Streptococcus ruminantium sp. nov. GUT-187T (= DSM 104980T = JCM 31869T), the type strain of S. ruminantium, and comparison with genome sequences of Streptococcus suis strains. Genome Biol Evol 10:1180–1184
Cruz Colque JI, Devriese LA, Haesebrouck F (1993) Streptococci and enterococci associated with tonsils of cattle. Lett Appl Microbiol 16:72–74
Ishigaki K, Nakamura A, Iwabuchi S, Kodera S, Ooe K, Kataoka Y, Aida Y (2009) A case of Streptococcus suis endocarditis, probably bovine-transmitted, complicated by pulmonary embolism and spondylitis. Kansenshogaku Zasshi 83:544–548 (in Japanese)
Li M, Wang C, Feng Y, Pan X, Cheng G, Wang J, Ge J, Zheng F, Cao M, Dong Y, Liu D, Wang J, Lin Y, Du H, Gao GF, Wang X, Hu F, Tang J (2008) SalK/SalR, a two-component signal transduction system, is essential for full virulence of highly invasive Streptococcus suis serotype 2. PLoS One 3:e2080
Okura M, Takamatsu D, Maruyama F, Nozawa T, Nakagawa I, Osaki M, Sekizaki T, Gottschalk M, Kumagai Y, Hamada S (2013) Genetic analysis of capsular polysaccharide synthesis gene clusters from all serotypes of Streptococcus suis: potential mechanisms for generation of capsular variation. Appl Environ Microbiol 79:2796–2806
Huang J, Ma J, Shang K, Hu X, Liang Y, Li D, Wu Z, Dai L, Chen L, Wang L (2016) Evolution and diversity of the antimicrobial resistance associated mobilome in Streptococcus suis: a probable mobile genetic elements reservoir for other Streptococci. Front Cell Infect Microbiol 6:118
Huang J, Liang Y, Guo D, Shang K, Ge L, Kashif J, Wang L (2016) Comparative genomic analysis of the ICESa2603 family ICEs and spread of erm(B)- and tet(O)-carrying transferable 89 K-subtype ICEs in swine and bovine isolates in China. Front Microbiol 7:55
Varela NP, Gadbois P, Thibault C, Gottschalk M, Dick P, Wilson J (2013) Antimicrobial resistance and prudent drug use for Streptococcus suis. Anim Health Res Rev 14:68–77
Acknowledgements
We thank Hiroyuki Egashira at the Sendai Livestock Hygiene Service Center and Yumiko Hiramatsu at the Western Center of Livestock Hygiene Service for their assistance with PFGE analysis.
Funding
A part of this work was supported by JSPS KAKENHI Grant Numbers 16H06279, 26870840, 18H02658, 16H05501, 16H01782, 221S0002.
Author information
Authors and Affiliations
Contributions
All authors contributed to preparing the manuscript. M Okura and DT designed the study. M Okura, DT and M Osaki identified the isolates from ruminants through the diagnostic service of NIAH-Japan. TT and YM collected and identified isolates from the tonsillar samples. A Osawa and M Okura performed PFGE analysis. AT, YO and TH conducted genome sequencing and assembled the reads. FM, A Ota and M Okura analysed the genome sequence data. SMS and M Okura performed antibiotic susceptibility testing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1
gdh-PCR positive Streptococcus strains from ruminants in the collection of the National Institute of Animal Health. Detailed information on the 64 strains listed in Table 1.
Additional file 2. PCR positive
Streptococcus strains collected from the tonsils of 110 cattle in this study. Detailed information on the additional 55 strains from 110 cattle is listed in Table 2.
Additional file 3.
Streptococcus spp. strains tested by the S. ruminantium-specific PCR assay developed in this study. Detailed information on the Streptococcus strains used for checking the specificity of the developed S. ruminantium-specific PCR.
Additional file 4. Whole-genome-sequenced strains and general properties of the genomes.
Information on the genome sequence assembly of the 20 S. ruminantium isolates.
Additional file 5. PCR primers for the typing of
cps gene clusters in S. ruminantium. Information on the primer sequences, target genes, and product sizes under the developed cps-typing approach for S. ruminantium.
Additional file 6. Alignment of partial regions of the 16S rRNA gene sequences of
S. suis serotype reference strains and S. ruminantium GUT-187T and DAT741. Primer binding sites for the PCR assay developed in this study and their flanking regions are displayed (forward primer, S. ruminantium GUT-187T positions 42–63; reverse primer, S. ruminantium GUT-187T positions 269–290; accession no. LC195038). Red boxes represent primer binding sites. Yellow shaded letters indicate the deviations from the consensus found in the tested S. ruminantium strains. The letters highlighted with a grey background also indicate deviations from the consensus but were not found in the tested S. ruminantium strains.
Additional file 7. Amplified products from the
S. ruminantium-specific PCR assay with serial dilutions of an S. ruminantium DAT741 DNA template. Lane 1, 2.7 × 107 CFU/mL; 2, 2.7 × 106 CFU/mL CFU/tube; 3, 2.7 × 105 CFU/mL; 4, 2.7 × 104 CFU/mL; 5, 2.7 × 103 CFU/mL; 6, 2.7 × 102 CFU/mL; 7, 27 CFU/mL; 8, Distilled water; M: molecular marker (100 bp + 3 k DNA Ladder, SMOBIO Technology, Taiwan).
Additional file 8.
cps types and sequence types (STs) of four S. suis clinical isolates from cattle used in this study. Information on cps types and allele types determined by MLST of the 4 S. suis clinical isolates from cattle.
Additional file 9. Presence/absence of genes of each COG among 21
S. ruminantium strains. All of the pangenome data of the 21 S. ruminantium strains analysed by Roary.
Additional file 10. Presence/absence of genes of each COG related to capsular polysaccharide synthesis among 21
S. ruminantium strains. Detailed information on the cps genes identified from pangenome data of the 21 S. ruminantium strains
Additional file 11. MICs of tetracycline, erythromycin, chloramphenicol, kanamycin and streptomycin in 14
S. ruminantium strains. MIC data of the 14 S. ruminantium isolates that carried antibiotic resistance genes.
Additional file 12. Presence/absence of the genes of each COG in the genomic islands carrying antibiotic resistance genes identified by CARD analysis with 20
S. ruminantium strains. Detailed information on the locations and genomic islands that included the antibiotic resistance genes identified by CARD.
Additional file 13. ICEs from the ICEberg database showing BLAST hits with the genomic islands carrying antibiotic resistance genes found in
S. ruminantium isolates in this study. First, three ICE hits with the genomic islands that included the identified antibiotic resistance genes.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Okura, M., Maruyama, F., Ota, A. et al. Genotypic diversity of Streptococcus suis and the S. suis-like bacterium Streptococcus ruminantium in ruminants. Vet Res 50, 94 (2019). https://doi.org/10.1186/s13567-019-0708-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13567-019-0708-1