Abstract
In the 2014–2015 Eurasian lineage clade 2.3.4.4A H5 highly pathogenic avian influenza (HPAI) outbreak in the U.S., backyard flocks with minor gallinaceous poultry and large commercial poultry (chickens and turkeys) operations were affected. The pathogenesis of the first H5N8 and reassortant H5N2 clade 2.3.4.4A HPAI U.S. isolates was investigated in six gallinaceous species: chickens, Japanese quail, Bobwhite quail, Pearl guinea fowl, Chukar partridges, and Ring-necked pheasants. Both viruses caused 80–100% mortality in all species, except for H5N2 virus that caused 60% mortality in chickens. The surviving challenged birds remained uninfected based on lack of clinical disease and lack of seroconversion. Among the infected birds, chickens and Japanese quail in early clinical stages (asymptomatic and listless) lacked histopathologic findings. In contrast, birds of all species in later clinical stages (moribund and dead) had histopathologic lesions and systemic virus replication consistent with HPAI virus infection in gallinaceous poultry. These birds had widespread multifocal areas of necrosis, sometimes with heterophilic or lymphoplasmacytic inflammatory infiltrate, and viral antigen in parenchymal cells of most tissues. In general, lesions and antigen distribution were similar regardless of virus and species. However, endotheliotropism was the most striking difference among species, with only Pearl guinea fowl showing widespread replication of both viruses in endothelial cells of most tissues. The expression of IFN-γ and IL-10 in Japanese quail, and IL-6 in chickens, were up-regulated in later clinical stages compared to asymptomatic birds.
Similar content being viewed by others
Introduction
The H5 A/goose/Guangdong/1/1996 (Gs/GD) lineage of highly pathogenic avian influenza (HPAI) virus has spread across multiple continents, affecting wild birds, poultry, and humans [1]. In 2014, Gs/GD lineage clade 2.3.4.4 Group A, also described as Buan2-like or icA, spread across Asia, Europe, and North America [2]. The initial detection of this viral lineage into North America was a reassortant H5N2 with five Eurasian avian influenza (AI) virus gene segments (including the H5 clade 2.3.4.4 hemagglutinin) and three North American wild bird lineage low pathogenic AI (LPAI) virus gene segments [3, 4] detected in November 2014 in British Columbia, Canada. Concurrently, an H5N8 HPAI virus with all 8 gene segments of Eurasian origin and the reassortant H5N2 HPAI virus were detected in a captive-reared gyrfalcon (Falco rusticolus) and a wild Northern pintail duck (Anas acuta), respectively, in Washington state, U.S. Over the next 7 months, the U.S. poultry industry experienced an unprecedented outbreak caused by these H5 HPAI viruses, with more than 7.5 million turkeys and 42.1 million chickens having died or culled during the control program [5] and widespread bans on exports of U.S. poultry and poultry products [6].
During the outbreak, virus was detected in 21 backyard flocks that included: (i) minor gallinaceous poultry species (quail, guinea fowl, pheasants, partridges, and grouse) on the same premise; (ii) different breeds of chickens and turkeys; and (iii) domestic ducks and geese [5, 7]. Minor gallinaceous poultry are commonly raised in small commercial outdoor operations and backyards, or are sometimes marketed in temporary or permanent live poultry markets (LPMs) [8], which highlights their importance as intermediary hosts in virus transmission under poor biosecurity conditions. Globally, two facts support their importance in AI epidemiology: (i) outdoor-raised systems often have higher potential of AI virus exposure from wild waterfowl, contributing to the increase of AI outbreaks and their impact [9]; and (ii) LPMs, with tendency for prevalent AI virus [10], contain a wide variety of live poultry and non-poultry species, providing the ideal environment for introduction, maintenance, and adaptation of viruses, as well as potential conditions for zoonotic transmission [11,12,13]. Although there is no direct epidemiological link between minor gallinaceous poultry from backyard flocks and chickens and turkeys from commercial farms in these U.S. clade 2.3.4.4A virus outbreaks [14], minor poultry have been identified or suggested as key link species in other outbreaks [15,16,17,18]. Moreover, the game bird poultry industry and backyard flocks in both developed and developing countries are known to suffer from HPAI epidemics [19].
Gallinaceous species infected with clade 2.3.4.4 viruses generally exhibit clinical disease, mortality, and pathological features that are indicative of HPAI virus infection [2], although several studies have pointed out that clade 2.3.4.4 H5 reassortants have reduced virulence compared to the parental Gs/GD H5N1 virus [2, 4, 20]. Lee et al. examined the pathogenicity of clade 2.3.4.4 viruses in Japanese quail and showed severe clinical disease, virus shedding, and contact transmission following challenge with 6 log10 EID50 of Korean Group A H5N8 virus [21]. Prior to the emergence of clade 2.3.4.4 viruses, numerous outbreaks of both LPAI and HPAI viruses had been reported in species such as Japanese quail, Pearl guinea fowl, and Ring-necked pheasants [19, 22]. Some studies suggest that certain gallinaceous species like Ring-necked pheasants and Japanese quail are more susceptible to LPAI viruses from free-living aquatic birds than chickens and turkeys [19, 23,24,25,26,27,28]. Others show that Japanese quail and European quail (Coturnix c. coturnix) may support the replication of almost all LPAI virus subtypes [26, 29]. Japanese quail are recognized as mixing vessels for avian and mammalian viruses [30,31,32,33] and facilitate the adaptation of duck viruses to chicken [34,35,36]. In addition, several studies have proven that HPAI viruses are able to infect and cause lesions and death in many gallinaceous species under experimental conditions [19, 37, 38]. Collectively, these findings highlight the relevance of avian species other than chickens, turkeys, and domestic ducks in the epidemiology of AI in small farming operations, village poultry, and LPMs.
Recently, we experimentally confirmed that the first U.S. Eurasian H5N8 and reassortant H5N2 clade 2.3.4.4A HPAI viruses lacked adaptation to chickens, i.e. 4.4 and 5.7 log10 mean bird infectious doses (BID50), respectively [39], but were more adapted to minor gallinaceous poultry, i.e. < 3.7 log10 BID50 for direct infection [40]. In addition, these higher BID50 required to produce chicken infections also resulted in greatly reduced contact transmission. Here we present the pathology results from our previously published infectivity studies [39, 40]. Specifically, we examined the severity and distribution of gross and microscopic lesions in experimentally infected birds and identified the organs and cell types with AI virus replication. In addition, we analyzed the innate immune response in chickens and Japanese quail at different clinical stages.
Materials and methods
Viruses
The influenza A isolates A/Gyrfalcon/Washington/40188-6/2014 (H5N8) and A/Northern pintail/Washington/40964/2014 (H5N2) were used as challenge viruses. These were the first two HPAI isolates from the U.S. outbreak and they are considered representative of the initial AI viruses from wild waterfowl introduction of both the Eurasian lineage H5N8 viruses and the reassortant Eurasian/North American lineage H5N2 viruses, respectively [40]. The viruses were propagated and titrated by allantoic sac inoculation of 9–10 day-old embryonated chicken eggs by standard methods [41].
Birds and housing
Six species of the order Galliformes were utilized: specific pathogen free White Leghorn chickens (Gallus domesticus; Southeast Poultry Research Laboratory [SEPRL], Athens, GA, USA), Japanese quail (Coturnix c. japonica; McMurray Hatchery, Webster City, IA, USA), Bobwhite quail (Colinus virginianus; M&M Quail Farm Inc., Gillsville, GA, USA), Pearl guinea fowl (Numida meleagris; McMurray Hatchery), Chukar partridges (Alectoris chukar; McMurray Hatchery), and Ring-necked pheasants (Phasianus colchicus; McMurray Hatchery). All birds were inoculated at 4 weeks of age. Prior to inoculation, 30–50% of birds were randomly sampled and confirmed negative for current infection with or previous exposure to AI virus [42, 43]. Each experimental group was housed separately in negative-pressure isolators with HEPA-filtered inlet air. Birds had ad libitum access to feed and water. All procedures were performed according to the requirements of the protocol approved by the Institutional Laboratory Animal Care and Use Committee.
Experimental design and sampling
Bird inoculation and sampling were performed as previously described [39, 40]. Briefly, each species was divided into three groups: H5N2 virus inoculated group, H5N8 virus inoculated group, and sham-inoculated group (10 to 17 birds/virus group and 5 birds/sham group). Birds were inoculated intrachoanally with approximately 6 log10 mean egg infectious doses (EID50) of H5N2 or H5N8 virus, or sterile allantoic fluid. This challenge dose was necessitated for comparison with previous HPAI pathogenesis studies [21, 25, 44,45,46,47,48]. Clinical signs were monitored twice a day during the first 4 days post-challenge (dpc) and daily thereafter. Two birds from each species exposed to each virus and showing severe clinical signs or found dead were necropsied at 2 and 3 dpc, except for chickens and Japanese quail that were necropsied at four time points based on clinical progression, as previously described [39, 40]: asymptomatic (twice, at 18 and 24 h post-challenge [hpc]), listless (showing mild to moderate clinical signs), and moribund or dead. One sham-inoculated bird of each species was euthanized and necropsied at the first and the last necropsy time points. At time of necropsy, portions of nasal cavity, brain, thymus, trachea, lung, proventriculus, duodenum, pancreas, jejunum-ileum, spleen, kidney, adrenal gland, gonad, liver, skeletal muscle, comb, and heart were collected in 10% buffered formalin (Thermo Fisher Scientific, Waltham, MA, USA) for histopathologic evaluation. Severely sick birds were euthanized. At 10 dpc, surviving birds were bled to evaluate antibody titers and euthanized.
Histopathology and immunohistochemistry
Tissues in 10% formalin were processed for routine hematoxylin and eosin (HE) staining and immunohistochemical (IHC) staining using a mouse-derived monoclonal antibody (P13C11, developed at SEPRL) specific for type A influenza virus nucleoprotein [39, 44].
Quantification of innate immune response genes
The mRNA expression of genes representative of different innate pathways, including type 1 interferon (IFN) (IFN-α), type 2 IFN (IFN-γ), Th1-type cytokine (interleukin (IL)-12, IL-18), Th2-type cytokine (IL-10), pro-inflammatory cytokine (IL-6), and toll-like receptor (TLR-7), was quantified from formalin-fixed paraffin-embedded (FFPE) lung and spleen tissues from chickens and Japanese quail necropsied at different clinical stages of infection. The FFPE tissue sections and RNA extraction were performed as previously described [49] with modifications. Briefly, ten 10-μm-thick sections were collected and deparaffinized, total RNA was extracted using the RNeasy FFPE Kit (Qiagen, Germantown, MD, USA), and RNA concentration and purity were measured on a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific). The cDNA was synthetized with the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific) using approximately 500 ng of RNA and following the manufacturer’s protocol and thermal cycling conditions. The mRNA expressions of the aforementioned genes were quantified by quantitative real time PCR (qRRT-PCR) using gene specific primers and conditions previously described [50, 51] with modifications. Briefly, qRRT-PCR was performed on a 7500 FAST Real-time PCR System (Applied Biosystems, Foster City, CA, USA). The qRRT-PCR reaction mixture contained 1.0 μL of sample cDNA, 0.1 to 0.5 μL of forward and reverse primers (1 to 5 μM each, standardized for efficient detection of target gene and absence of dimers) (Additional file 1), 5 μL of KAPA SYBR FAST MasterMix (KAPA Biosystems, Wilmington, MA, USA), and nuclease free water for a final reaction volume of 10.0 μL. The thermal profile consisted of one cycle for 3 min of polymerase activation at 95 °C, followed by 45 cycles of PCR at 95 °C for 10 s and specific annealing temperature of 60 °C for 30 s. After the completion of amplification step, the dissociation (melting) curve was performed at 95 °C for 10 s and 60 °C for 30 s. The relative expression of each target gene was normalized using the housekeeping gene β-actin (Additional file 1). The expression of β-actin was constant within each species regardless of clinical stage and tissue. The relative quantification of gene expression was done by the 2 − ΔΔct formula and expressed as fold change in infected birds compared to sham (negative control) birds. Results from H5N2 and H5N8 virus infected birds were pooled due to similar mRNA expression levels for all the genes. Similarly, results from moribund and dead birds were pooled due to similar mRNA expression levels for all the genes. After normalization with β-actin, gene expression results in infected birds were analyzed based on species, tissue, and clinical stage and compared to sham birds. Our data had a non-parametric distribution and was analyzed with Kruskal–Wallis test and Dunn’s Multiple Comparison Test using Prism 7 (GraphPad software, San Diego, CA, USA). A p value of < 0.05 was considered to be significant.
Results
Clinical signs, mortality, and gross lesions
Previously, we determined that intrachoanal inoculation of 6 log10 EID50 of either H5N2 or H5N8 virus caused 80–100% mortality in the six gallinaceous species [39, 40], with the exception of H5N2 virus that caused 60% mortality in chickens [39] (Table 1). Mean death times (MDTs) ranged from 2.5 to 5.2 days and were not significantly different among them [39, 40] (Table 1). The surviving birds were considered uninfected based on lack of clinical disease and lack of HA antibodies at the end of the experiment [39, 40]. Among the infected birds, clinical signs and gross lesions are described in detail for chickens [39] and the other gallinaceous species [40] elsewhere.
Microscopic findings
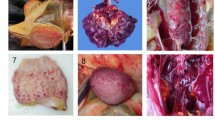
Multifocal areas of necrosis, sometimes accompanied by heterophilic or lymphoplasmacytic inflammatory infiltrate, with viral antigen were widespread in the parenchymal cells of most tissues (Tables 2, 3, 4 and Figure 1). In general, asymptomatic or listless chickens and Japanese quail did not show significant histopathological lesions or antigen staining, with some exceptions like severe vacuolation and necrosis of the pancreatic acinar epithelium of a listless H5N2 virus infected Japanese quail (Figure 1A) and nucleoprotein-positive pancreatic acinar cells (Figure 1D). More severe lesions and widespread viral staining were observed in moribund and dead birds, especially in lung, heart, brain, pancreas, spleen, and adrenal gland.
Histological lesions in gallinaceous following experimental infection with clade 2.3.4.4A HPAI viruses. A/Northern pintail/Washington/40964/2014 (H5N2); A/Gyrfalcon/Washington/40188-6/2014 (H5N8). Magnification ×40. Japanese quail, listless (3 dpc), H5N2 virus, pancreas, HE staining (A) and IHC staining (D). Bobwhite quail, 3 dpc, H5N2 virus, respiratory epithelium, HE staining (B) and IHC staining (E). Pearl guinea fowl, 2 dpc, H5N8 virus, lung (endothelium), HE staining (C) and IHC staining (F). Ring-necked pheasant, 3 dpc, H5N2 virus, cerebrum, IHC staining (G). Ring-necked pheasant, 3 dpc, H5N2 virus, kidney, IHC staining (H). Chukar partridge, 3 dpc, H5N8 virus, adrenal gland, IHC staining (I).
Collectively, similar type and severity of histological lesions and antigen staining were observed with all species and both viruses, although some differences between the two viruses were observed (Tables 2 and 3). In the pancreas, lesions and staining were mild and infrequent in H5N2 virus infected birds, but were severe and widespread in H5N8 virus infected birds; in the thymus and cloacal bursa, the opposite was observed. A remarkable difference among species was observed in the kidney: Chukar partridges and Ring-necked pheasants (Figure 1H) displayed mild to moderate nephrosis and widespread staining, while chickens, Japanese quail, and Bobwhite quail had generally no lesions or staining, and lesions and staining in Pearl guinea fowl tissues were in between in extent. Another difference among species was found in nasal cavity, which was especially affected in Bobwhite quail (Figures 1B–E) and Pearl guinea fowl. Virus staining in vascular endothelial cells was infrequent in all the species examined except for Pearl guinea fowl, which showed extensive endothelial cell staining with both viruses. In particular, vascular endothelial cells expressed virus antigen in the following tissues: sheath arterioles of spleen of H5N2 virus inoculated Japanese quail; comb of H5N8 and nasal cavity of H5N2 virus inoculated Bobwhite quail; systemically in Pearl guinea fowl (Figure 1F); ovary of H5N8 virus inoculated Ring-necked pheasants; spleen and proventriculus of H5N2 and brain of H5N8 virus inoculated Chukar partridges.
Immune gene expression profiles
The mRNA expression of innate immune response genes is summarized in Figure 2. The mRNA levels of IFN-γ were significantly up-regulated in lung of moribund/dead Japanese quail compared to asymptomatic birds. Similarly, the mRNA levels of IL-10 were significantly up-regulated in both lung and spleen of Japanese quail in later clinical stages compared to earlier clinical stages. In chickens, IL-6 was the only gene with significant variation among clinical stages, as its mRNA levels were significantly up-regulated in spleen of moribund/dead chickens compared to asymptomatic ones. Several genes were down-regulated in some clinical stages when compared to shams, like IFN-α, IL-12, and IL-18 for both species; IFN-γ and IL-10 for chickens; and TLR-7 for Japanese quail.
Relative RNA expression of innate immune genes in chicken (Ck) and Japanese quail (JQ). The fold change was calculated relative to levels of mRNA expression in infected birds compared to sham birds. Data plotted in green represents down-regulation (significantly lower than shams). Blue (*) shows significant differences between clinical stages ● asymptomatic, ■ listless, and ▲ moribund/dead. Significance p < 0.05.
Discussion
Since 2014, Gs/GD H5 HPAI clade 2.3.4.4 viruses have spread rapidly and globally by migratory aquatic birds and have evolved through reassortment with prevailing local LPAI viruses [2]. Under both experimental and natural conditions, a wide range of avian species including wild and domestic waterfowl, domestic poultry, and even zoo birds appear to be permissive for infection by and/or transmission with these viruses [2]. In the present study, the pathobiology of the first U.S. clade 2.3.4.4A HPAI viruses in the 2014–2015 outbreak was investigated in six gallinaceous species. In addition, innate immune responses to infection with these viruses were analyzed in chickens and Japanese quail.
We previously confirmed that these two viruses were poorly adapted to chickens, based on high mean chicken infectious dose (i.e. 4.4 and 5.7 log10 BID50) and poor transmissibility [39], but were more adapted to minor gallinaceous poultry (< 3.7 BID50) [40]. As expected with 6 log10 EID50 challenge dose, some chickens did not become infected, as evident by only 60% mortality with H5N2, and the survivors were confirmed as not being infected because they lacked clinical disease, virus shedding, and HA antibodies [39]. By contrast, high morbidity and mortality rates and severe pathobiology upon challenge with 6 log10 EID50 confirmed that these viruses were highly pathogenic for minor gallinaceous poultry [40].
In the present study, chickens and Japanese quail were necropsied at four time-points based on clinical progression. Similar type and severity of histological lesions and antigen staining were observed in both species and for both viruses at each clinical stage, but differences were observed between clinical stages. Asymptomatic and listless chickens and Japanese quail (necropsied from 18 hpc to 2–3 dpc) lacked histopathological lesions or antigen staining in the tissue samples tested, while moribund or dead birds (necropsied from 2 to 5 dpc) displayed more severe lesions and widespread viral staining in known HPAI virus-target tissues and cells [47]. Although rapidity of clinical progression is highly dependent on the HPAI virus strain [47], our findings are in line with previous studies showing lack of antigen staining when clinical signs are absent, but severe histopathological lesions and widespread antigen staining in moribund or dead gallinaceous birds [44, 52]. Our observations suggest that there is a short time window between the initial virus replication in epithelial cells of the respiratory tract and the subsequent viremia and dissemination to multiple organs, where the virus replicates in parenchymal cells [53]. The other four species were necropsied when showing severe clinical signs or found dead (at 2 and 3 dpc), with both viruses showing similar type and severity of histological lesions and antigen detection in tissues across species.
Among the few discrepancies observed in virus replication in tissues of different species, the vascular endothelium showed the most remarkable variations. We observed that chickens, Japanese quail, Bobwhite quail, Chukar partridges, and Ring-necked pheasants lacked widespread virus replication in capillary endothelial cells at any clinical stage, with consequent lack of severe edematous and hemorrhagic lesions. In contrast, Pearl guinea fowl presenting severe clinical signs or found dead had endothelial cell staining for both viruses in capillary endothelium of almost all tissues. Previous studies have shown that endotheliotropism is common in gallinaceous poultry infections with HPAI viruses [46, 54]. Such tropism has been extensively studied in chickens infected with early H5N1 HPAI Gs/GD viruses, which typically show widespread virus replication in vascular endothelium alongside edematous, hemorrhagic, and necrotic cutaneous lesions [44, 45, 48]. Noteworthy, these early H5N1 HPAI Gs/GD viruses are well adapted to and remarkably virulent for chickens [38, 55]. Peracute HPAI virus infections tend to be more endotheliotropic than subacute infections, with subacute infections having more extensive virus replication in parenchymal cells of visceral organs [38, 53]. Therefore, it is unclear why the clade 2.3.4.4A HPAI viruses tested in the present study replicated systemically and extensively in endothelial cells of Pearl guinea fowl but had infrequent endothelial replication and in a limited number of tissues in the other gallinaceous species, even in peracute infections. Interestingly, Pearl guinea fowl not only showed some of the lowest BID50 among the gallinaceous species tested, but also some of the shortest MDTs [40], indicating that the virus was well adapted to this host. Further studies are needed to elucidate the mechanisms that determine either restrictive or permissive replication of clade 2.3.4.4A viruses in endothelial cells of different gallinaceous species.
Several studies have shown that different avian species display differential innate immune responses to AI infection [51, 56,57,58,59,60], and that these responses tend to correlate with different pathobiology outcomes [57, 60, 61]. Here, the innate immune responses of chickens and Japanese quail infected with two H5Nx HPAI viruses were compared in order to elucidate any potential link between cytokine responses and clinical or pathological progression of infection. Type II IFN-γ and Th2-type cytokine IL-10 in Japanese quail, and pro-inflammatory IL-6 in chickens, were up-regulated in later clinical stages compared to asymptomatic birds, probably in response to widespread virus replication in parenchymal cells. Previously, Uno et al. found up-regulation of IFN-γ, IL-10, and IL-6 genes in peripheral blood mononuclear cells of Japanese quail collected 24 h after Gs/GD H5N1 HPAI virus challenge and before showing severe neurologic signs around 3 dpc [51]. This supports the idea that the pro-inflammatory cytokine IL-6 is produced early after infection as part of the induced innate immune response and has been associated with the recruitment of inflammatory cells and severe pathology [58, 62, 63]. Similarly, IL-6 was up-regulated in lung and spleen collected from H5 or H7 HPAI virus inoculated chickens [57, 58]. Besides IL-6, innate responses of infected chickens were similar or down-regulated compared to shams, which differs from other studies that find up-regulation in the expression of IFN-α, IFN-γ, and IL-12 in lung and spleen of chickens infected with H5N1 HPAI viruses, H7 HPAI and LPAI viruses, or H9N2 LPAI [56,57,58, 60, 64, 65]. However, in line with our findings, TLR-7 remained stable in lung of chickens infected with H7 HPAI virus [57], and IL-10 remained stable in lung and spleen of chickens infected with Gs/GD H5 HPAI virus clade 1 [66]. In the present study, chickens appeared to elicit weaker innate immune responses than Japanese quail, suggesting that the lower infectivity and replication of these viruses in chickens may trigger weaker antiviral immune responses. It is worth mentioning that, initially, the innate immune gene expression analysis was performed separately for moribund and dead birds. All genes analyzed had similar mRNA expression levels in moribund and dead birds within each species, confirming that tissue necrosis did not alter cytokine expression. Also, it is worth emphasizing that quantification of innate immune genes was performed on FFPE tissues. Despite the improved isolation methods, RNA obtained from FFPE tissues can be of lower quality and smaller size (less than 200 bp) than fresh tissues due to formalin-induced cross-linking and deterioration of RNA during fixation and storage [67, 68]. Consequently, smaller amplicon sizes are needed for optimal sensitivity [67], with targets in the 70–150-bp being the ideal range [69]. Yet, FFPE tissues have been commonly used for the analysis of RNA expression because of the high availability of FFPE-preserved clinical samples and the direct correlation of these analyses with clinical data [67, 70]. Therefore, although many of our results corresponded with previous data, they should be interpreted with caution.
In conclusion, although the first U.S. clade 2.3.4.4A HPAI viruses in the 2014–2015 outbreak were differently adapted to the six gallinaceous species studied here [39, 40], we observed similar type and severity of histopathological lesions and antigen distribution in those birds that became infected, regardless of virus and species. Asymptomatic or listless infected chickens and Japanese quail lacked microscopic findings, emphasizing the risk of unrecognized virus spread if only passive surveillance is practiced. These viruses appear to have high mortality in minor gallinaceous poultry without prior adaptation, supporting the relevance of minor poultry species in the epidemiology of HPAI as intermediate hosts between wild waterfowl and major commercial gallinaceous poultry. The striking endotheliotropism in Pearl guinea fowl but not the other species calls for further investigation.
Availability of data and materials
The datasets generated and/or analyzed during the current study are included in this published article and its supplementary information files, or are available from the corresponding author on reasonable request.
Abbreviations
- AI:
-
avian influenza
- BID50 :
-
mean bird infectious dose
- dpc:
-
day post-challenge
- EID50 :
-
mean egg infectious dose
- FFPE:
-
formalin-fixed paraffin-embedded
- Gs/GD:
-
A/goose/Guangdong/1/1996
- HE:
-
hematoxylin and eosin
- HPAI:
-
highly pathogenic avian influenza
- IFN:
-
interferon
- IL:
-
interleukin
- IHC:
-
immunohistochemistry
- LPM:
-
live poultry market
- LPAI:
-
low pathogenic avian influenza
- MDT:
-
mean death time
- nt:
-
no tissue
- qRRT-PCR:
-
quantitative real-time RT-PCR
- SEPRL:
-
Southeast Poultry Research Laboratory
- TLR:
-
toll-like receptor
References
Sims LD, Brown IH (2016) Multi-continental panzootic of H5 highly pathogenic avian influenza (1996–2015). In: Swayne DE (ed) Animal influenza. Wiley Blackwell, Ames
Lee DH, Bertran K, Kwon JH, Swayne DE (2017) Evolution, global spread, and pathogenicity of highly pathogenic avian influenza H5Nx clade 2.3.4.4. J Vet Sci 18:269–280
Ip HS, Torchetti MK, Crespo R, Kohrs P, DeBruyn P, Mansfield KG, Baszler T, Badcoe L, Bodenstein B, Shearn-Bochsler V, Killian ML, Pedersen JC, Hines N, Gidlewski T, DeLiberto T, Sleeman JM (2015) Novel Eurasian highly pathogenic avian influenza A H5 viruses in wild birds, Washington, USA, 2014. Emerg Infect Dis 21:886–890
Lee DH, Bahl J, Torchetti MK, Killian ML, Ip HS, DeLiberto TJ, Swayne DE (2016) Highly pathogenic avian influenza viruses and generation of novel reassortants, United States, 2014–2015. Emerg Infect Dis 22:1283–1285
United States Department of Agriculture, Animal and Plant Health Inspection Service (2015) Highly Pathogenic Avian Influenza Infected Premises 2014–2015. https://www.aphis.usda.gov/animal_health/animal_dis_spec/poultry/downloads/hpai-positive-premises-2014-2015.pdf
United States Department of Agriculture, Food Safety and Inspection Service (2015) Export library—requirements by country. http://www.fsis.usda.gov/wps/portal/fsis/topics/international-affairs/exporting-products/export-library-requirements-by-country
World Organisation for Animal Health (OIE) (2017) Update on avian influenza in animals (types H5 and H7). http://www.oie.int/en/animal-health-in-the-world/update-on-avian-influenza/2017/
Sims LD, Weaver J, Swayne DE (2016) Epidemiology of avian influenza in agricultural and other man-made systems. In: Swayne DE (ed) Animal Influenza. Blackwell Publishing, Ames
Alexander DJ (2007) An overview of the epidemiology of avian influenza. Vaccine 25:5637–5644
Ni X, He F, Hu M, Zhou X, Wang B, Feng C, Wu Y, Li Y, Tu J, Li H, Liu M, Chen H, Chen S (2015) Investigation of avian influenza virus in poultry and wild birds due to novel avian-origin influenza A(H10N8) in Nanchang City, China. Microbes Infect 17:48–53
Lee SS, Wong NS, Leung CC (2013) Exposure to avian influenza H7N9 in farms and wet markets. Lancet 381:1815
Lai S, Qin Y, Cowling BJ, Ren X, Wardrop NA, Gilbert M, Tsang TK, Wu P, Feng L, Jiang H, Peng Z, Zheng J, Liao Q, Li S, Horby PW, Farrar JJ, Gao GF, Tatem AJ, Yu H (2016) Global epidemiology of avian influenza A H5N1 virus infection in humans, 1997-2015: a systematic review of individual case data. Lancet Infect Dis 16:e108–e118
World Organisation for Animal Health (OIE) (2018) Cumulative number of confirmed human cases of avian influenza A(H5N1) reported to WHO. http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/
United States Department of Agriculture, Animal and Plant Health Inspection Service (2015) Epidemiologic and other analyses of HPAI-affected poultry flocks, September 2015. https://www.aphis.usda.gov/animal_health/animal_dis_spec/poultry/downloads/Epidemiologic-Analysis-Sept-2015.pdf
Peiris M, Yuen KY, Leung CW, Chan KH, Ip PL, Lai RW, Orr WK, Shortridge KF (1999) Human infection with influenza H9N2. Lancet 354:916–917
Guan Y, Shortridge KF, Krauss S, Chin PS, Dyrting KC, Ellis TM, Webster RG, Peiris M (2000) H9N2 influenza viruses possessing H5N1-like internal genomes continue to circulate in poultry in southeastern China. J Virol 74:9372–9380
Bosco-Lauth AM, Bowen RA, Root JJ (2016) Limited transmission of emergent H7N9 influenza A virus in a simulated live animal market: do chickens pose the principal transmission threat? Virology 495:161–166
Turner JC, Feeroz MM, Hasan MK, Akhtar S, Walker D, Seiler P, Barman S, Franks J, Jones-Engel L, McKenzie P, Krauss S, Webby RJ, Kayali G, Webster RG (2017) Insight into live bird markets of Bangladesh: an overview of the dynamics of transmission of H5N1 and H9N2 avian influenza viruses. Emerg Microbes Infect 6:e12
Bertran K, Dolz R, Majo N (2014) Pathobiology of avian influenza virus infection in minor gallinaceous species: a review. Avian Pathol 43:9–25
Sun H, Pu J, Hu J, Liu L, Xu G, Gao GF, Liu X, Liu J (2016) Characterization of clade 2.3.4.4 highly pathogenic H5 avian influenza viruses in ducks and chickens. Vet Microbiol 182:116–122
Lee DH, Kwon JH, Noh JY, Park JK, Yuk SS, Erdene-Ochir TO, Lee JB, Park SY, Choi IS, Lee SW, Song CS (2016) Pathogenicity of the Korean H5N8 highly pathogenic avian influenza virus in commercial domestic poultry species. Avian Pathol 45:208–211
Carnaccini S, Crossley B, Breitmeyer R, Charlton BR, Bland M, Fowler K, De La Torre F, Torchetti MK, Wong SS, Wilson D, Jones A, Senties-Cue CG (2015) Diagnosis and control of a LPAI H5N8 outbreak in a Japanese quail (Coturnix coturnix japonica) commercial flock in the Central Valley of California. Avian Dis 59:344–348
Senne DA, Pederson JC, Panigraphy B (2005) Live-bird markets in the Northeastern United States: a source of avian influenza in commercial poultry. In: Koch G, Schrijver RS (eds) Avian Influenza. Prevention and Control. Springer, Dordrecht
Humberd J, Boyd K, Webster RG (2007) Emergence of influenza A virus variants after prolonged shedding from pheasants. J Virol 81:4044–4051
Bertran K, Perez-Ramirez E, Busquets N, Dolz R, Ramis A, Darji A, Abad FX, Valle R, Chaves A, Vergara-Alert J, Barral M, Hofle U, Majo N (2011) Pathogenesis and transmissibility of highly (H7N1) and low (H7N9) pathogenic avian influenza virus infection in red-legged partridge (Alectoris rufa). Vet Res 42:24
Bertran K, Dolz R, Busquets N, Gamino V, Vergara-Alert J, Chaves AJ, Ramis A, Abad FX, Hofle U, Majo N (2013) Pathobiology and transmission of highly and low pathogenic avian influenza viruses in European quail (Coturnix c. coturnix). Vet Res 44:23
Bonfante F, Patrono LV, Aiello R, Beato MS, Terregino C, Capua I (2013) Susceptibility and intra-species transmission of the H9N2 G1 prototype lineage virus in Japanese quail and turkeys. Vet Microbiol 165:177–183
Pantin-Jackwood MJ, Miller PJ, Spackman E, Swayne DE, Susta L, Costa-Hurtado M, Suarez DL (2014) Role of poultry in the spread of novel H7N9 influenza virus in China. J Virol 88:5381–5390
Makarova NV, Ozaki H, Kida H, Webster RG, Perez DR (2003) Replication and transmission of influenza viruses in Japanese quail. Virology 310:8–15
Castrucci MR, Donatelli I, Sidoli L, Barigazzi G, Kawaoka Y, Webster RG (1993) Genetic reassortment between avian and human influenza A viruses in Italian pigs. Virology 193:503–506
Perez DR, Lim W, Seiler JP, Yi G, Peiris M, Shortridge KF, Webster RG (2003) Role of quail in the interspecies transmission of H9 influenza A viruses: molecular changes on HA that correspond to adaptation from ducks to chickens. J Virol 77:3148–3156
Perez DR, Webby RJ, Hoffmann E, Webster RG (2003) Land-based birds as potential disseminators of avian mammalian reassortant influenza A viruses. Avian Dis 47:1114–1117
Obadan AO, Kimble BJ, Rajao D, Lager K, Santos JJ, Vincent A, Perez DR (2015) Replication and transmission of mammalian-adapted H9 subtype influenza virus in pigs and quail. J Gen Virol 96:2511–2521
Tashiro M, Reinacher M, Rott R (1987) Aggravation of pathogenicity of an avian influenza virus by adaptation to quails. Arch Virol 93:81–95
Cilloni F, Toffan A, Giannecchini S, Clausi V, Azzi A, Capua I, Terregino C (2010) Increased pathogenicity and shedding in chickens of a wild bird-origin low pathogenicity avian influenza virus of the H7N3 subtype following multiple in vivo passages in quail and turkey. Avian Dis 54:555–557
Giannecchini S, Clausi V, Di Trani L, Falcone E, Terregino C, Toffan A, Cilloni F, Matrosovich M, Gambaryan AS, Bovin NV, Delogu M, Capua I, Donatelli I, Azzi A (2010) Molecular adaptation of an H7N3 wild duck influenza virus following experimental multiple passages in quail and turkey. Virology 408:167–173
Spickler AR, Trampel DW, Roth JA (2008) The onset of virus shedding and clinical signs in chickens infected with high-pathogenicity and low-pathogenicity avian influenza viruses. Avian Pathol 37:555–577
Pantin-Jackwood MJ, Swayne DE (2009) Pathogenesis and pathobiology of avian influenza virus infection in birds. Rev Sci Tech 28:113–136
Bertran K, Swayne DE, Pantin-Jackwood MJ, Kapczynski DR, Spackman E, Suarez DL (2016) Lack of chicken adaptation of newly emergent Eurasian H5N8 and reassortant H5N2 high pathogenicity avian influenza viruses in the U.S. is consistent with restricted poultry outbreaks in the Pacific flyway during 2014–2015. Virology 494:190–197
Bertran K, Lee DH, Pantin-Jackwood MJ, Spackman E, Balzli C, Suarez DL, Swayne DE (2017) Pathobiology of clade 2.3.4.4 H5Nx high-pathogenicity avian influenza virus infections in minor gallinaceous poultry supports early backyard flock introductions in the Western United States in 2014–2015. J Virol 91:e00960
Swayne DE, Senne DA, Beard CW (1998) Influenza. In: Swayne DE, Glisson JR, Jackwood MW, Pearson JE, Reed WM (eds) Isolation and Identification of Avian Pathogens. American Association of Avian Pathologists, Kennett Square
Spackman E, Senne DA, Myers TJ, Bulaga LL, Garber LP, Perdue ML, Lohman K, Daum LT, Suarez DL (2002) Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J Clin Microbiol 40:3256–3260
Pedersen JC (2008) Hemagglutination-inhibition test for avian influenza virus subtype identification and the detection and quantitation of serum antibodies to the avian influenza virus. Methods Mol Biol 436:53–66
Perkins LE, Swayne DE (2001) Pathobiology of A/chicken/Hong Kong/220/97 (H5N1) avian influenza virus in seven gallinaceous species. Vet Pathol 38:149–164
Antarasena C, Sirimujalin R, Prommuang P, Blacksell SD, Promkuntod N, Prommuang P (2006) Tissue tropism of a Thailand strain of high-pathogenicity avian influenza virus (H5N1) in tissues of naturally infected native chickens (Gallus gallus), Japanese quail (Coturnix coturnix japonica) and ducks (Anas spp.). Avian Pathol 35:250–253
Swayne DE (2007) Understanding the complex pathobiology of high pathogenicity avian influenza viruses in birds. Avian Dis 51:242–249
Swayne DE, Pantin-Jackwood MJ (2008) Pathobiology of avian influenza virus infections in birds and mammals. In: Swayne DE (ed) avian influenza. Blackwell Publishing, Ames
Jeong OM, Kim MC, Kim MJ, Kang HM, Kim HR, Kim YJ, Joh SJ, Kwon JH, Lee YJ (2009) Experimental infection of chickens, ducks and quails with the highly pathogenic H5N1 avian influenza virus. J Vet Sci 10:53–60
Butt SL, Dimitrov KM, Zhang J, Wajid A, Bibi T, Basharat A, Brown CC, Rehmani SF, Stanton JB, Afonso CL (2019) Enhanced phylogenetic resolution of Newcastle disease outbreaks using complete viral genome sequences from formalin-fixed paraffin-embedded tissue samples. Virus Genes 55:502–512
Uno Y, Usui T, Fujimoto Y, Ito T, Yamaguchi T (2012) Quantification of interferon, interleukin, and Toll-like receptor 7 mRNA in quail splenocytes using real-time PCR. Poult Sci 91:2496–2501
Uno Y, Usui T, Soda K, Fujimoto Y, Takeuchi T, Ito H, Ito T, Yamaguchi T (2013) The pathogenicity and host immune response associated with H5N1 highly pathogenic avian influenza virus in quail. J Vet Med Sci 75:451–457
Chaves AJ, Busquets N, Valle R, Rivas R, Vergara-Alert J, Dolz R, Ramis A, Darji A, Majo N (2011) Neuropathogenesis of a highly pathogenic avian influenza virus (H7N1) in experimentally infected chickens. Vet Res 42:106
Kuiken T, van den Brand J, van Riel D, Pantin-Jackwood M, Swayne DE (2010) Comparative pathology of select agent influenza a virus infections. Vet Pathol 47:893–914
Short KR, Veldhuis Kroeze EJ, Reperant LA, Richard M, Kuiken T (2014) Influenza virus and endothelial cells: a species specific relationship. Front Microbiol 5:653
Swayne DE, Slemons RD (2008) Using mean infectious dose of high- and low-pathogenicity avian influenza viruses originating from wild duck and poultry as one measure of infectivity and adaptation to poultry. Avian Dis 52:455–460
Cornelissen JB, Post J, Peeters B, Vervelde L, Rebel JM (2012) Differential innate responses of chickens and ducks to low-pathogenic avian influenza. Avian Pathol 41:519–529
Cornelissen JB, Vervelde L, Post J, Rebel JM (2013) Differences in highly pathogenic avian influenza viral pathogenesis and associated early inflammatory response in chickens and ducks. Avian Pathol 42:347–364
Kuchipudi SV, Tellabati M, Sebastian S, Londt BZ, Jansen C, Vervelde L, Brookes SM, Brown IH, Dunham SP, Chang KC (2014) Highly pathogenic avian influenza virus infection in chickens but not ducks is associated with elevated host immune and pro-inflammatory responses. Vet Res 45:118
Cao Y, Huang Y, Xu K, Liu Y, Li X, Xu Y, Zhong W, Hao P (2017) Differential responses of innate immunity triggered by different subtypes of influenza a viruses in human and avian hosts. BMC Med Genomics 10(Suppl 4):70
Vidana B, Dolz R, Busquets N, Ramis A, Sanchez R, Rivas R, Valle R, Cordon I, Solanes D, Martinez J, Majo N (2018) Transmission and immunopathology of the avian influenza virus A/Anhui/1/2013 (H7N9) human isolate in three commonly commercialized avian species. Zoonoses Public Health 65:312–321
Vidana B, Martinez J, Martinez-Orellana P, Garcia Migura L, Montoya M, Martorell J, Majo N (2014) Heterogeneous pathological outcomes after experimental pH1N1 influenza infection in ferrets correlate with viral replication and host immune responses in the lung. Vet Res 45:85
Fukuyama S, Kawaoka Y (2011) The pathogenesis of influenza virus infections: the contributions of virus and host factors. Curr Opin Immunol 23:481–486
Kuribayashi S, Sakoda Y, Kawasaki T, Tanaka T, Yamamoto N, Okamatsu M, Isoda N, Tsuda Y, Sunden Y, Umemura T, Nakajima N, Hasegawa H, Kida H (2013) Excessive cytokine response to rapid proliferation of highly pathogenic avian influenza viruses leads to fatal systemic capillary leakage in chickens. PLoS One 8:e68375
Ranaware PB, Mishra A, Vijayakumar P, Gandhale PN, Kumar H, Kulkarni DD, Raut AA (2016) Genome wide host gene expression analysis in chicken lungs infected with avian influenza viruses. PLoS One 11:e0153671
Wang J, Cao Z, Guo X, Zhang Y, Wang D, Xu S, Yin Y (2016) Cytokine expression in three chicken host systems infected with H9N2 influenza viruses with different pathogenicities. Avian Pathol 45:630–639
Karpala AJ, Bingham J, Schat KA, Chen LM, Donis RO, Lowenthal JW, Bean AG (2011) Highly pathogenic (H5N1) avian influenza induces an inflammatory T helper type 1 cytokine response in the chicken. J Interferon Cytokine Res 31:393–400
Ecco R, Brown C, Susta L, Cagle C, Cornax I, Pantin-Jackwood M, Miller PJ, Afonso CL (2011) In vivo transcriptional cytokine responses and association with clinical and pathological outcomes in chickens infected with different Newcastle disease virus isolates using formalin-fixed paraffin-embedded samples. Vet Immunol Immunopathol 141:221–229
Nam SK, Im J, Kwak Y, Han N, Nam KH, Seo AN, Lee HS (2014) Effects of fixation and storage of human tissue samples on nucleic Acid preservation. Korean J Pathol 48:36–42
McKinney MD, Moon SJ, Kulesh DA, Larsen T, Schoepp RJ (2009) Detection of viral RNA from paraffin-embedded tissues after prolonged formalin fixation. J Clin Virol 44:39–42
Penland SK, Keku TO, Torrice C, He X, Krishnamurthy J, Hoadley KA, Woosley JT, Thomas NE, Perou CM, Sandler RS, Sharpless NE (2007) RNA expression analysis of formalin-fixed paraffin-embedded tumors. Lab Invest 87:383–391
Acknowledgements
The authors gratefully acknowledge Kira Moresco, Roger Brock, Jerry Damron, Keith Crawford, Suzanne DeBlois, Marisela Rodriguez, Diane Smith, Mar Costa-Hurtado, and Eric DeJesus for their excellent technical assistance. The authors also thank Dr Alba for statistical assistance.
Funding
This research was supported by USDA/ARS Research Project 6612-32000-066-00D, by the NIAID-funded Center of Excellence in Influenza Research and Surveillance (CEIRS; Contract HHSN272201400008C), by the ARS/Animal and Plant Health Inspection Service (APHIS) interagency agreement Project 60-6040-6-005, and by the DELTA-FLU Project 58-6040-7-012FN. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the USDA or NIH. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA.
Author information
Authors and Affiliations
Contributions
MPJ, ES, DLS, and DES conceived this project. KB, MFC, DHL, CLB, and MPJ conducted the animal experiments and sample processing. KB, MFC, and DHL analyzed the data. KB, MFC, MPJ, and DES drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bertran, K., Pantin-Jackwood, M.J., Criado, M.F. et al. Pathobiology and innate immune responses of gallinaceous poultry to clade 2.3.4.4A H5Nx highly pathogenic avian influenza virus infection. Vet Res 50, 89 (2019). https://doi.org/10.1186/s13567-019-0704-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13567-019-0704-5