Abstract
The principal virulence factor of Shiga toxin (Stx)-producing Escherichia coli (STEC), the eponymous Stx, modulates cellular immune responses in cattle, the primary STEC reservoir. We examined whether immunization with genetically inactivated recombinant Shiga toxoids (rStx1MUT/rStx2MUT) influences STEC shedding in a calf cohort. A group of 24 calves was passively (colostrum from immunized cows) and actively (intra-muscularly at 5th and 8th week) vaccinated. Twenty-four calves served as unvaccinated controls (fed with low anti-Stx colostrum, placebo injected). Each group was divided according to the vitamin E concentration they received by milk replacer (moderate and high supplemented). The effective transfer of Stx-neutralizing antibodies from dams to calves via colostrum was confirmed by Vero cell assay. Serum antibody titers in calves differed significantly between the vaccinated and the control group until the 16th week of life. Using the expression of activation marker CD25 on CD4+CD45RO+ cells and CD8αhiCD45RO+ cells as flow cytometry based read-out, cells from vaccinated animals responded more pronounced than those of control calves to lysates of STEC and E. coli strains isolated from the farm as well as to rStx2MUT in the 16th week. Summarized for the entire observation period, less fecal samples from vaccinated calves were stx1 and/or stx2 positive than samples from control animals when calves were fed a moderate amount of vitamin E. This study provides first evidence, that transfer to and induction in young calves of Stx-neutralizing antibodies by Shiga toxoid vaccination offers the opportunity to reduce the incidence of stx-positive fecal samples in a calf cohort.
Similar content being viewed by others
Introduction
Enterohemorrhagic Escherichia coli (EHEC), a subset of Shiga toxin-producing E. coli (STEC), is a food-borne pathogen that can evoke life-threatening diseases in humans such as hemorrhagic colitis and hemolytic-uremic syndrome. EHEC serotype O157:H7/H− is implicated in most EHEC outbreaks worldwide. However, human infections by non-O157:H7 serotypes (e.g. O91, O26, O113) frequently occur in Germany and other European countries [1]. The only virulence marker common to all STEC serotypes is the possession of a gene encoding for Shiga toxin (Stx). Calves get infected orally with a plethora of different STEC strains present in their environment early in life, but rarely develop clinical signs of infection. Many STEC strains are able to colonize the bovine intestine [2,3,4,5,6], including non-O157:H7 [7,8,9]. Cattle may shed these bacteria for several month in quantities that may be considerably high at some sampling points [10,11,12,13] making cattle, besides other ruminants, an important reservoir for STEC strains associated with human diseases. To reduce the risk of STEC entering the food chain, interventions must be applied at several stages starting at cow and herd level and continuing in slaughterhouses, processing plants, distributors, and households [14, 15].
Even though previous attempts to develop vaccination strategies in cattle were promising they only partially reduce STEC excretion and the effect was mostly restricted to single subpopulations of STEC, e.g. O157 strains [14, 16]. Long-term studies on anti-Stx antibody titers in serum and fecal STEC shedding by cattle unveiled significantly delayed humoral immune responses following experimental STEC infection [17] and natural exposure [12]. Delayed adaptive cellular immune responses was also shown after experimental STEC infection [17]. The principal STEC virulence factor, the eponymous Stx, modulates cellular immune responses in cattle [18,19,20,21]. In vitro and in vivo studies revealed that Stx operates during the early phases of immune activation rather than depressing an established immunity [17, 20, 22, 23]. Current knowledge of STEC shedding dynamics and influences of Stx on immune responses suggests, that Stx may hinder the development of an effective immune response by hitherto immunologically naïve animals upon first STEC contact at early calves’ ages.
Inactivation of Stx by genetic modification located within the enzymatically active cleft of Stx resulted in toxoids (rStx1MUT and rStx2MUT) with retained antigenicity and immunogenicity but lost immunomodulatory properties in cattle [24]. Immunisation of sows with Stx2e toxoid [25, 26] was shown to trigger maternal immunity which protects offspring against edema disease [27] and fully protected the animals when challenged with native Stx2e [28]. Induction of humoral and cellular immune responses by Stx toxoids was also achieved in mice [29, 30].
We therefore hypothesized that passive (maternal) and active vaccination against Stx1 and Stx2 confers protection against the toxins’ immunomodulating effect and subsequently enables calves to actively mount a rapid immune response against STEC strains circulating in the respective cohort. In order to follow a novel approach to add on current vaccination strategies aiming at reducing STEC shedding by cattle, this study wanted to assess if active and passive immunization with Stx toxoid-based vaccines enables calves (i) to gain and produce Stx-neutralizing antibodies and (ii) to mount a more rapid and effective cellular immune response against STEC strains than unvaccinated controls in a respective cohort (iii) resulting in reduction of STEC shedding. As an essential antioxidant for maintaining the stability of biological membranes and the function of the immune system vitamin E (vit E) is considered to support adaptive and humoral immune responses [31,32,33,34]. Therefore, milk replacer fed to some animals deployed in the study was supplemented with vit E in higher amounts as usually included in commercially available products to support the effect of the vaccine.
Materials and methods
Generation of recombinant Shiga toxins and Shiga toxoids
Recombinant Stx (rStx1WT and rStx2WT) and genetically inactivated recombinant Stx toxoids (rStx1MUT and rStx2MUT) were previously generated and tested by Kerner et al. [24]. rStx1MUT and rStx2MUT preparation were adjusted separately with NaCl solution (0.89%) to 0.75 Mio verocytotoxic doses 50% (CD50) equivalents [24] each in 1.4 mL and frozen at −20 °C.
Vaccination of cows for colostrum production
In October and November 2012, 14 cows (Deutsche Holstein) from the dairy herd at the experimental station, FLI Brunswick, served as donors for colostrum to be used in the subsequent year. Five cows had been vaccinated at 9 and 6 weeks before the calculated calving date. To this end, cows received by separate injection in the left and right M. gluteus 0.75 Mio CD50 equivalents of rStx1MUT and of rStx2MUT each in 1.4 mL NaCl solution (0.89%) freshly mixed with 0.6 mL aluminum hydroxide (Alu-Gel-S, Serva Electrophoresis GmbH, Heidelberg, Germany). Further 9 cows served as unvaccinated controls. Colostrum was collected from the first 3 milkings after parturition and screened for Stx-neutralizing antibodies by Vero cell neutralization assay (VNA). Pools of colostrum with high (pool VAC+; anti-Stx1 titer 51 001, anti-Stx2 titer 32 856; determined by VNA, see below) and with lower anti-Stx titer (pool VAC−; anti-Stx1 titer 11 005, anti-Stx2 titer 15 811) were collected, aliquoted and stored (−20 °C).
Experimental design, housing and sampling of calves
In October and November 2013, a total of 48 male Holstein calves born in the dairy herd of FLI Brunswick were alternately assigned to groups of to be rStxMUT-vaccinated animals (VAC+) and placebo-treated animals (VAC−), as well as to vit E high (VitEH) and vit E moderate (VitEM) feeding groups. The treatments were arranged according to a two by two complete 2-factorial design and consequently resulted in four experimental groups: VAC+ VitEH (n = 11), VAC− VitEH (n = 11), VAC+ VitEM (n = 13) and VAC− VitEM (n = 13).
Directly after birth, calves were separated from dams. In the first 9 (± 1.5) days of life animals were kept separately in calf huts on straw. Thereafter calves were kept in 2 groups in accordance with vit E feeding assignment with straw bedding. From weaning until the end of the observation period, animals were housed in groups of different sizes irrespective of their group affiliation together with other bull calves of the same age, not included in the trial. Fecal samples were collected in weeks 3, 16, 26 and 55. Serum samples were collected before initial colostrum intake (pre-colostral), between 6 to 24 h after initial colostrum intake (post-colostral), as well as in weeks 3, 11, 16, 26, 55. For PBMC preparation 16 mL blood (week 16 and 26) were drawn from the jugular vein into tubes containing 4 mL 3.8% sodium citrate dihydrate (Sigma-Aldrich GmbH, Deisenhofen, Germany) solution. The vaccination protocol included a combination of passive and active vaccination with recombinant Shiga toxoids (rStxMUT). Directly after birth, VAC+ calves were fed 3 L of a colostrum pool previously collected from rStxMUT vaccinated cows as described above while VAC− calves received colostrum from non-vaccinated cows. In the 5th and 8th week of life VAC+ animals were actively vaccinated with rStx1MUT and rStx2MUT as described above. VAC− animals were placebo-injected with NaCl solution and adjuvant. After feeding 3 L colostrum, calves were offered 875 g/day of milk replacer (MR) supplying 188 and 200 IU all-rac-α-tocopheryl acetate/kg dry matter (DM) in group VitEM and VitEH, respectively, dissolved in 6 L of water split into two equal portions (bucket feed) daily until the 9th (± 1.5) day of live. The vit E dose fed to VitEM group was referred to as “moderate” as the vit E concentration supplied with the feed was above levels of international recommendations for calves of the respective age [35]. However, the dose used was equivalent to the degree of supplementation of milk replacer commercially distributed and used in modern livestock farming. From 10th to 49th day of life VitEH calves were offered 840 g/day MR supplemented with 188 IU and 354 IU all-rac-α-tocopheryl acetate/kg DM in group VitEMand VitEH by automatic calf feeders (Förster-Technik GmbH, Engern, Germany). Vit E fed groups split by vaccination took up comparable average amounts of all-rac-α-tocopheryl acetate (IU/day: VAC+ VitEH 279.2; VAC− VitEH 276.9; VAC+ VitEM 152.2; VAC− VitEM 154.7) throughout the entire period of performance acquisition. From the 9th day calves received hay ad libitum and concentrate up to 2 kg/day in addition. Drinking water was available for ad libitum intake during the whole experiment. From the 50th day until the end of observation period animals were fed conventionally.
Vero cell cytotoxicity assay (VCA) and Vero cell neutralization assay (VNA)
The VCA was performed in 96-well microtiter plates (Nunc GmbH, Wiesbaden, Germany) using Vero cells (ATCC CRL 1587, LGC-Promochem GmbH, Wesel, Germany) to determine Vero cytotoxic doses 50% (CD50/mL) [36]. The VNA was used for the quantitation of the neutralizing activity in serum and colostrum against rStx1WT or rStx2WT as described previously [12]. Serum samples were tested at pre-dilutions of 1:30 and 1:90, colostrum samples at 1:300 and 1:900. At the pre-colostral sampling only a subset of animal’s sera (n = 23) was tested, at the other samplings all animals were included (n = 48). Neutralizing antibody (nAb) titers were determined by multiplying the relative cell activity with the dilution factor when the relative cell activity was > 30% (rStx1WT) or > 20% (rStx2WT). Samples with a relative cell activity below the detection limit (nAb rStx1 = 900; nAb rStx2 = 600) were given arbitrary nAb titer of half of the lowest detectable value.
PBMC stimulation assay
Five E. coli and 2 Listeria monocytogenes strains isolated from dams of the experimental herd were cultivated in LB broth (lysogeny broth, Lennox), adjusted to 2 × 107 colony forming units (cfu)/mL by optical density and heat inactivated (100 °C, 10 min). Aliquots of each lysate were tested for sterility on sheep blood agar (SIFIN diagnostics GmbH, Berlin, Germany) and stored at −20 °C until use. E. coli lysates were tested for cytotoxicity by VCA and for stx1 and stx2 by PCR. E. coli strain lysates were not cytotoxic except E. coli strain 2 (430 CD50/mL).
Peripheral blood mononuclear cells (PBMC) were isolated as described previously without erythrocytes lysis step [20]. PMBC were suspended in cell culture medium (RPMI 1640 supplemented with 10% fetal calf serum, 3 μM 2-mercaptoethanol, 100 IE/mL Penicillin, 2 mM l-glutamine) and seeded into Cellstar® 24-well suspension plates (Greiner Bio-One GmbH, Frickenhausen, Germany) at 1 mL/well (2 × 106 cells). Pure cell culture medium (MC) or cell culture medium supplemented with stimulants (lysates equivalent to final concentration (f.c.) of 1 × 105 cfu/mL [17], Concanavalin A (ConA) f.c. 0.75 µg/mL, or rStx1MUT or rStx2MUT f.c. 200 CD50 equivalent [24] were added in 1 mL/well. Plates were incubated for 5 days at 5% CO2 and 37 °C.
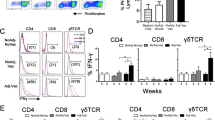
After 5-day incubation, supernatants were collected for further use (see below) and cells gently resuspended with washing buffer (PBS-Dulbecco without Ca and Mg, Biochrom supplemented with 0.01% sodium azide). Cell suspension was transferred to V-shape microtiter plates (Greiner Bio-One GmbH, Frickenhausen, Germany), incubated (10 min), and centrifuged (400 × g, 3 min, 4 °C). Supernatants were removed and cells resuspended with 30 µL of the primary monoclonal antibody dilution (anti-CD45RO clone IL-A116 [Bio-Rad AbD Serotec GmbH, Puchheim, Germany], 1:100). Cells were incubated (30 min, 4 °C, in the dark), washed twice, and resuspended with directly labelled antibodies (anti-CD4 Alexa Fluor 647 clone CC8; anti-CD8α Alexa Fluor 647 clone CC63; anti-CD25 FITC clone IL-A111 [Bio-Rad AbD Serotec GmbH, Puchheim, Germany], 1:200) and secondary antibody solutions (PE labelled α-rat IgG2a ([QIAGEN Leipzig GmbH, Leipzig, Germany], 1:1000; for detection of anti-CD45RO) and incubated again. For isotype control, mouse IgG2a (Alexa Fluor 647 [Bio-Rad AbD Serotec GmbH, Puchheim, Germany], 1:200) and mouse IgG1 (FITC [Bio-Rad AbD Serotec GmbH, Puchheim, Germany], 1:200) was used. Addition of PE-labelled α-rat IgG2a to a control sample without anti-CD45RO was used to exclude non-specific binding of the indirectly labelled antibody. After washing, dead cells were quantified in the first set of experiments by incubating 50 µL cell suspension with 0.75 µL propidium iodide (Merck KGaA, Darmstadt, Germany) within MC. The proportions of dead cells were approx. 6% on average without group differences. At least 5000 cells with unaltered morphology (i.e. events which fell into the non-blast and blast region in the FSC vs. SSC plot) per sample, were assessed by use of a BD FACS Canto™ II analyzer (BD Biosciences, Heidelberg, Germany). CD4+CD45RO+ and CD8αhiCD45RO+ blast cells were analyzed for CD25 expression (FlowJo; Tree Star, Inc., San Carlos, CA, USA; see Figure 1 for the gating strategy). Results were expressed as percentage change compared to MC using the formula: (Geometric mean of fluorescence intensity [Geomean] stimulation—Geomean MC)/Geomean MC × 100%.
Gating strategy used to quantify CD25 expression on inating vitro re-stimulated CD4+CD45RO+ and CD8αhiCD45RO+ cells by flow cytometry. Representative dot plots obtained with PBMC after 5 days of in vitro cultivation with Concanavalin A and subsequent tri-colour immunolabelling. A In a first analytical step, the non-blast cell (Region R0) and blast cell population (R1) were acquired for gating based on its forward versus side scatter (FSC/SSC) characteristics. B In samples labelled with anti-bovine CD4 antibodies, CD4+ cells within the blast cell population were defined by R2 as such that less than 2% of events were considered positive in the corresponding isotype control sample. C In samples labelled with anti-bovine CD8α antibodies, CD8αhi cells were defined by R3, thereby excluding the majority of CD8αlo cells. D Cells within R2 (CD4+) and R3 (CD8αhi) were displayed on CD45RO/CD25 dot plots and the geometric mean for detection of CD25 expression was assessed for all CD45RO+ within the CD4+ and CD8αhi, respectively, as defined by R4 (shaded area).
IFN-γ ELISA of cell culture supernatants
PBMC supernatants were tested for IFN-γ protein by ID Screen® ELISA test kit (ID.vet, Grabels) and evaluated following instructions provided by the manufacturer. Optical density (OD) was converted to S/P ratio following the equation: S/P ratio = [ODstimulated sample – ODmedium controll/ODpositive control × 100]. IFN-γ positive bovine activated plasma for positive control were obtained from the manufacturer.
Estimation of quantity and quality of fecal STEC shedding
Approximately 10 g of fecal matter per animal was obtained directly from rectal lumen using sterile gloved fingers and stored at −80 °C. One gram was diluted in 9 mL sterile PBS buffer, homogenized, log10 diluted over five steps, and plated on Gassner agar (SIFIN diagnostics GmbH, Berlin, Germany). After incubation (18 h, 37 °C), representative agar plates were enumerated and cfu/g feces calculated. All coliform colonies from one 10−1 dilution plate were washed off with 1 mL LB broth supplemented with 30% glycerin, boiled 10 min at 100 °C, put on ice for 5 min and used as PCR template. E. coli strain EDL933 (kindly provided by Prof. R. Bauerfeind, Institute for Hygiene and Infectious Diseases of Animals, Justus Liebig University Giessen, Germany), positive for stx1 and stx2, served as a positive control. PCR primer pairs for detection of stx1 and stx2 were designed with reference to published sequence data [37]. The detection limit was determined to be 1 × 104 cfu/g feces when two stx-negative fecal samples were spiked with 10−1 to 105 cfu/g feces of viable E. coli EDL933 bacteria.
Stx-negative samples were excluded from further analysis. Colonies from stx-positive samples (stx1- and/or stx2-positive) were isolated by DNA–DNA colony hybridization as described by Geue et al. [13] with the following variations. DNA probes were labeled with digoxigenin with MP4/MP3 primers [37] using the PCR DIG Probe Synthesis Kit (Roche, Deutschland Holding GmbH, Grenzach Wyhlen, Germany) as specified by the manufacturer. Each stx-positive signal was assigned to a colony and up to 10 stx-positive colonies per blot were individually cultured in 200 µL LB broth, incubated (18 h, 37 °C), 30% glycerin added, and stored at −80 °C. The number of total stx-positive colonies on each blot was counted and cfu STEC/g feces calculated. Samples with stx-positive culture but no STEC detection by colony blot were given arbitrary STEC cfu/g feces of half of the dilution step used for blotting.
STEC isolates were characterized as to their possession of four virulence markers by multiplex PCR specific for stx1 and stx2 [37], eae [38], ehxA (= EHEC hlyA) [39]. Profiles were defined by the presence of eae and/or ehxA in addition to stx1 and/or stx2. The numbers of different STEC profiles detectable in single fecal samples were recorded.
Statistical analysis
Statistical analysis was done with “IBM SPSS statistics” (version 19, IBM Corporation, New York, USA) and XLSTAT-Pro (version 2015.1. Addinsoft, Paris, France). Fisher’s exact test was used to compare the PCR result of fecal stx status between the groups and to analyze if specific stx types detected in fecal cultures were differently distributed among trial groups. Friedman test was used to validate development of E. coli cfu and STEC cfu over time. Dunn’s test after a Kruskal–Wallis test and Mann–Whitney U test was performed for group comparison of E. coli cfu, STEC cfu, anti-Stx titer, INF-γ production and PBMC stimulation assay. Changes over time in anti-Stx titer were performed by Wilcoxon test. A value of p < 0.05 was considered statistically significant.
Results
Anti-Stx1 and anti-Stx2 titers in calf sera after passive and active immunization
Stx1- and Stx2-neutralizing antibodies (nStx1Ab/nStx2Ab) were detectable in pre-colostral sera of 2 of 9 tested VAC+ and 3 of 14 tested VAC− calves (Figure 2). After colostrum intake, nStx1Ab and nStx2Ab titers started to differ significantly between the VAC+ and the VAC− group. The nStx2Ab titer in 21 of 24 VAC− animals even remained below the detection limit. Regardless of vit E supplementation, the nStx1Ab and nStx2Ab serum titers continued to differ significantly between the VAC+ and the VAC− groups until 16th week of life.
Temporal pattern of Stx neutralizing titers in calves’ sera. Antibody titers were determined before (ac = ante colostrum) and after (pc = post-colostral) colostrum intake as well as in the 3rd, 11th, 16th, 26th and 55th week of life. Animals were grouped according to their vaccination status (VAC+ = rStxMUT-vaccinated; VAC− = placebo control) and the supplementation of vitamin E (VitEH = high supplementation; VitEM = moderate supplementation). Arrows indicate active vaccination in the 5th and 8th week of life. A Temporal pattern of Stx1 neutralizing titers. A titer of 450 was attributed to those samples with a relative cell activity < 30% (detection limit, dashed line). B Temporal pattern of Stx2 neutralizing titers. A titer of 300 was attributed to those samples with a relative cell activity < 20% (detection limit, dashed line). Results of Vero cell neutralization assay expressed as box plots with 5–95% interquartile ranges. Different letters indicate significant differences between the groups at each time point separately based on Dunn’s test after a Kruskal–Wallis test.
The nStx1Ab titer declined within the first 11 weeks in both groups (Figure 2A). In the VAC− group, nStx1Ab titers dropped below the detection limit in the 11th week. In the 16th week, most VAC− animals underwent a nStx1Ab seroconversion with titers peaking in week 26. VAC+ animals showed a similar trend with clearly detectable nStx1Ab titers throughout and a slight increase from week 16 to week 26. nStx1Ab titers declined in both groups from week 26 to week 55. The feeding with standard (“moderate”) or elevated (“high”) amounts of vit E had no influence on nStx1Ab titers, except within the VAC− group in the 16th week. nStx1Ab titers had increased significantly to levels indistinguishable from titers of VAC+ animals in the VAC− VitEM sub-group (9 of 13 animals seroconverted) but not in the VAC− VitEH sub-group (9 of 11 seroconverted).
Independent of vit E supplementation, maternal nStx2Ab titers in VAC+ animals had already declined until week 3. Application of the vaccines between week 3 and 11 led to a clear increase in nStx2Ab titers, which only lasted until week 16, however. VAC− animals had very low to undetectable levels of nStx2Ab from week 11 onwards.
IFN-γ production by PBMC after in vitro stimulation
PBMC isolated from calves in the 16th week of life secreted IFN-γ in higher amounts upon 24 h stimulation with ConA than PBMC in the medium control. Thereof, PBMC from VitEH animals produced and secreted more IFN-γ than PBMC from VitEM animals irrespective of rStxMUT vaccination (Figure 3). Cultivation with lysates from stx-negative E. coli strains previously isolated from the farm, or with rStx1MUT or rStx2MUT did not induce significantly increased IFN-γ secretion by PBMC from animals of all groups. After cultivation with stx1-positive E. coli strain lysates, IFN-γ concentrations even tended to decrease in PBMC supernatants of the VitEM fed animals compared to VitEH fed animals. Differences reached statistical significance after stimulation with E. coli strain 5.
IFN-γ production by PBMC after in vitro stimulation. PBMC were isolated from calves in 16th week of life, 8 weeks after active immunization with rStxMUT. Animals were grouped according to their vaccination status (VAC+ = rStxMUT-vaccinated; VAC− = placebo control) and the supplementation of vitamin E (VitEH = high supplementation; VitEM = moderate supplementation). PBMC were stimulated with lysates of 5 E. coli strains previously isolated at the farm and positive (stx1 +) or negative (stx −) for stx. ELISA values obtained with cell culture supernatants after 24 h of stimulation are depicted in box plots (5–95% interquartile ranges) as percentages of positive controls (S/P). *Asterisk indicates statistical significance (p < 0.05) using Kruskal–Wallis test.
CD25 expression on CD4+CD45RO+ and CD8αhiCD45RO+ cells after in vitro stimulation
CD4+CD45RO+and CD8αhiCD45RO+ cells responses to ConA, rStx1MUT or Listeria strain lysates did not differ between calves of the VAC+ and the VAC− group (Figure 4). In contrast, cells obtained from VAC+ animals 8 weeks after vaccination (16th week of life) responded to rStx2MUT re-stimulation in vitro with an increase in the number of CD25 molecules on the cellular surface, whereas CD25 expression on CD4+CD45RO+and CD8αhiCD45RO+ of VAC− animals was indistinguishable from that of cells in the medium control. The effect of vaccination was significant for CD4+CD45RO+ from VitEH and VitEM animals, but for CD8αhiCD45RO+ cells in the VitEM group only. Such an effect of rStx2MUT re-stimulation on CD25 expression by CD8αhiCD45RO+ cells was also observed with PBMC obtained in the 26th week but did not reach significant levels. Incubation with lysates of 1 out of 5 E. coli strains isolated from the herd (E. coli 1) did not affect CD25 expression (Figure 5). Even though group differences did not always reach significant levels, CD4+CD45RO+and CD8αhiCD45RO+ cells of VAC+ animals responded to lysates from the other 4 E. coli isolates with CD25 up-regulation, while respective cells from VAC− animals did not. Differences in responsiveness occurred in week 16, but not in week 26 and were more often significantly different in the VitEM as in the VitEH group.
Expression of CD25 on CD4 +CD45RO+ and CD8αhiCD45RO+ cells after cultivation with ConA, Shiga toxoids or Listeria monocytogenes lysates. PBMC were isolated from calves in their 16th and 26th week of life, corresponding 8 and 18 weeks after active immunization with rStxMUT. Animals were grouped according to their vaccination status (VAC+ = rStxMUT-vaccinated; VAC− = placebo control) and the supplementation of vitamin E (VitEH = high supplementation; VitEM = moderate supplementation). Data obtained by flow cytometric analysis of the PBMC cultures after 5 days cultivation is depicted in box plots (5–95% interquartile ranges) as change of the geometric mean of fluorescence intensity of CD25 on CD4+CD45RO+ and CD8αhiCD45RO+ relative to unstimulated control cells (the latter values defined as 0, indicated by the dashed line). Different letters indicate significant differences between the groups in CD4+CD45RO+ (lower case letters) and CD8αhiCD45RO+ (capital letters) cells (p < 0.05, Kruskal–Wallis test with post hoc Dunn’s test).
Expression of CD25 on CD4+CD45RO+ and CD8αhiCD45RO+ cells after cultivation with E. coli lysates. For details see legend to Figure 4.
Fecal cultures and stx 1-/stx 2-PCR
Coliform colony-forming units per gram feces (cfu/g) in samples collected in the 3rd week of life (median with 95% interquartile range: 3.3 × 106 [1.3 × 106–9.0 × 106]) were significantly higher (p < 0.001) than in samples from the 16th (1.1 × 105 [4.1 × 104–2.6 × 105]), 26th (1.4 × 105 [7.7 × 104–2.6 × 105]), or 55th (4.0 × 104 [3.5 × 103–8.0 × 104]) week of life independent of group affiliation (Additional file 2).
In an attempt to unveil potential qualitative and quantitative changes in STEC shedding resulting from Stx toxoid vaccination, quantification of shedding as well as isolation and characterization of strains was conducted by applying an analytical workflow based on (i) detection of stx genes in the feces, (ii) stx-specific colony blotting, isolation and quantification of signal-positive colonies, and (iii) characterization of STEC isolates by multiplex PCR (see subsequent paragraph).
Taking into account all samples from VAC+ (n = 95) and VAC− calves (n = 96) over time, significantly (p = 0.040) less fecal samples collected from VAC+ animals were stx-positive by PCR than samples from VAC− animals (32/95 [33.7%] versus 47/96 [49.0%] samples in the VAC+ and the VAC− group, respectively). At each individual sampling point, less samples isolated from VAC+ animals were positive for stx-specific DNA than samples from VAC− animals, but those differences did not reach statistical significance (Figure 6A). The level of vit E supplementation did not influence the frequency of stx-positive fecal samples on its own. However, significant (p = 0.017) less samples collected from VAC+ VitEM animals (16/52 [30.8%]) were stx-positive than from VAC− VitEM animals (29/52 [55.8%]) over the whole observation period. In contrast, samples from VAC+ VitEH (16 stx-positive of 43 [37.2%]) and from VAC− VitEH (18/44 [40.9%]) animals did not differ significantly (p = 0.827). Three and two animals in the VAC+ and the VAC− group remained stx-negative, respectively, throughout the entire observation study. All other animals shed STEC, as deduced from a positive PCR result for stx1 or stx2, at least on one occasion. It cannot be ruled out that single PCR-positive fecal samples result from passaging of the bacteria rather than true infection. As a proxy of infection, 7 and 14 animals in the VAC+ and in the VAC− group, respectively, were found to be fecal-positive for at least one toxin type on at least two consecutive sampling times. Again, the VitEH and VitEM groups differed in their STEC shedding pattern. The number of animals that remained stx-negative were evenly distributed (VAC+ VitEH: 1/11, VAC− VitEH: 2/13; VAC+ VitEM: 1/11; VAC− VitEM: 1/13). However, 10 calves in the VAC− VitEM group but only 5, 2 and 4 calves in the VAC+ VitEH, the VAC− VitEH and the VAC+ VitEM groups, respectively, were fecal-positive for stx on at least two consecutive sampling times (Additional file 1).
Quantitative and qualitative assessment of fecal STEC shedding. Animals were grouped according to their vaccination status (VAC+ = rStxMUT-vaccinated; VAC− = placebo control) and the supplementation of vitamin E (VitEH = high supplementation; VitEM = moderate supplementation). A Fecal cultures (n = 191) were screened for stx1/stx2 by PCR and grouped into stx-negative, stx1-positive, stx2-positive and stx1 plus stx2-positive. B Samples detected by stx-PCR of fecal cultures as stx 1 and stx 2 negative samples were not further analyzed (white part of the bar). Nylon membrane blots of agar plates of positively tested feces samples were hybridized with stx1/stx2 probes. Single colonies tested positive by hybridization (up to 10 per blot) were picked and further analyzed by PCR. A STEC profile is defined as single colony testing positive for stx (stx 1 and/or stx 2 ) plus facultative eae and/or ehxA. Different STEC profiles are illustrated with differently colored bars. Samples with no detectable stx1-/stx2-positive signal by hybridization (dark grey bars) and samples with single colonies positive detected by hybridization but negative signal by multiplex PCR (bright grey bars) are illustrated as such.
Considering stx-positive fecal samples only, such samples from VAC+ animals contained significantly (p = 0.003) more often stx1 plus stx2 (14/32; 43.8%) than samples from VAC− animals (6/47; 12.8%; Figure 6B). In turn, single stx1- or single stx2-positive samples were detected more often in VAC− (27/47 [51.1%] and 17/47 [36.2%], respectively) than in VAC+ (11/32 [34.4%] and 7/32 [21.9%], respectively) animals but differences did not reach statistical different levels (p ≥ 0.171). STEC shedding significantly varied with vit E supplementation also. A greater proportion of fecal samples from VAC+ VitEM animals was stx1/stx2-positive (11/16; 68.8%) compared to VAC+ VitEH (3/16; 18.8%; p = 0.011), VAC− VitEH (4/28; 22.2%; p = 0.014), and VAC− VitEM (2/29; 6.9%; p < 0.001). In contrast, significantly (p = 0.013) less fecal samples were single stx1-positive (3/16) in the VAC+ VitEM group compared to the VAC− VitEM (17/29; 18.8% versus 58.6%) group. For individual pattern of stx-positive fecal cultures see Additional file 1.
Isolation and characterization of STEC strains
The percentage of stx PCR-positive fecal samples of which the subsequently conducted colony blotting did not yield a positive signal was different between the VAC+ (29.0%) and the VAC− groups (46.7%). STEC cfu per g of stx PCR-positive fecal samples did not differ (p = 0.216) between the trial groups over the entire observation period (Additional file 2). From a total of 40 fecal samples, that had at least one positive signal on the colony blot, 215 single colonies (VAC+ n = 108, VAC− n = 107 colonies) were analyzed to assess their virulence gene profile in order to assess possible implications of the vaccination for the composition and zoonotic potential of the STEC strains shed. As a result, isolates could be grouped into 12 virulence gene profiles based on the possession of stx1, stx2, eae or ehxA. Continuing with only one strain per virulence gene profile within a single sample, 64 individual STEC isolates were further analyzed (Table 1). More stx1–positive isolates were also positive for eae (59.1%) than stx2- and stx1/stx2-positive isolates (28.0 and 28.6%). The ehxA gene was detected in 77.3% of stx1-, 84.0% of stx2- and 28.6% of stx1-/stx2-positive isolates. Ten isolates lost the stx gene upon sub-culturing as they were originally positive by colony blot. The relative number of stx-blot positive samples harbouring more than one gene profile varied between VAC+ and VAC− animals (38.9 and 19.0%, respectively) (Figure 5B), indicating that vaccination had a qualitative impact on STEC shedding.
Discussion
Up to now, experimental and licensed vaccines for reduction of STEC shedding (Econiche Corp, Belleville, Canada; Epitopix, Willmar, USA) were only partially successful and effects were mostly restricted to subpopulations of STEC, e.g. O157:H7/H– [14,15,16]. This proof-of-concept study provides first evidence that immunization with Stx toxoid-based vaccines under field-like conditions enables calves to actively mount a more effective immune response against STEC strains circulating in the cohort. Observed effects of rStxMUT immunization on the incidence of stx-positive fecal samples were moderate compared to effects reportedly seen after immunization of cattle with STEC adhesins and subsequent homologous experimental challenge [14, 16]. However, the immune response primarily analysed herein was only indirectly achieved through natural exposure to a bacterial pathogen known to asymptomatically colonize the bovine intestine. Furthermore, vaccinated and control animals were kept together and participated in the same network of animal-to-animal and environmental-to-animal transitional events. The study did not include an immunological naïve control group, i.e. the colostrum pool administered to control calves was not free of anti-Stx antibodies. These confounding factors make the degree of differences in cellular immune responses and STEC shedding between animals groups highly plausible.
Colostral nStx1Ab and nStx2Ab were effectively transferred to calves with nStx1Ab titers clearly exceeding nStx2Ab titers in the VAC+ as well as the VAC− group similar to what was observed after natural Stx exposure and after rStxMUT vaccination [12, 24, 40]. Active rStxMUT immunization in week 5 and 8 did not result in a detectable increase of nStx1Ab in calves’ sera, different from calves vaccinated after vanishing of maternal antibodies [24]. High maternal titers may have impaired the success of the rStx1MUT vaccination but a significant humoral immune response was achieved by rStx2MUT immunization of calves with no or low serum nStx2Ab titers at the time of active vaccination. A modified vaccination scheme, consisting of maternal vaccination, prime vaccination of calves not earlier than week 11 and booster application in the second half of the first year of life might overcome these obstacles.
Applying the quantitation of CD25 expression on CD45RO+ lymphocytes for monitoring antigen-specific T cell responses in cattle, as suggested by Koo et al. [41], we found that peripheral CD4+CD45RO+ and CD8αhiCD45RO+ cells from VAC+ calves responded better to re-stimulation with STEC antigens. Eight weeks after active vaccination, PBMCs from VAC+ animals responded more pronounced to rStx2MUT re-stimulation in vitro suggesting that vaccination directly induced an adaptive immune response against Stx2. The missing T cell responsiveness to rStx1MUT in vitro may result from high maternal humoral protection at the time of active vaccination as discussed for the humoral response. Of note, VAC+ calves were capable of mounting an earlier adaptive immune response to E. coli antigens other than Stx1 as well. This became apparent by re-stimulation with stx1 +/stx2 − E. coli lysates in the 16th week of life. The CD4+ subset analysed may have comprised different TH subsets as well as Treg cells. Bovine NK cells express CD45RO, CD8αα and CD8αβ [42] and are able to respond to bacterial molecular patterns without MHC restriction [43,44,45]. By gating on CD8αhi cells, we aimed at excluding NK as well as γδT cells which both may express CD8αlo [43, 46]. PBMC of the calves in the VAC+ and the VAC− group responded differently to some but not all bacterial lysates implying that the response detected was not due to an innate-like immune response but, in support of our hypothesis, indicative of an elevated adaptive cellular immune response to STEC strains circulating in the herd. Antibodies to STEC antigens may not be sufficient to protect cattle from STEC colonization [47]. Corbishley et al. showed by characterizing the epitopes recognized by CD4+ T-cells that STEC-infected calves develop a specific immunological response at the infection site [48]. In light of these findings, the relative importance of functionally different immune cell populations for the control of STEC colonization in cattle’s intestine urgently needs to be unveiled.
Hoffmann et al. showed that calves inoculated twice with stx-negative O157 strain, but not the calves inoculated with stx2-positive O157 strain, developed cellular immune responses against the latter strain [17]. Under less standardized field conditions in the current study, VAC+ animals also responded to one of the two stx-negative E. coli strains better than VAC− animals, implying that rStxMUT vaccination enables calves to build an infection immunity towards other antigens that are common in STEC and other E. coli strains. In order to determine if this supportive effect expands to infections with other enteric bacterial agents, immune control of which heavily relies on cellular immune responses, we also re-stimulated PBMC of the calves with lysates of Listeria strains previously isolated on the experimental farm. A beneficial effect of StxMUT vaccination on the immune response against Listeria monocytogenes in the cohort of calves under study could not be confirmed. We cannot rule out that this resulted from lack of exposure as we did not monitor the Listeria infection status of the calves. Nevertheless, T-cells from VAC+ animals, which we consider to have been less exposed to Stx, tended to respond more vigorously to ConA in week 16. Stx inhibits the immunogenic effect of systemically administered antigen [49] and Kieckens et al. as well as Corbishley et al. showed that STEC colonization affect cattle’s general immune response [50, 51] suggesting that Stx impairs immune responses in cattle in a more general manner than currently recognized.
The vaccine deployed in this study contained rStx1MUT and rStx2MUT, respectively, but is not devoid of antigens derived from the laboratory E. coli K-12 strain used for recombinant protein expression. The control calves were placebo injected with NaCl solution and adjuvant to exclude the adjuvant’s influence. Registered O157-vaccines (Econiche Corp, Belleville, Canada; Epitopix, Willmar, USA) contain proteins from the LEE locus and/or siderophore proteins as the main vaccine component. Both potentially protective STEC antigens are not included in the genome of the E. coli K-12 strain. Moreover, residual E. coli antigens other than rStx1MUT and rStx2MUT in the vaccine preparations are unlikely to have had an effect on the parameters assessed. Firstly, the general coliform flora in the calves was not affected by vaccination as no differences in the colony count were measured. Secondly, T-cells of VAC+ calves responded to rStx2MUT containing vaccine preparations derived from the laboratory E. coli K-12 strain in vitro but not to the rStx1MUT containing preparation strongly arguing against a general anti-E. coli immunity induced by the vaccination itself.
The hypothesis that high vit E supplementation stimulates the immune system and thereby supports the effect of rStxMUT vaccination to reduce STEC shedding could not be substantiated. The higher vit E supplementation had no positive effect on CD25 expression by CD4+D45RO+ and CD8αhiCD45RO+ after stimulation with STEC and E. coli and rather a negative effect on STEC shedding in VAC+ calves. Nevertheless, 354 IU compared to 188 IU daily vit E supplementation via milk replacer exhibited effects on STEC shedding within the VAC+ group for reasons that have not been unveiled yet and interactive effects of vit E supply and rStxMUT vaccination on, e.g., feed intake (N. Schmidt, T. Luhmann, L. Hüther, U. Meyer, S. A. Barth, L. Geue, C. Menge, J. Frahm, S. Dänicke, submitted for publication) have also to be taken into consideration.
Shiga toxoid immunization did not influence the absolute amount of STEC in fecal samples that still were stx PCR-positive. Moreover, our data indicate that samples from VAC+ calves tended to have higher STEC cfu in relation to total coliform counts. As the methodical workflow allowed isolation of STEC via colony blotting only from samples with STEC cfu/g feces maximally 2 log10 levels lower than coliform flora, we assume that rStxMUT vaccination reduced the incidence of STEC primarily in animals with low numbers of STEC within the coliform flora rather than affecting the shedding of animals with relatively high levels of STEC shedding, referred to as supershedders [52].
Descriptive analysis of virulence gene profiles of STEC isolates showed that VAC+ animals had higher number of different STEC profiles per fecal sample and that STEC strains isolated from VAC+ animals were significantly more often both stx1 and stx2 positive than isolates from VAC−. The effect of rStxMUT vaccination is mainly indirect and the success of the vaccination approach depends on sufficient exposure to a relevant number of bacteria of all strains implicated in STEC transmission dynamics in a respective cattle herd. In order to avoid selection of more virulent STEC strains by vaccination of cattle, the immunogenic capacity (dose) of the toxoids, the vaccine formulation, and the time and route of application have to be optimized and other virulence factors conserved among STEC strains like intimin [51], EspB [53] or associated with the colonization type of STEC strains [6] have to be included in the vaccine and/or the feed composition. Nevertheless, data presented here support our hypothesis that the immune modulatory effect of Stx should be considered for integration in strategies aiming at reducing STEC shedding in cattle.
Abbreviations
- Stx:
-
Shiga toxin
- STEC:
-
Shiga toxin-producing Escherichia coli
- rStx1MUT/rStx2MUT :
-
recombinant Shiga toxoids
- EHEC:
-
enterohemorrhagic Escherichia coli
- Vitamin E:
-
vit E
- VNA:
-
Vero cell neutralization assay
- VAC+:
-
rStxMUT-vaccinated animals
- VAC−:
-
placebo-treated animals
- VitEH :
-
vit E high feeding group
- PBMC:
-
peripheral blood mononuclear cells
- VitEM :
-
vit E moderate feeding group
- MR:
-
milk replacer
- DM:
-
dry matter
- VCA:
-
Vero cell cytotoxicity assay
- nAb:
-
neutralizing antibody
- cfu:
-
colony forming units
- ConA:
-
Concanavalin A
- MC:
-
medium control
- nStx1Ab/nStx2Ab:
-
Stx1-/Stx2-neutralizing antibodies
References
EFSA (European Food Safety Authority), ECDC (European Centre for Disease Prevention and Control) (2017) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2016. EFS2 15:148
Krogfelt KA (1991) Bacterial adhesion: genetics, biogenesis, and role in pathogenesis of fimbrial adhesins of Escherichia coli. Rev Infect Dis 13:721–735
Jordan DM, Cornick N, Torres AG, Dean-Nystrom EA, Kaper JB, Moon HW (2004) Long polar fimbriae contribute to colonization by Escherichia coli O157:H7 in vivo. Infect Immun 72:6168–6171
Ackers ML, Mahon BE, Leahy E, Goode B, Damrow T, Hayes PS, Bibb WF, Rice DH, Barrett TJ, Hutwagner L, Griffin PM, Slutsker L (1998) An outbreak of Escherichia coli O157:H7 infections associated with leaf lettuce consumption. J Infect Dis 177:1588–1593
Barth S, Tscholshiew A, Menge C, Weiss R, Baljer G, Bauerfeind R (2007) Virulence and fitness gene patterns of Shiga toxin-encoding Escherichia coli isolated from pigs with edema disease or diarrhea in Germany. Berl Munch Tierarztl Wochenschr 120:307–316
Barth SA, Menge C, Eichhorn I, Semmler T, Wieler LH, Pickard D, Belka A, Berens C, Geue L (2016) The accessory genome of Shiga toxin-producing Escherichia coli defines a persistent colonization type in cattle. Appl Environ Microbiol 82:5455–5464
Hussein HS, Sakuma T (2005) Prevalence of shiga toxin-producing Escherichia coli in dairy cattle and their products. J Dairy Sci 88:450–465
Menrath A, Wieler LH, Heidemanns K, Semmler T, Fruth A, Kemper N (2010) Shiga toxin producing Escherichia coli: identification of non-O157:H7-Super-Shedding cows and related risk factors. Gut Pathog 2:7
Hamm K, Barth SA, Stalb S, Geue L, Liebler-Tenorio E, Teifke JP, Lange E, Tauscher K, Kotterba G, Bielaszewska M, Karch H, Menge C (2016) Experimental infection of calves with Escherichia coli O104:H4 outbreak strain. Sci Rep 6:32812
Naylor SW, Gally DL, Low JC (2005) Enterohaemorrhagic E. coli in veterinary medicine. Int J Med Microbiol 295:419–441
Caprioli A, Morabito S, Brugere H, Oswald E (2005) Enterohaemorrhagic Escherichia coli: emerging issues on virulence and modes of transmission. Vet Res 36:289–311
Fröhlich J, Baljer G, Menge C (2009) Maternally and naturally acquired antibodies to Shiga toxins in a cohort of calves shedding Shiga-toxigenic Escherichia coli. Appl Environ Microbiol 75:3695–3704
Geue L, Segura-Alvarez M, Conraths FJ, Kuczius T, Bockemuhl J, Karch H, Gallien P (2002) A long-term study on the prevalence of shiga toxin-producing Escherichia coli (STEC) on four German cattle farms. Epidemiol Infect 129:173–185
Vande Walle K, Vanrompay D, Cox E (2013) Bovine innate and adaptive immune responses against Escherichia coli O157:H7 and vaccination strategies to reduce faecal shedding in ruminants. Vet Immunol Immunopathol 152:109–120
Callaway TR, Anderson RC, Edrington TS, Genovese KJ, Harvey RB, Poole TL, Nisbet DJ (2004) Recent pre-harvest supplementation strategies to reduce carriage and shedding of zoonotic enteric bacterial pathogens in food animals. Anim Health Res Rev 5:35–47
Snedeker KG, Campbell M, Sargeant JM (2012) A systematic review of vaccinations to reduce the shedding of Escherichia coli O157 in the faeces of domestic ruminants. Zoonoses Public Health 59:126–138
Hoffman MA, Menge C, Casey TA, Laegreid W, Bosworth BT, Dean-Nystrom EA (2006) Bovine immune response to Shiga-toxigenic Escherichia coli O157: H7. Clin Vaccine Immunol 13:1322–1327
Stamm I, Mohr M, Bridger PS, Schropfer E, Konig M, Stoffregen WC, Dean-Nystrom EA, Baljer G, Menge C (2008) Epithelial and mesenchymal cells in the bovine colonic mucosa differ in their responsiveness to Escherichia coli Shiga toxin 1. Infect Immun 76:5381–5391
Moussay E, Stamm I, Taubert A, Baljer G, Menge C (2006) Escherichia coli Shiga toxin 1 enhances il-4 transcripts in bovine ileal intraepithelial lymphocytes. Vet Immunol Immunopathol 113:367–382
Menge C, Wieler LH, Schlapp T, Baljer G (1999) Shiga toxin 1 from Escherichia coli blocks activation and proliferation of bovine lymphocyte subpopulations in vitro. Infect Immun 67:2209–2217
Menge C, Stamm I, van Diemen PM, Sopp P, Baljer G, Wallis TS, Stevens MP (2004) Phenotypic and functional characterization of intraepithelial lymphocytes in a bovine ligated intestinal loop model of enterohaemorrhagic Escherichia coli infection. J Med Microbiol 53:573–579
Menge C, Stamm I, Wuhrer M, Geyer R, Wieler LH, Baljer G (2001) Globotriaosylceramide (Gb(3)/CD77) is synthesized and surface expressed by bovine lymphocytes upon activation in vitro. Vet Immunol Immunopathol 83:19–36
Stamm I, Wuhrer M, Geyer R, Baljer G, Menge C (2002) Bovine lymphocytes express functional receptors for Escherichia coli Shiga toxin 1. Microb Pathog 33:251–264
Kerner K, Bridger PS, Kopf G, Frohlich J, Barth S, Willems H, Bauerfeind R, Baljer G, Menge C (2015) Evaluation of biological safety in vitro and immunogenicity in vivo of recombinant Escherichia coli Shiga toxoids as candidate vaccines in cattle. Vet Res 46:38
Hovde CJ, Calderwood SB, Mekalanos JJ, Collier RJ (1988) Evidence that glutamic acid 167 is an active-site residue of Shiga-like toxin I. Proc Natl Acad Sci U S A 85:2568–2572
Yamasaki S, Furutani M, Ito K, Igarashi K, Nishibuchi M, Takeda Y (1991) Importance of arginine at position 170 of the A subunit of Vero toxin 1 produced by enterohemorrhagic Escherichia coli for toxin activity. Microb Pathog 11:1–9
Oanh TKN, Nguyen VK, de Greve H, Goddeeris BM, Urban JF (2011) Protection of piglets against Edema disease by maternal immunization with Stx2e toxoid. Infect Immun 80:469–473
Makino S, Watarai M, Tabuchi H, Shirahata T, Furuoka H, Kobayashi Y, Takeda Y (2001) Genetically modified Shiga toxin 2e (Stx2e) producing Escherichia coli is a vaccine candidate for porcine edema disease. Microb Pathog 31:1–8
Ohmura-Hoshino M, Yamamoto M, Yuki Y, Takeda Y, Kiyono H (2004) Non-toxic Stx derivatives from Escherichia coli possess adjuvant activity for mucosal immunity. Vaccine 22:3751–3761
Ishikawa S, Kawahara K, Kagami Y, Isshiki Y, Kaneko A, Matsui H, Okada N, Danbara H (2003) Protection against Shiga toxin 1 challenge by immunization of mice with purified mutant Shiga toxin 1. Infect Immun 71:3235–3239
Reddy PG, Morrill JL, Frey RA (1987) Vitamin E requirements of dairy calves. J Dairy Sci 70:123–129
Reddy PG, Morrill JL, Minocha HC, Stevenson JS (1987) Vitamin E is immunostimulatory in calves. J Dairy Sci 70:993–999
Pekmezci D, Cakiroglu D (2009) Investigation of immunomodulatory effects of levamisole and vitamin E on Immunity and some blood parameters in newborn Jersey calves. Vet Res Commun 33:711–721
Samanta AK, Dass RS, Rawat M, Mishra RC, Mehra UR (2006) Effect of dietary vitamin E supplementation on serum α-tocopherol and immune status of crossbred calves. Asian-Aust J Anim Sci 19:500–506
National Research Council (2001) Nutrient requirements of dairy cattle: Seventh revised edition, 2001//Nutrient requirements of dairy cattle. The National Academies Press; National Academies Press, Washington, DC
Gentry MK, Dalrymple JM (1980) Quantitative microtiter cytotoxicity assay for Shigella toxin. J Clin Microbiol 12:361–366
Muller D, Greune L, Heusipp G, Karch H, Fruth A, Tschape H, Schmidt MA (2007) Identification of unconventional intestinal pathogenic Escherichia coli isolates expressing intermediate virulence factor profiles by using a novel single-step multiplex PCR. Appl Environ Microbiol 73:3380–3390
Franck SM, Bosworth BT, Moon HW (1998) Multiplex PCR for enterotoxigenic, attaching and effacing, and Shiga toxin-producing Escherichia coli strains from calves. J Clin Microbiol 36:1795–1797
Paton AW, Paton JC (1998) Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J Clin Microbiol 36:598–602
Pirro F, Wieler LH, Failing K, Bauerfeind R, Baljer G (1995) Neutralizing antibodies against Shiga-like toxins from Escherichia coli in colostra and sera of cattle. Vet Microbiol 43:131–141
Koo HC, Park YH, Hamilton MJ, Barrington GM, Davies CJ, Kim JB, Dahl JL, Waters WR, Davis WC (2004) Analysis of the immune response to Mycobacterium avium subsp. paratuberculosis in experimentally infected calves. Infect Immun 72:6870–6883
Graham EM, Thom ML, Howard CJ, Boysen P, Storset AK, Sopp P, Hope JC (2009) Natural killer cell number and phenotype in bovine peripheral blood is influenced by age. Vet Immunol Immunopathol 132:101–108
Endsley JJ, Endsley MA, Estes DM (2006) Bovine natural killer cells acquire cytotoxic/effector activity following activation with IL-12/15 and reduce Mycobacterium bovis BCG in infected macrophages. J Leukoc Biol 79:71–79
Hope JC, Sopp P, Howard CJ (2002) NK-like CD8(+) cells in immunologically naive neonatal calves that respond to dendritic cells infected with Mycobacterium bovis BCG. J Leukoc Biol 71:184–194
Olsen I, Boysen P, Kulberg S, Hope JC, Jungersen G, Storset AK (2005) Bovine NK cells can produce gamma interferon in response to the secreted mycobacterial proteins ESAT-6 and MPP14 but not in response to MPB70. Infect Immun 73:5628–5635
Menge C, Dean-Nystrom EA (2008) Dexamethasone depletes gammadelta T cells and alters the activation state and responsiveness of bovine peripheral blood lymphocyte subpopulations. J Dairy Sci 91:2284–2298
Johnson RP, Cray WC, Johnson ST (1996) Serum antibody responses of cattle following experimental infection with Escherichia coli O157:H7. Infect Immun 64:1879–1883
Corbishley A, Ahmad NI, Hughes K, Hutchings MR, McAteer SP, Connelley TK, Brown H, Gally DL, McNeilly TN (2014) Strain-dependent cellular immune responses in cattle following Escherichia coli O157:H7 colonization. Infect Immun 82:5117–5131
Chu A (2010) Immunomodulation by Shiga toxin 2. PhD Thesis, University of Saskatchewan http://hdl.handle.net/10388/etd-08252010-163545
Kieckens E, Rybarczyk J, Li RW, Vanrompay D, Cox E (2016) Potential immunosuppressive effects of Escherichia coli O157:H7 experimental infection on the bovine host. BMC Genomics 17:1049
Corbishley A, Connelley TK, Wolfson EB, Ballingall K, Beckett AE, Gally DL, McNeilly TN (2016) Identification of epitopes recognised by mucosal CD4(+) T-cell populations from cattle experimentally colonised with Escherichia coli O157:H7. Vet Res 47:90
Matthews L, Low JC, Gally DL, Pearce MC, Mellor DJ, Heesterbeek JAP, Chase-Topping M, Naylor SW, Shaw DJ, Reid SWJ, Gunn GJ, Woolhouse MEJ (2006) Heterogeneous shedding of Escherichia coli O157 in cattle and its implications for control. Proc Natl Acad Sci U S A 103:547–552
Vande Walle K, Yekta MA, Verdonck F, de Zutter L, Cox E (2011) Rectal inoculation of sheep with E. coli O157:H7 results in persistent infection in the absence of a protective immune response. Vet Microbiol 147:376–382
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CM, SD, SB and LG were involved in hypothesis generation and experimental design. NS, SB, JF, and UM were involved in organizing and conducting the experiments. CM, SB, LG, NS were involved in interpreting and analyzing the results and NS was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank Prof. Dr. Rolf Bauerfeind (Justus-Liebig University Giessen, Institute for Hygiene and Infectious Diseases of Animals) for kind provision of vaccine antigen and EDL933 strain as well as the staff of the experimental facilities (Friedrich-Loeffler-Institut, Brunswick) for their support during the animal trial. We acknowledge the excellent technical support by Nicola Mickenautsch, Lara Lindner and Anke Hinsching.
Availability of data and materials
The datasets used and/or analyzed during the current study is provided in detail in the results section of the manuscript.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was carried out in strict accordance with German law for the care and use of experimental animals. Experiments were approved by the Lower Saxony State Office for Consumer Protection and Food Safety (LAVES), Oldenburg, Germany (Reg Number 33.9-42,502.04.12/0933 and 33.9-42502-04-11/0444, vaccination and vit E treatment, respectively).
Funding
Nadine Schmidt was financial supported in part by the H. Wilhelm Schaumann foundation, Hamburg.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1.
Individual pattern of stx-positive fecal cultures in the 3rd, 16th, 26th and 55th week of life. Animals were grouped according to their vaccination status (VAC+ = rStxMUT-vaccinated; VAC− = placebo control) and the supplementation of vitamin E (VitEH = high supplementation; VitEM = moderate supplementation). White boxed depict stx 1 -/stx 2 -fecal cultures analyzed by multiplex PCR. Detection of stx 1 and stx 2 is marked in gray and black, respectively. White cross-out boxes mark a gap in sampling.
Additional file 2.
Quantitative assessment of fecal E. coli and STEC shedding. Fecal E. coli colony forming units (cfu) are shown with respect to the total feces sampling pool (Shiga toxin positive and negative feces) and Shiga toxin positive pool of each trial group at each sampling.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Schmidt, N., Barth, S.A., Frahm, J. et al. Decreased STEC shedding by cattle following passive and active vaccination based on recombinant Escherichia coli Shiga toxoids. Vet Res 49, 28 (2018). https://doi.org/10.1186/s13567-018-0523-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13567-018-0523-0