Abstract
Purpose
Metabolic parameters are increasingly being used to characterize tumors. Motion artifacts due to patient respiration introduce uncertainties in quantification of metabolic parameters during positron emission tomography (PET) image acquisition. The present study investigates the impact of amplitude-based optimal respiratory gating (ORG) on quantification of PET-derived image features in patients with pancreatic ductal adenocarcinoma (PDAC), in correlation with overall survival (OS).
Methods
Sixty-nine patients with histologically proven primary PDAC underwent 2′-deoxy-2′-[18F]fluoroglucose ([18F]FDG) PET/CT imaging during diagnostic work-up. Standard image acquisition and reconstruction was performed in accordance with the EANM guidelines and ORG images were reconstructed with a duty cycle of 35%. PET-derived image features, including standard parameters, first- and second-order texture features, were calculated from the standard and corresponding ORG images, and correlation with OS was assessed.
Results
ORG significantly impacts the quantification of nearly all features; values of single-voxel parameters (e.g., SUVmax) showed a wider range, volume-based parameters (e.g., SUVmean) were reduced, and texture features were significantly changed. After correction for motion artifacts using ORG, some features that describe intra-tumoral heterogeneity were more strongly correlated to OS.
Conclusions
Correction for respiratory motion artifacts using ORG impacts the quantification of metabolic parameters in PDAC lesions. The correlation of metabolic parameters with OS was significantly affected, in particular parameters that describe intra-tumor heterogeneity. Therefore, interpretation of single-voxel or average metabolic parameters in relation to clinical outcome should be done cautiously. Furthermore, ORG is a valuable tool to improve quantification of intra-tumoral heterogeneity in PDAC.
Similar content being viewed by others
Introduction
Pancreatic malignancies have a dismal prognosis. The primary treatment for patients with a resectable tumor is surgery, resulting in a 23% 5-year survival. In patients with unresectable tumor or metastatic disease, 5-year survival decreases to 6% [1]. Although FOLFIRINOX [2] and nab-paclitaxel [3] have shown slightly better outcome as compared to gemcitabine-containing regimen, no effective systemic treatment options are available for irresectable or distant metastatic disease [4].
2′-deoxy-2′-[18F]fluoroglucose ([18F]FDG) PET/CT scanning is not standard in the diagnostic work-up for pancreatic cancer. In most institutes, this modality is mainly used to rule out distant metastases prior to planned surgery. Recent literature suggests that metabolic features of the primary tumor might associate with overall survival (OS) in patients with pancreatic ductal adenocarcinoma (PDAC). For many other malignancies, e.g., endometrial cancer [5], non-small cell lung carcinoma [6, 7], sarcomas [8] and bone metastatic breast cancer [9], higher [18F]FDG uptake in the primary tumor tends to associate with worse OS. For PDAC, recent studies use a manifold of different metabolic parameters to correlate to OS, yielding conflicting results.
Some studies showed a significant association between standardized uptake value (SUV), mostly SUVmax, and OS [10, 11], but results are equivocal [12, 13]. Measures for intratumoral heterogeneity may provide alternative biomarkers with prognostic impact [14, 15].
Several factors should be considered when metabolic parameters are assessed in PDAC. First, respiratory motion-related artifacts complicate the quantification of metabolic parameters. It is known that the movement of the diaphragm during image acquisition (with relatively long acquisition times, typically 3–4 min per bed position) causes motion artifacts, which results in blurring of anatomical borders, overestimation of lesion volume, and underestimation of radiotracer uptake in PET/CT-imaging [16,17,18]. Several methods of respiratory-gated imaging have been studied, including amplitude-based optimal respiratory gating (ORG) with HD Chest [18], resulting in significant decrease in lesion volume and increase in SUVmean values when compared to non-gated imaging. Results changed more drastically for lesions closer to the diaphragm. So, evaluation of metabolic behavior of pancreatic lesions could gain accuracy using ORG.
Secondly, recent literature suggests that specific genetic mutations affect tumor metabolism [19]. For example, in colorectal cancer, KRAS-mutated tumors show increased SUVmax values as compared to KRAS wild-type tumors [20]. In prostate cancer, AKT1 activation was associated with accumulation of aerobic glycolysis metabolites, whereas MYC overexpression was associated with dysregulated lipid metabolism [21]. In parallel, PDAC tumors have a heterogeneous genetic mutation profile that might affect their metabolic phenotype [22,23,24]. Since intra-tumoral heterogeneity, as visualized and assessed on imaging studies, can play an important role in defining tumor biology [25], SUVmax or SUVmean might, intrinsically to their physical definitions, not always be the most relevant parameter.
In this study, we analyzed a large cohort of patients with proven PDAC scanned with [18F]FDG PET/CT during diagnostic work-up. To investigate the impact of respiratory motion correction on the quantification of metabolic parameters, we analyzed ORG-gated [18F]FDG PET/CT and the corresponding standard [18F]FDG PET/CT for commonly used metabolic parameters, first and second-order texture features, and their correlation with OS.
Materials and methods
Study design and population
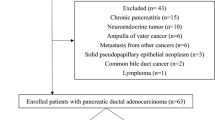
In this retrospective explorative study, data from all patients who underwent an [18F]FDG PET/CT scan at the Radboudumc in Nijmegen for suspected pancreatic malignancy between November 2004 and January 2015 were collected (Table 1). We also included patients who were scanned elsewhere and were referred for further diagnostic evaluation. The available histopathological data acquired by either biopsy or surgery were analyzed and 158 patients with histologically proven pancreatic ductal adenocarcinoma were identified (Additional file 1: Figure S1, CONSORT flowchart). In sixty-nine of these patients, an ORG reconstructed scan was available. We used this group to test the additional value of ORG scanning on the quantification of metabolic parameters. Both non-ORG (according to EANM guidelines) and ORG scans were correlated to OS. Given the retrospective nature of this study and the anonymized handling of data, informed consent was waived by the institutional review board (protocol CMO2018-4420). Scans were anonymized before review and the readers were unaware of the clinical or histopathological data.
Image acquisition
All patients fasted for a minimum of 6 h prior to intravenous administration of [18F]FDG. Subsequently, patients rested for approximately 60 min before imaging.
Whole-body PET images were acquired using a Biograph 40 mCT (Siemens Medical Solutions, Knoxville Tennessee, USA) PET/CT scanner equipped with a dual-slice CT. PET images were reconstructed according to the EANM procedure guidelines for tumor PET imaging [26]. A TrueX algorithm was used with point spread function (PSF) and time of flight (TOF) measurements, using three iterations, 21 subsets, matrix size 200 × 200 (pixel spacing of 4.07 mm), full width half maximum (FWHM) of 3 mm, and using 2 min of PET data. Post-processing was performed using a 3D Gaussian filter kernel, 3.0 mm. For respiratory gating, we used an amplitude-based ORG algorithm, which is integrated in the Syngo 2012A MI.PET/CT software by Siemens, designated HD Chest. The procedures were performed as previously described [18]. In short, the main user input for the ORG algorithm is the percentage duty cycle, which is the percentage of the total acquired true coincidences used for image reconstruction. The ORG algorithm calculates an optimal amplitude range for a given duty cycle, by calculating the amplitude range for different values of the lower limit (L). With each value of L, the upper limit (U) is adjusted to include the specified percentage of the acquired PET data, and an amplitude range (W) is calculated through a simple subtraction (U − L). The optimal amplitude range is defined as the smallest amplitude range obtained and calculated by minimizing W.
The reconstruction settings were kept constant between ORG and non-ORG images; the percentage of duty cycles (total acquired true coincidences used for image reconstruction) was set at 35%, corresponding with 2 min of PET data.
Low-dose CT scans for attenuation corrections were reconstructed using a B19f convolution kernel, slice thickness 5.0 mm, and CT images for anatomical reference were reconstructed using a B31f convolution kernel, slice thickness 3.0 mm.
Image analysis
All [18F]FDG PET/CT scans were evaluated using the Inveon Research Workspace 4.2 (Preclinical Solutions, Siemens Medical Solutions USA, Knoxville Tennessee, USA). After manual annotation of tumor region, a 40% SUVmax isocontour was used to delineate the metabolic tumor volume. Uptake of [18F]FDG projecting over adjacent structures, for example, uptake in the duodenum or biliary stent, was manually excluded from the VOIs, unless this was identified as tumor on contrast-enhanced CT (ceCT) or magnetic resonance cholangiopancreatography (MRCP). The first and second-order features were extracted using the PyRadiomics toolbox [27]. For the texture feature extraction, the images were discretized using a fixed number of 255 bins. The texture features were extracted from the co-occurrence (GLCM), gray-level run-length (GLRLM), and gray-level size-zone texture matrices (GLSZM). These matrices were generated by considering 26 connected voxels, 13 directions in three dimensions with a distance of 1 voxel, as described previously [27].
Statistical analysis
Statistical analysis was performed using R Software. Quantification of metabolic parameters measured on non-ORG versus ORG scans was compared using the Wilcoxon paired t test (no Gaussian distribution assumed).
Optimal cutoff values were determined for each individual parameter on both ORG and non-ORG scans, based on Cox proportional hazard model and log-rank testing in Kaplan Meier survival curves. Overall survival of a patient was defined from the date of the PET/CT acquisition until the date of the patient’s death.
Univariate analysis of the data was performed on all 69 non-ORG and ORG scans using the Cox proportional hazard model, after scaling the features using the standard-scaler of Scikit-learn [28]. Additionally, all respiratory-gated scans were compared to their corresponding non-ORG counterpart to test the additional value of respiratory-gating on quantification of metabolic parameters.
Results
ORG improves the detection of pancreatic lesions and assessment of intra-tumoral heterogeneity
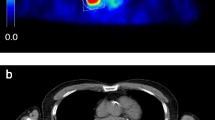
Pancreatic lesions can present in a wide range of variations on [18F]FDG PET/CT, but commonly, ductal adenocarcinomas are FDG avid beyond the level of liver uptake, with corresponding hypodense lesions on ceCT. Smaller lesions can be overlooked as physiological [18F]FDG accumulation in the duodenum. Small size and low absolute level of [18F]FDG accumulation renders small lesions particularly prone to partial-volume effects and motion artifacts. Correction for respiratory motion artifacts improved the visual detection of small pancreatic ductal adenocarcinomas in the pancreatic head and ampullary carcinomas (Fig. 1).
Optimal respiratory gating improves detection of small pancreatic and ampullary lesions. A [18F]FDG PET/CT scan reconstructed according to EANM guidelines (a, c) and with optimal respiratory gating (b, d), demonstrating markedly improvement of a small ampullary lesion in a 45-year old male who presented with jaundice. Endoscopic retrograde cholangiopancreatography (ERCP) showed a polyp-like lesion located at the ampulla of Vater, which was histologically characterized as metaplastic mucosa
Using ORG reconstruction, the visual assessment of intra-tumoral regional differences in [18F]FDG accumulation changed, revealing regions of absent [18F]FDG accumulation or multiple foci of increased [18F]FDG accumulation (Fig. 2).
ORG significantly impacts quantification of metabolic parameters
Next, we compared pairs of standard and ORG reconstructed scans to explore the impact of respiratory motion on the quantification of metabolic parameters. In concordance with previous studies using respiratory gating techniques, the parameters SUVmax, SUVmean, and SUVdiff showed significantly higher values on ORG scans compared to the corresponding non-ORG scans. The other metabolic parameters, e.g., MTV40%, significantly decreased (Additional file 2: Table S1, Fig. 3).
Optimal respiratory gating impacts the quantification of [18F]FDG PET-derived image parameters. The quantification of PET-derived parameters changes significantly after compensating for respiratory motion, as demonstrated for a SUVmax, b SUVmean, c skewness, d Kurtosis, e difference entropy, and f joint entropy
First-order features that describe intratumoral heterogeneity changed, for example, skewness, kurtosis, standard deviation, and entropy of the histogram increased significantly in the ORG reconstructed scan. Except for the features difference entropy and gray level variance, second-order features also showed a significant increase or decrease in the ORG reconstructed scans, for example, dissimilarity and joint entropy.
Prognostic impact of metabolic parameters is affected by ORG
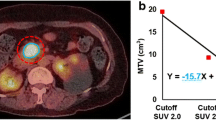
As the quantification of metabolic parameters changed considerably using ORG, we investigated whether their correlation with OS was consequently affected. For both standard and ORG reconstructed scans, optimal cutoff values for correlation with OS were defined. Logistic regression analysis demonstrated that the correlation with OS of most metabolic parameters changed considerably, but in an inconsistent pattern (Table 2). Standard PET-derived parameters SUVmax, SUVmean, and SUVdiff were no longer associated with OS after ORG reconstruction (Table 2, Fig. 4), whereas parameters that describe heterogeneity (sum entropy) became significant, or borderline significant (difference entropy, joint entropy), associated with OS (Table 2, Fig. 5).
Optimal respiratory gating impacts the correlation of standard [18F]FDG PET-derived image parameters with overall survival. Kaplan-Meier survival curves demonstrating the impact of compensation of respiratory motion on the correlation of PET-derived parameters with overall survival for standard PET-derived parameters, a–b SUVmax, c–d SUVmean, and e–f SUVdiff
Optimal respiratory gating impacts the correlation of [18F]FDG PET-derived texture features with overall survival. Kaplan-Meier survival curves demonstrating the impact of compensation of respiratory motion on the correlation of PET-derived parameters with overall survival for PET-derived texture features, a–b sum entropy, c–d joint entropy, and e–f difference entropy
Discussion
Current literature shows equivocal results for the correlation of metabolic parameters measured by [18F]FDG PET/CT and survival in patients with pancreatic cancer. In this study, we retrospectively analyzed a cohort of histologically proven PDAC patients to (1) study the impact of optimal respiratory gating on the quantification of the most commonly used metabolic parameters and (2) assess whether the correlation of these metabolic parameters and OS is affected.
In a relatively large cohort of patients, we noted that ORG results in the improved visibility of especially smaller sized pancreatic lesions, together with better visual assessment of intratumoral heterogeneity. Furthermore, we demonstrated that ORG imaging has a significant impact on quantification of all measured metabolic parameters, including the commonly used SUVmax and SUVmean, and also first- and second-order texture features that describe intratumoral heterogeneity. As expected, ORG imaging more accurately allocated measured activity to the corresponding voxel and therefore resulted in smaller measured tumor volumes with a wider range of values. Importantly, the correlation of metabolic parameters with OS significantly changed using ORG; some parameters did now correlate whereas other parameters did no longer correlate with OS.
Recent studies on metabolic parameters and their predictive value in PDAC have yielded equivocal findings [10, 12, 13, 29], and no single parameter was confirmed to predict behavior of PDAC. The uncertainties in these observations can be addressed from different angles; first, tumor metabolism is only one feature of a highly complex tumor microenvironment that involves a multitude of different functional phenotypes [30]. Other hallmarks of cancer than tumor metabolism perhaps are more critical to clinical outcome. Secondly, PDAC are generally characterized by high stromal content, which impacts pathways of tumor metabolism. Other metabolic processes, e.g., glutaminolysis or autophagy, are also involved to different degrees [19]. This might in part be reflected by heterogeneous patterns of glucose metabolism and renders a single PET parameter less relevant in this cancer type. Our study might help to explain the contradictory results from a technical standpoint, as it illustrates the difficulties with accurate quantification of metabolic parameters in organs just below the diaphragm and subjected to respiratory motion during acquisition.
The impact of ORG on the prognostic impact of PET-derived metabolic parameters or clinical decision-making was beyond the scope of this study and in general involves more information than the metabolic parameters investigated here. The retrospective nature of this study and the cohort size does not allow a comprehensive multivariate analysis to investigate whether ORG-gated measurements result in improved prognostic impact. Further prospective studies are needed to establish whether [18F]FDG PET imaging has a prognostic role and which (set of) parameters are most accurate. Furthermore, it is highly interesting to investigate in further studies whether, for example, detection of metastases in liver and lymph nodes or local tumor growth is impacted by ORG. Moreover, we investigated a homogeneous cohort with histologically proven PDAC to rule out variations by different etiologies, whereas in the clinical setting, patients with (peri-) ampullary lesions or (focal) pancreatitis will also be referred for [18F]FDG PET/CT imaging under the suspicion of pancreatic cancer.
Although correction for respiratory motion impacts the quantification of metabolic parameters derived from PET imaging, we could not demonstrate a consistent pattern in correlation with overall survival. Given the heterogeneous nature of cancer, it can be hypothesized that metabolic parameters that assess intratumoral heterogeneity better classify the underlying tumor biology than single-voxel parameters like SUVmax or averaged parameters like SUVmean or TLG. Studies in esophagus [31], breast [32] and other cancers [33, 34] support this hypothesis and illustrate the potential of PET texture features to predict survival and treatment outcome. However, extracting texture features from PET images is not standardized and subject to variations in image reconstruction, tumor delineation, SUV normalization, and SUV discretization [35,36,37]. In this study, we adhered to the 40% SUVmax threshold for tumor delineation which is widely accepted and recommended by the EANM guidelines [38]. This isocontour corresponds best with the actual dimensions of the tumor in non-ORG images. As there is no gold standard as to assess the actual tumor volume, e.g., CT or MRCP, and anatomical imaging was not standardized in this cohort, our data does not allow cross-validation of this tumor delineation method for ORG scans.
A recent study in a similar cohort of patients with PDAC suggests that first-order entropy is an independent predictor for 2-year survival [14]. As texture features are increasingly being reported to correlate with gene expression profiles [39], it is tempting to speculate that ORG imaging further improves the assessment of intratumoral heterogeneity using advanced image analyses and facilitates in vivo classification of tumor behavior in relevant clinical settings [40].
Conclusions
The present study demonstrates that correction for respiratory motion artifacts significantly changes the quantitative measurement of metabolic parameters, including first- and second-order measures for intratumoral heterogeneity, in primary pancreatic ductal adenocarcinoma, with direct impact on their correlation with overall survival.
Abbreviations
- [18F]FDG:
-
2′-Deoxy-2′-[18F]fluoro-D-glucose
- ceCT:
-
Contrast-enhanced computed tomography
- EANM:
-
European Association for Nuclear Medicine
- ERCP:
-
Endoscopic retrograde cholangiopancreatography
- FWHM:
-
Full width half maximum
- MRCP:
-
Magnetic resonance cholangiopancreatography
- ORG:
-
Optimal respiratory gating
- OS:
-
Overall survival
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PET:
-
Positron emission tomography
- PSF:
-
Point spread function
- SUV:
-
Standardized uptake value
- TLG:
-
Total lesion glycolysis
- TOF:
-
Time of flight
References
Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(22):2140–1.
Conroy T, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25.
Von Hoff DD, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29(34):4548–54.
Gresham GK, et al. Chemotherapy regimens for advanced pancreatic cancer: a systematic review and network meta-analysis. BMC Cancer. 2014;14:471.
Walentowicz-Sadlecka M, et al. The preoperative maximum standardized uptake value measured by 18F-FDG PET/CT as an independent prognostic factor of overall survival in endometrial cancer patients. Biomed Res Int. 2014;2014:234813.
Ulger S, et al. High FDG uptake predicts poorer survival in locally advanced nonsmall cell lung cancer patients undergoing curative radiotherapy, independently of tumor size. J Cancer Res Clin Oncol. 2014;140(3):495–502.
Tong AN, et al. Prognostic value of FDG uptake in primary inoperable non-small cell lung cancer. Med Oncol. 2014;31(1):780.
Eary JF, et al. Sarcoma mid-therapy [F-18]fluorodeoxyglucose positron emission tomography (FDG PET) and patient outcome. J Bone Joint Surg Am. 2014;96(2):152–8.
Ulaner GA, et al. Prognostic value of quantitative fluorodeoxyglucose measurements in newly diagnosed metastatic breast cancer. Cancer Med. 2013;2(5):725–33.
Sperti C, et al. 18-Fluorodeoxyglucose positron emission tomography in predicting survival of patients with pancreatic carcinoma. J Gastrointest Surg. 2003;7:953–60.
Chen BB, et al. Multiparametric PET/MR imaging biomarkers are associated with overall survival in patients with pancreatic cancer. Eur J Nucl Med Mol Imaging. 2018;45(7):1205–17.
Epelbaum R, et al. Tumor aggressiveness and patient outcome in cancer of the pancreas assessed by dynamic 18F-FDG PET/CT. J Nucl Med. 2013;54(1):12–8.
Heinrich S, et al. Neoadjuvant chemotherapy generates a significant tumor response in resectable pancreatic cancer without increasing morbidity: results of a prospective phase II trial. Ann Surg. 2008;248(6):1014–22.
Hyun SH, et al. Intratumoral heterogeneity of F-FDG uptake predicts survival in patients with pancreatic ductal adenocarcinoma. Eur J Nucl Med Mol Imaging. 2016;43(8):1461–8. https://doi.org/10.1007/s00259-016-3316-6.
Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501(7467):346–54.
Callahan J, et al. Motion effects on SUV and lesion volume in 3D and 4D PET scanning. Australas Phys Eng Sci Med. 2011;34(4):489–95.
Chang G, et al. Implementation of an automated respiratory amplitude gating technique for PET/CT: clinical evaluation. J Nucl Med. 2010;51(1):16–24.
Grootjans W, et al. Amplitude-based optimal respiratory gating in positron emission tomography in patients with primary lung cancer. Eur Radiol. 2014;24(12):3242–50.
Halbrook CJ, Lyssiotis CA. Employing metabolism to improve the diagnosis and treatment of pancreatic cancer. Cancer Cell. 2017;31(1):5–19.
Kawada K, et al. Relationship between 18F-fluorodeoxyglucose accumulation and KRAS/BRAF mutations in colorectal cancer. Clin Cancer Res. 2012;18(6):1696–703.
Priolo C, et al. AKT1 and MYC induce distinctive metabolic fingerprints in human prostate cancer. Cancer Res. 2014;74(24):7198–204.
Waddell N, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518(7540):495–501.
Sahin IH, Iacobuzio-Donahue CA, O'Reilly EM. Molecular signature of pancreatic adenocarcinoma: an insight from genotype to phenotype and challenges for targeted therapy. Expert Opin Ther Targets. 2016;20(3):341–59.
Bailey P, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531(7592):47–52.
O'Connor JP, et al. Imaging intratumor heterogeneity: role in therapy response, resistance, and clinical outcome. Clin Cancer Res. 2015;21(2):249–57.
Boellaard R, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010;37(1):181–200.
van Griethuysen JJM, et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017;77(21):e104–7.
Pedregosa F, et al. Scikit-learn: machine learning in python. J Mach Learn Res. 2011;12:2825–30.
Topkan E, et al. Predictive value of metabolic 18FDG-PET response on outcomes in patients with locally advanced pancreatic carcinoma treated with definitive concurrent chemoradiotherapy. BMC Gastroenterol. 2011;11:123.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Tixier F, et al. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med. 2011;52(3):369–78.
Soussan M, et al. Relationship between tumor heterogeneity measured on FDG-PET/CT and pathological prognostic factors in invasive breast cancer. PLoS One. 2014;9(4):e94017.
Hatt M, et al. 18F-FDG PET uptake characterization through texture analysis: investigating the complementary nature of heterogeneity and functional tumor volume in a multi-cancer site patient cohort. J Nucl Med. 2015;56(1):38–44.
Grootjans W, et al. The impact of optimal respiratory gating and image noise on evaluation of intratumor heterogeneity on 18F-FDG PET imaging of lung cancer. J Nucl Med. 2016;57(11):1692–8.
Leijenaar RT, et al. The effect of SUV discretization in quantitative FDG-PET Radiomics: the need for standardized methodology in tumor texture analysis. Sci Rep. 2015;5:11075.
Galavis PE, et al. Variability of textural features in FDG PET images due to different acquisition modes and reconstruction parameters. Acta Oncol. 2010;49(7):1012–6.
Orlhac F, et al. Tumor texture analysis in 18F-FDG PET: relationships between texture parameters, histogram indices, standardized uptake values, metabolic volumes, and total lesion glycolysis. J Nucl Med. 2014;55(3):414–22.
Boellaard R, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–54.
Aerts HJ, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006.
Coroller TP, et al. CT-based radiomic signature predicts distant metastasis in lung adenocarcinoma. Radiother Oncol. 2015;114(3):345–50.
Acknowledgements
Not applicable.
Funding
This work was supported by a grant from the Radboud Oncologie Fonds, partner of the Dutch Cancer Society (KUN2015-8106).
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
MG and EHJGA designed the study. EMMS and DSW retrieved the data. JJH, LFdGO, and KvL interpreted and analyzed the scans. EMMS, DSW, WG, MG, and EHJGA analyzed and interpreted the data. EMMS, DSW, and EHJGA wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the medical ethical review board (region Arnhem-Nijmegen) under protocol number CMO2018-4420. The medical ethical review board waived the need of informed consent because of the retrospective nature of the study and anonymized handling of data.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Figure S1. CONSORT flow chart. (PNG 137 kb)
Additional file 2:
Table S1. Optimal respiratory gating impacts the quantification of [18F]FDG PET-derived image features. (DOCX 23 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Smeets, E.M.M., Withaar, D.S., Grootjans, W. et al. Optimal respiratory-gated [18F]FDG PET/CT significantly impacts the quantification of metabolic parameters and their correlation with overall survival in patients with pancreatic ductal adenocarcinoma. EJNMMI Res 9, 24 (2019). https://doi.org/10.1186/s13550-019-0492-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13550-019-0492-y