Abstract
Background
Greig cephalopolysyndactyly syndrome is a rare multiple congenital anomaly syndrome characterized by the triad of polysyndactyly (preaxial or mixed preaxial and postaxial), macrocephaly, and ocular hypertelorism. Little is known about the neuropsychological phenotype and the developmental features of this syndrome.
Case presentation
We describe the clinical features of a 7-year-old Italian white boy affected by Greig cephalopolysyndactyly syndrome in comorbidity with autism spectrum disorder and the case of his 45-year-old white father, carrying the same point deletion (c.3677del) in the GLI3 gene and showing subclinical autistic symptoms. We performed a neuropsychiatric assessment of cognitive, adaptive, socio-communicative, and behavioral skills of the child. Concurrently, the father underwent his first psychiatric evaluation of cognitive skills and autistic symptoms.
Conclusions
We report the first clinical description of an association between autistic symptoms and Greig cephalopolysyndactyly syndrome in two members of the same family with the same genetic point deletion. Further research is required in order to draw an accurate conclusion regarding the association between Greig cephalopolysyndactyly syndrome and autism.
Similar content being viewed by others
Background
Greig cephalopolysyndactyly syndrome (GCPS), Online Mendelian Inheritance in Man (OMIM) 175700, is a rare (estimated incidence 1–9/1,000,000) multiple congenital anomaly syndrome inherited in an autosomal dominant pattern [1]. Loss of function mutations on chromosome 7p14.1 in the transcription factor gene GLI3 determine GCPS, which is allelic to Pallister–Hall syndrome (OMIM 146510) [2]. The GLI3 gene regulates cell growth, proliferation, and specialization (limbs, brain) and its expression is essential during early stages of development [3].
This syndrome is clinically characterized by the typical triad of polysyndactyly (preaxial or mixed preaxial and postaxial), macrocephaly, and ocular hypertelorism [4]. However, a wide spectrum of phenotypic variation has been described with a high variability of major clinical findings in terms of the expressivity and severity of the disease [1, 4].
Little is known about the neuropsychological phenotype and the developmental features of this syndrome. Intellectual disability and developmental delay are not common manifestations of the classical syndrome [1]. Up to now and to the best of our knowledge, there is only one study in the literature reporting mild-severe developmental delay in 11–20% of patients with GCPS [5]. Intellectual disability and developmental delay together with hydrocephalus and seizures are included in a more severe clinical phenotype [4, 6, 7] associated with a variant disorder characterized by a worse prognosis: the Greig cephalopolysyndactyly-contiguous gene syndrome (GCPS-CGS), caused by mutations larger than 1 Mb in the GLI3 gene [8, 9].
To the best of our knowledge, autistic symptoms have not been previously described in association with GCPS neither in the classical syndrome nor in its variant form (GCPS-CGS).We describe the case of a 7-year-old boy affected by GCPS and autism spectrum disorder (ASD) and the case of his 45-year-old father, also affected by the syndrome and with subclinical autistic symptoms.
Case presentation
Child
A 7-year-old Italian white boy affected by GCPS and ASD was referred to our Child Psychiatry Unit for a neuropsychiatric assessment.
The child, born of non-consanguineous white parents, was born at 40 weeks of gestation by vaginal delivery. A previous spontaneous miscarriage was reported. His birth weight was 3070 g (15–50th centile), length 49 cm (15–50th centile), head circumference 34 cm (15–50th centile), and APGAR Index 9–10. He was born with postaxial polysyndactyly of his hands (right hand had two extra fingers, partial syndactyly of finger 5–6; left hand had one extra finger) and of his right foot (one extra toe), surgically corrected at 6 months of age. In the early perinatal period, due to the observed dysmorphic features, the child underwent brain ultrasound (referred as normal) and genetic counseling without specific indication for subsequent genetic screening.
Motor developmental milestones were normally achieved. A history of language delay was reported: first words at 18 months with a following regression of the verbal development. At around 30 months of age, restricted and repetitive behaviors (RRBs), social withdrawal, lack of pretending game together with poor communicative skills were the main parental worries. Based on these clinical features, at 3 years of age a diagnosis of ASD was made and for this reason he started applied behavior analysis (ABA) behavioral therapy (12 hours per week).
The diagnosis of GCPS was clinically suspected in both the child and his father respectively at 3 and 42 years of age, and later molecularly confirmed through direct sequencing and multiplex ligation-dependent probe amplification (MLPA): “heterozygous for the single nucleotide deletion c.3677del, point mutation paternally transmitted, not previously described, localized in gene’s region associated with GCPS, resulting in a truncated GLI3 protein caused by the frameshift mutation and the insertion of a premature stop codon (Pro1226Glnfs4)” (see Fig. 1 for the chromatogram).
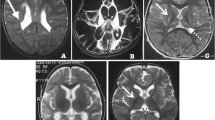
Asymmetry of the ventricular supratentorial system (right major representation) and lateral deviation of a septum pellucidum were present on brain magnetic resonance imaging performed at 6 years of age; whereas a routine and sleep-induced electroencephalogram recorded diffused paroxysmal abnormal activity during the falling asleep phase and decreasing during sleep.
No history of clinical seizures was reported. Sleep/wake cycle was regular. Food selectivity was reported since 30 months of age.
On our clinical examination at 7 years and 5 months of age, he weighed 26 kg (50–85th centile), his height was 126 cm (50–85th centile), and his head circumference around was 54.3 cm (98th centile); frontal bossing, a prominent forehead, hypertelorism, a flat nasal bridge, and low-set ears were present. A neurological examination showed normal cranial nerves, and regular muscular tropism and tone. No sensory or autonomic involvement was observed. Deep tendon reflexes of superior and lower limbs were present and normal. The Finger-to-Nose test, performed with open eyes, due to lack of collaboration, showed mild hesitation. A widespread ligament lassitude and a mild deficiency of superior limbs’ strength were observed.
We performed a neuropsychiatric assessment of cognitive, adaptive, socio-communicative, and behavioral skills through standardized tools (see Table 1); in detail, the Leiter International Performance Scale, Third Edition (Leiter-3) [10] and the Coloured Progressive Matrices (CPM) [11] were administered revealing a non-verbal intellectual quotient (IQ) of 71 and inclusion between 10 and 25th centile (range 75–85).
The Adaptive Behavior Assessment System, Second Edition (ABAS-II) [12], a questionnaire filled in by the caregivers evaluating ten adaptive areas organized in four main domains (General Adaptive, Conceptual, Social, Practical), showed adaptive skills below the average in all the fields evaluated (see Table 1).
The previous diagnosis of ASD was confirmed through clinical observation and the administration of the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2), which is the gold standard instrument for the evaluation and diagnosis of autism [13]. We performed Module 1, which is suitable for children beyond 30 months of age with a verbal language composed of single words. The diagnostic algorithm is organized in two main areas: social affect (SA) and RRB. The total score obtained (SA 16 + RRB 7 = 23) exceeded the cut-off (16) for the diagnosis of autism. Finally, a moderate level of ASD symptom severity was measured through the Calibrated Severity Score (ADOS-CSS).
A Social Responsiveness Scale (SRS) [14], a questionnaire filled in by the parents, showed a moderate deficiency of the child’s social relationship, which compromised his general functioning.
Finally, no significant problematic behavior emerged from the caregiver report, Child Behavior Checklist (CBCL) [15], except for a borderline score in the area investigating anxiety problems (see Table 1).
Father
The father’s clinical and molecular diagnosis of GCPS was made together with his son’s genetic consultation. Both carried the same single nucleotide deletion in the GLI3 gene (c.3677del). Until 42 years of age he underwent no genetic examination.
He was born of Italian non-consanguineous white parents with postaxial polydactyly of the hands and of the right foot and congenital clubfoot, which were surgically operated on after birth. No genetic counseling and screening were performed in the perinatal and postnatal period. Developmental milestones were referred as normal. No academic difficulties were reported and he graduated with success. No family history of neuropsychiatric diseases emerged.
Concurrently with the child’s evaluation, we performed a neuropsychological assessment of the 45-year-old father. Until our clinical examination, he had never undergone a psychiatric evaluation. In particular, a cognitive assessment and a specific evaluation of autistic symptoms were performed (see Table 2). His non-verbal IQ, measured by Standard Progressive Matrices (SPM) [16], turned out to be above average (IQ 128). Autistic symptoms were measured with the ADOS-2 [13]. We performed Module 4, which is suitable for adults with fluent speech. The diagnostic algorithm was composed of two main domains: Communication domain (C domain) and Social Relationship domain (SR domain); the algorithm revealed a total score of 5 (C domain 2 + SR domain 3) which does not exceed the general cut-off for the “spectrum” (7) or for “autism” (10). The partial score of the C domain, however, reached the cut-off for the “spectrum” (2).
Discussion
GCPS is a rare autosomal dominant condition characterized by high variability of the phenotype. However, a minor intrafamilial severity is generally described among multiplex families (more than one member affected) [1]. A good prognosis of the patient is frequently reported and the risk of intellectual disability is usually related to the size of the deletions in the GLI3 gene. The involvement of contiguous genes (GCPS-CGS) is associated with a worse prognosis and a major risk of cognitive impairment.
We reported the first description of autistic symptoms within individuals affected by GCPS. In particular, we described the case of two members of the same family, father and son, both carrying the same point deletion (c.3677del) in the GLI3 gene and showing autistic symptoms, but at a different level. As a matter of fact, the child presented a definite diagnosis of ASD characterized by a moderate level of severity in association with cognitive and language impairment.
On the other hand, sub-threshold autistic symptoms emerged from the father’s clinical interview and the administration of the ADOS-2 in the C domain, with results highlighting a mild impairment. During the clinical observation, a prosodic deficit with low volume of the voice and a lacking of facial expressions directed to the examiner were observed; few voluntary emotional, instrumental gestures were associated with verbal language. Moreover, he described himself as a shy person with difficulties in maintaining eye contact during conversation. These communication difficulties have partially impaired his social functioning especially during adolescence.
Furthermore, although they both carried the same point mutation (smaller than 1 Mb), as emerged from the cognitive and adaptive evaluation, the child was affected by a mild intellectual disability, whereas the father’s IQ was above average. These differences in the severity of the neuropsychological phenotype (cognitive and socio-communicative) within the same family are in contrast with the literature that refers to less intrafamilial variability [1].
Conclusions
This clinical case is the first description of an association between autistic symptoms and GCPS in two members of the same family with the same genetic point deletion. Autistic symptoms, either at a clinical or subclinical level, should be considered and investigated within the GCPS population in order to perform an early diagnosis and to begin the early intervention required. Therefore neuropsychiatric assessment is recommended in early stages of development.
Further descriptions of the cognitive and socio-communicative phenotype of the affected patients will enable us to better delineate a specific neuropsychological profile and developmental trajectory of the syndrome.
Abbreviations
- ABA:
-
Applied behavior analysis
- ABAS-II:
-
Adaptive Behavior Assessment System, Second Edition
- ADOS-2:
-
Autism Diagnostic Observation Schedule, Second Edition
- ASD:
-
Autism spectrum disorder
- CBCL:
-
Child Behavior Checklist
- C domain:
-
Communication domain
- CPM:
-
Coloured Progressive Matrices
- CSS:
-
Calibrated Severity Score
- GCPS:
-
Greig cephalopolysyndactyly syndrome
- GCPS-CGS:
-
Greig cephalopolysyndactyly-contiguous gene syndrome
- IQ:
-
Intellectual quotient
- MLPA:
-
Multiplex ligation-dependent probe amplification
- OMIM:
-
Online Mendelian Inheritance in Man
- RRB:
-
Restricted and repetitive behavior
- SA:
-
Social affect
- SPM:
-
Standard Progressive Matrices
- SR domain:
-
Social Relationship domain
- SRS:
-
Social Responsiveness Scale
References
Biesecker LG. The Greig cephalopolysyndactyly syndrome. Orphanet J Rare Dis. 2008;3:10.
Kang S, Graham JM Jr, Olney AH, Biesecker LG. GLI3 frameshift mutations cause autosomal dominant Pallister-Hall syndrome. Nat Genet. 1997;15:266–8.
Vortkamp A, Gessler M, Grzeschik K-H. GLI3 zinc finger gene interrupted by translocations in Greig syndrome families. Nature. 1991;352:539–40.
Balk K, Biesecker LG. The clinical atlas of Greig cephalopolysyndactyly syndrome. Am J Med Genet Part A. 2008;146A:548–57.
Démurger F, Ichkou A, Mougou-Zerelli S, et al. New insights into genotype–phenotype correlation for GLI3 mutations. Eur J Hum Genet. 2015;23(1):92–102. https://doi.org/10.1038/ejhg.2014.62. Epub 2014 Apr 16.
Duncan PA, Klein RM, Wilmot PL, Shapiro LR. Greig cephalopolysyndactyly syndrome. Am J Dis Child. 1979;133:818–21.
Gorlin RJ, Cohen MM Jr, Hennekam RCM. Greig cephalopolysyndactyly syndrome. In: Syndromes of the Head and Neck. 4th ed. New York: Oxford University Press; 2001. p. 995–6.
Johnston JJ, Olivos-Glander I, Turner J, Aleck K, Bird LM, Mehta L, Schimke RN, Heilstedt H, Spence JE, Blancato J, Biesecker LG. Clinical and molecular delineation of the Greig cephalopolysyndactyly contiguous gene deletion syndrome and its distinction from acrocallosal syndrome. Am J Med Genet Part A. 2003;123A:236–42.
Johnston J, Walker R, Davis S, Facio F, Turner J, Bick D, Daentl D, Ellison J, Meltzer P, Biesecker L. Zoom-in comparative genomic hybridisation arrays for the characterisation of variable breakpoint contiguous gene syndromes. J Med Genet. 2007;44:e59.
Roid GH, Miller LJ, Pomplun M, Koch C. Leiter International Performance Scale – Third Edition. Florence: Giunti Psychometrics; 2016.
Raven JC. Coloured Progressive Matrices. Florence: Giunti Psychometrics; 1984.
Harrison PT, Oakland T. Adaptive Behavior Assessment System – Second Edition. Florence: Giunti Psychometrics; 2014.
Lord C, Rutter R, DiLavore PM, Risi S, Luyster RJ, Gotham K, Bishop SL, Guthrie W. Autism Diagnostic Observation Schedule. 2nd. Oxford: Hogrefe; 2013.
Costantino JN, Gruber PC. Social Responsiveness Scale. Florence: Giunti Psychometrics; 2010.
Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington: University of Vermont, Research Center for Children, Youth, & Families; 2001.
Raven JC. Standard Progressive Matrices. Florence: Giunti Psychometrics; 1954.
Funding
There is no funding to declare.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Contributions
MS, AR, AB, and LM examined, assessed, and were involved in the management of the patients. MB performed the genetic analysis and provided the sequence chromatogram. MS, AR, and LM analyzed the literature and wrote the manuscript. LM and PC read and approved the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Ethical approval was not required for the publication of this case report as this does not involve sharing of the personal details of the patient.
Consent for publication
Written informed consent was obtained from the patient’s legal guardians for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Siracusano, M., Riccioni, A., Baratta, A. et al. Autistic symptoms in Greig cephalopolysyndactyly syndrome: a family case report. J Med Case Reports 13, 100 (2019). https://doi.org/10.1186/s13256-019-2043-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-019-2043-6