Abstract
Background
X-linked ichthyosis is a dermatological condition caused by deficiency for the enzyme steroid sulfatase. Previously, X-linked ichthyosis/steroid sulfatase deficiency has been associated with developmental and neurological phenotypes. Here, we show for the first time, that X-linked ichthyosis may be comorbid with an additional psychiatric phenotype (psychosis).
Case presentation
We report the case of an 11-year-old Saudi Arabian boy with X-linked ichthyosis associated with psychosis, mental retardation, autism spectrum disorder, inattentive attention deficit hyperactivity disorder, and epilepsy. Genetic analysis revealed a 1.68 Mb deletion encompassing STS in 95% of cells while biochemical analysis revealed correspondingly low steroid sulfatase activity consistent with a diagnosis of X-linked ichthyosis. The psychotic symptoms could be reasonably well controlled by administration of an atypical antipsychotic.
Conclusions
This report describes a case of comorbid X-linked ichthyosis and psychosis (most closely corresponding to early-onset schizophrenia) for the first time, and suggests that deficiency for steroid sulfatase and contiguous genes may increase vulnerability to psychosis as well as other psychological disorders.
Similar content being viewed by others
Background
The ichthyoses are a group of dermatological conditions characterized by dry scaly skin; most of these disorders are hereditary, but examples of sporadic or acquired forms have been reported. Inherited ichthyoses are usually apparent during the first year of life, often at birth, and continue to affect a person throughout life [1]. In 1965, Wells and Kerr first recognized X-linked ichthyosis (XLI) in 81 affected males [2]. Approximately 90% of cases of XLI are caused by deletions within, or encompassing, the steroid sulfatase (STS) gene on chromosome Xp22.31, with ~ 10% caused by point mutations within the STS gene [3]; deletions may be “typical” (encompassing STS and a small number of adjacent genes), or “atypical” (encompassing many contiguous genes). XLI may occur solely as a skin disorder or may be associated with other physical findings such as corneal opacities (up to 50% of cases), cryptorchidism (20% of cases), chondrodysplasia punctata, and nephrotic syndrome [4,5,6]. Deletions encompassing STS have been reported to be associated with multiple behavioral, cognitive, and neurological phenotypes notably: mental retardation, developmental conditions including autism spectrum disorders (ASDs), attention deficit hyperactivity disorder (ADHD), and seizures [7]. In the first systematic behavioral study of boys with XLI, Kent et al. showed high rates of ADHD (particularly inattentive ADHD) and autism-related conditions; the latter were seen when the deletions were large and included the neuroligin 4 gene (NLGN4X) [8]. Consistent with these data, genetic variants within STS are associated with inattentive symptoms in children with ADHD [9, 10] while mice lacking the Sts gene exhibit behavioral phenotypes associated with developmental disorders including inattention, hyperactivity, elevated emotional reactivity, behavioral perseveration, and aggression [11,12,13]. STS is expressed in brain regions associated with attention, impulsivity, and the integration of, and response to, sensory information [10]. XLI rarely interferes with the activities of daily living and individuals with mild forms may not require treatment [4]. The skin condition can be managed relatively effectively, but some patients may experience a significantly reduced quality of life due to self-consciousness and social embarrassment or due to comorbid behavioral, cognitive, or neurological conditions; such patients may require lifelong treatment [14, 15].
Case presentation
A summary of the case presentation and management is provided in Table 1. An 11-year-old Saudi Arabian boy, in Grade Three of primary school, initially presented with a year-long history of epileptic fits in the form of abnormal jerky movement of upper limbs accompanied by fixed eye gaze. The episodes typically lasted for a few seconds each, with up to 15 episodes per day, and were often preceded by episodes of unexplained crying.
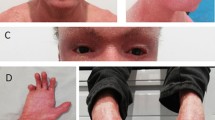
The boy was born at 28 weeks’ gestation by spontaneous vaginal delivery after an uneventful and uncomplicated pregnancy. He had a low birth weight of 1.5 kg (below the 5th centile). During pregnancy, his mother took no medication apart from folic acid supplementation during the first trimester. Following delivery, he was kept in the intermediate care unit for 3 months until he gained sufficient weight. At birth, he was diagnosed with congenital ichthyosis although the subtype was not defined; from early childhood, his parents were advised by the dermatologist of the importance of daily bathing and regular application of emollient creams containing urea. His language and gross motor milestones were delayed; he had poor social interaction with others and restricted play. There was a family history of developmental disorders (speech and language problems and learning disability), and the boy’s 13-year-old sister exhibited mild mental retardation. There was no family history of ichthyosis, or psychotic or mood disorder.
The boy’s parents reported that his academic performance was poor, that he had a learning disability and that he required substantial support in school. His parents also reported that, coincidentally with the epileptic fits, he started exhibiting behavioral changes, becoming socially withdrawn, unresponsive, and preoccupied. He also became restless and wandered aimlessly, shouted and made odd noises, began to believe that others were watching him, and was seen talking to himself and laughing without any reason. His parents initially sought help from traditional healers who provided him with herbal medicine for a short period of time (honey and herbal oil). After no improvement in the boy’s symptoms, his parents arranged for him to be seen by a local psychiatrist and a neurologist. According to the boy’s father, initial blood test results (which included full blood counts, liver function test, urea and electrolytes, and thyroid function test) were normal; however, an electroencephalograph (EEG) showed epileptiform discharges. Carbamazepine (100 mg per day) and olanzapine treatment (5 mg, twice per day) were instigated to treat the boy’s EEG abnormalities and his disturbed behavior respectively; carbamazepine was considered to be the optimum treatment to alleviate his brief episodes of staring with unresponsiveness which were suggestive of complex partial seizures. However, his adherence to the treatment regime was poor: he did not take his medication as prescribed and had no regular follow-up. Hence there was minimal improvement in his condition.
One month after his initial presentation to local doctors, the boy was urgently referred and admitted to a tertiary hospital. He was extremely aggressive and attempted to bite his parents and siblings; he was also verbally abusive, highly vocal (screaming), and restless. His sleep pattern was extremely disturbed; he remained awake for the majority of the 3 days prior to admission. He became doubly incontinent. On examination, the admitting doctor documented microcephaly and dry skin with scales over his body (which the patient was peeling off and eating); there was no erythema. His vital signs were stable, and there were no neurological signs apart from eye twitches. He was shouting and uncooperative, and was looking around as if responding to visual stimuli. He was also talking to himself and mumbling. He was disoriented with regard to time and place, and demonstrated poor general knowledge, even in a simple color-naming task. He was restless and aggressive, required sedation, and lorazepam (0.5 mg twice per day) was administered orally from the day of admission. He showed immediate improvements in his sleep pattern and became less agitated and restless. However, he persisted in talking to himself and continued to be suspicious and paranoid. His presentation and symptoms were considered to best resemble the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5.0) diagnostic criteria for early-onset schizophrenia [16]. Therefore, he continued to be treated with lorazepam for a 2-week period, with treatment tapering off within the third week.
He was subsequently referred to several subspecialties including dermatology, genetics, neurology, and child psychiatry. During the hospital course, a number of physiological and metabolic measures were taken (Table 2).
Most of our patient’s results were within the normal range, although relatively low levels of hemoglobin and relatively high values for erythrocyte sedimentation rate (ESR) and pyruvate were noted.
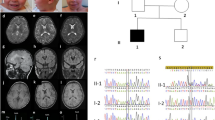
On the basis of family history and skin appearance, dermatologists suspected XLI and continued prescribing emollient creams containing urea. Chromosomal G-banding and fluorescence in situ hybridization (FISH) analysis on peripheral blood and fibroblast cultures revealed an interstitial deletion within Xp22.31 on 95% of the examined cells with a low level of mosaicism (5% of cells with normal karyotype). Consistent with the FISH analysis, STS activity in fibroblasts was low at 3.5 nmol/hour/protein (normal range, 5 to 32 nmol/hour/protein). The boy’s genomic deoxyribonucleic acid was assayed on a combined comparative genomic hybridization (CGH) and single nucleotide polymorphism (SNP) array in an attempt to specify a genetic basis for his clinical presentation. The array CGH study confirmed a 1.68 Mb deletion within the cytogenetic band Xp22.31 (hg 19: 6,456,036-8,139,238) that contains the STS gene in addition to VCX3A, PUDP, VCX, and PNPLA4 genes, and two microRNAs (MIR4767 and MIR651) [17]. His mother was tested for mutation carrier status, but his siblings were not genetically tested. Eight regions of homozygosity spanning ~ 111Mb in total were detected within our patient across multiple autosomes (Table 3). Subsequent targeted exome sequencing of these homozygosity blocks using next generation sequencing and capillary Sanger sequencing did not reveal any pathological, likely pathological, or other sequence change of possible clinical relevance in the coding regions of the genes within these regions.
Radiological and neurological investigations using computed tomography (CT) revealed a structurally normal brain. A CT venogram showed no evidence of cerebral venous thrombosis. Magnetic resonance imaging (MRI) indicated mild periventricular leukomalacia, possibly related to our patient’s premature birth. Two EEGs were performed; the first one performed initially during his stay in the hospital and the second one was 2 months after his discharge. The first EEG showed mild slowing of the background with no evidence of any epileptiform discharges; the second EEG showed mild, but improved, slowing with no evidence of epileptiform discharge. An autoimmune etiology was entertained by the neurologist; this was partially on the basis of a clear cerebrospinal fluid (CSF) and acute behavioral symptoms; thus, a methylprednisolone pulse therapy regime (50 mg/kg) to treat suspected encephalitis was initiated for a period of 5 days, in addition to intravenously administered immunoglobulin over another 5 days. These therapies did not improve our patient’s symptoms of disorientation, cognitive dysfunction, and psychosis. He showed some improvement in symptoms with a subsequent management regime of 5 mg olanzapine twice per day, but he demonstrated poor compliance with this. The psychiatry team opted to continue him on this drug but at higher doses (the dose was increased from 10 mg to 20 mg per day gradually over a 2-week period) to control his psychotic symptoms, as per prescribing guidelines [18]; olanzapine is a second-generation antipsychotic which has been shown to have robust clinical benefits for treating early-onset schizophrenia [19]. With this revised treatment regime, his psychiatric symptoms were brought under control, and eventually he was discharged with follow-up appointments in ophthalmology (for bilateral iris mammillation), psychiatric, pediatric neurology, and dermatology clinics.
The follow-up by the psychiatric clinic revealed no psychotic symptoms as indexed by the mental state examination; he was maintained on olanzapine 15 mg per day for almost 1 year after he was discharged from our hospital. Neuropsychological testing revealed a non-verbal intelligence quotient (IQ) of 57 with Test of Nonverbal Intelligence, Second Edition (TONI-2), consistent with a mild intellectual disability, and an ASD on the basis of the Autism Diagnostic Observation Schedule (ADOS). A diagnosis of ADHD (inattentive subtype) was also made on the basis of parental history, academic reports, and Conners Questionnaire; psychostimulant medication was not considered to have been beneficial in treating his ADHD symptoms.
Discussion
Our patient presented with dry scaly skin and pronounced behavioral and neurological symptoms that included: psychotic features, disorganized behavior, ADHD (inattentive subtype), ASD, and a learning disability. His EEG showed epileptiform discharges. Pharmacological treatments to control the psychotic symptoms and epilepsy were relatively effective. Genetic and biochemical analysis confirmed the diagnosis of XLI.
It is feasible that the mutation encompassing the STS gene and some, if not all, of the behavioral and neurological features seen in our patient are causally related. The limited clinical literature documents several cases of children in which XLI is comorbid with epilepsy/EEG abnormalities, mental retardation, and ADHD or an autism-related condition (particularly in the case of large chromosomal deletions) [8, 20, 21], while animal studies have emphasized a link between Sts deficiency and cognitive and behavioral phenotypes associated with developmental disorders [11,12,13].
To the best of our knowledge, this is the first report of psychosis consistent with a diagnosis of early-onset schizophrenia in a child with confirmed XLI. Given the comparative rarity of both XLI and psychosis in the general population, our observations, together with previous literature reporting paranoid schizophrenia in two females with deletions spanning STS [22] and implicating STS deficiency in postpartum psychosis [23, 24], suggest that, in addition to influencing risk of developmental disorders, STS deficiency may also predispose to risk of psychotic illness; this notion is consistent with the latest genome-wide genetic screens which show that individual polymorphisms or mutations may act as risk factors in multiple developmental and affective conditions [25].
Our patient’s behavioral and neurological presentation could feasibly be modulated by other genetic or environmental factors segregating within his family; this idea is supported by the strong family history of learning disability and the communication deficits in our patient’s eldest sister. In addition, autosomal runs of homozygosity could theoretically have contributed toward our patient’s clinical presentation through revealing recessive deleterious mutations of relevance to brain function [26]; however, the fact that exome sequencing detected no coding mutations of clinical significance within these regions makes this explanation unlikely. Finally, it is possible that living with a congenital skin condition and/or treatment for that condition, for example retinoids or poorly specified traditional and herbal remedies, may influence psychological function. There is a case report of a woman with ichthyosis vulgaris who presented with a schizophrenia-like psychosis and EEG abnormalities similar to our patient [27]; however, she also had a strong family history of psychosis and learning disability.
Conclusions
This case and the previous limited literature highlight the severe behavioral, psychiatric, and neurological symptoms that may be comorbid with XLI. Clearly, given the prevalence of the condition (~1 in 3000 to 6000 men, equivalent to over 1.2 million individuals worldwide [4]), there is a need for the physical and psychological phenotypes associated with it (including the prevalence of psychosis) to be comprehensively described. Such an endeavor will allow individuals such as our case to have their condition and potential comorbidities recognized and treated at an early stage, and to be monitored closely by appropriate multidisciplinary teams.
Abbreviations
- ADHD:
-
Attention deficit hyperactivity disorder
- ADOS:
-
Autism diagnostic observation schedule
- ASDs:
-
Autism spectrum disorders
- CGH:
-
Comparative genomic hybridization
- CSF:
-
Cerebrospinal fluid
- CT:
-
Computed tomography
- DSM-5.0:
-
Diagnostic and statistical manual of mental disorders, fifth Edition
- EEG:
-
Electroencephalograph
- FISH:
-
Fluorescence in situ hybridization
- IQ:
-
Intelligence quotient
- MRI:
-
Magnetic resonance imaging
- NLGN4X :
-
Neuroligin 4 gene
- SNP:
-
Single nucleotide polymorphism
- STS:
-
Steroid sulfatase
- TONI-2:
-
Test of nonverbal intelligence, second edition
- XLI:
-
X-linked ichthyosis
References
Marukian NV, Choate KA. Recent advances in understanding ichthyosis pathogenesis. F1000Res. 2016;5. doi:10.12688/f1000research.8584.1.
Wells RS, Kerr CB. Genetic classification of ichthyosis. Arch Dermatol. 1965;92(1):1–6.
Takeichi T, Akiyama M. Inherited ichthyosis: Non-syndromic forms. J Dermatol. 2016;43(3):242–51. doi:10.1111/1346-8138.13243.
Fernandes NF, Janniger CK, Schwartz RA. X-linked ichthyosis: an oculocutaneous genodermatosis. J Am Acad Dermatol. 2010;62(3):480–5. doi:10.1016/j.jaad.2009.04.028.
Hung C, Ayabe RI, Wang C, Frausto RF, Aldave AJ. Pre-descemet corneal dystrophy and X-linked ichthyosis associated with deletion of Xp22.31 containing the STS gene. Cornea. 2013;32(9):1283–7. doi:10.1097/ICO.0b013e318298e176.
Mishra K, Batra VV, Basu S, Rath B, Saxena R. Steroid-resistant nephrotic syndrome associated with steroid sulfatase deficiency-x-linked recessive ichthyosis: a case report and review of literature. Eur J Pediatr. 2012;171(5):847–50. doi:10.1007/s00431-012-1712-x.
Chatterjee S, Humby T, Davies W. Behavioural and psychiatric phenotypes in men and boys with X-linked ichthyosis: evidence from a worldwide online survey. PLoS One. 2016;11(10):e0164417. doi:10.1371/journal.pone.0164417.
Kent L, Emerton J, Bhadravathi V, Weisblatt E, Pasco G, Willatt LR, et al. X-linked ichthyosis (steroid sulfatase deficiency) is associated with increased risk of attention deficit hyperactivity disorder, autism and social communication deficits. J Med Genet. 2008;45(8):519–24. doi:10.1136/jmg.2008.057729.
Brookes KJ, Hawi Z, Kirley A, Barry E, Gill M, Kent L. Association of the steroid sulfatase (STS) gene with attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8):1531–5. doi:10.1002/ajmg.b.30873.
Stergiakouli E, Langley K, Williams H, Walters J, Williams NM, Suren S, et al. Steroid sulfatase is a potential modifier of cognition in attention deficit hyperactivity disorder. Genes Brain Behav. 2011;10(3):334–44. doi:10.1111/j.1601-183X.2010.00672.x.
Davies W, Humby T, Kong W, Otter T, Burgoyne PS, Wilkinson LS. Converging pharmacological and genetic evidence indicates a role for steroid sulfatase in attention. Biol Psychiatry. 2009;66(4):360–7. doi:10.1016/j.biopsych.2009.01.001.
Trent S, Dennehy A, Richardson H, Ojarikre OA, Burgoyne PS, Humby T, et al. Steroid sulfatase-deficient mice exhibit endophenotypes relevant to attention deficit hyperactivity disorder. Psychoneuroendocrinology. 2012;37(2):221–9. doi:10.1016/j.psyneuen.2011.06.006.
Trent S, Dean R, Veit B, Cassano T, Bedse G, Ojarikre OA, et al. Biological mechanisms associated with increased perseveration and hyperactivity in a genetic mouse model of neurodevelopmental disorder. Psychoneuroendocrinology. 2013;38(8):1370–80. doi:10.1016/j.psyneuen.2012.12.002.
Ganemo A, Sjoden PO, Johansson E, Vahlquist A, Lindberg M. Health-related quality of life among patients with ichthyosis. Eur J Dermatol. 2004;14(1):61–6.
Ganemo A. Quality of life in Swedish children with congenital ichthyosis. Dermatol Rep. 2010;2(1):e7. doi:10.4081/dr.2010.e7.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington. D.C: American Psychiatric Association; 2013.
Ensembl. http://www.ensembl.org. Accessed 9 Sept 2016.
David T, Carol P, Shitij K. The Maudsley prescribing guidelines in psychiatry. 12th ed. 2015.
Harvey RC, James AC, Shields GE. A systematic review and network meta-analysis to assess the relative efficacy of antipsychotics for the treatment of positive and negative symptoms in early-onset schizophrenia. CNS Drugs. 2016;30(1):27–39. doi:10.1007/s40263-015-0308-1.
van Steensel MA, Vreeburg M, Engelen J, Ghesquiere S, Stegmann AP, Herbergs J, van Lent J, Smeets B, Vles JH. Contiguous gene syndrome due to a maternally inherited 8.41 Mb distal deletion of chromosome band Xp22.3 in a boy with short stature, ichthyosis, epilepsy, mental retardation, cerebral cortical heterotopias and Dandy-Walker malformation. Am J Med Genet A. 2008;146A(22):2944–9. doi:10.1002/ajmg.a.32473.
Doherty MJ, Glass IA, Bennett CL, Cotter PD, Watson NF, Mitchell AL, Bird TD, Farrell DF. An Xp; Yq translocation causing a novel contiguous gene syndrome in brothers with generalized epilepsy, ichthyosis, and attention deficits. Epilepsia. 2003;44(12):1529–35.
Milunsky J, Huang XL, Wyandt HE, Milunsky A. Schizophrenia susceptibility gene locus at Xp22.3. Clin Genet. 1999;55(6):455–60.
Davies W. Does steroid sulfatase deficiency influence postpartum psychosis risk? Trends Mol Med. 2012;18(5):256–62. doi:10.1016/j.molmed.2012.03.001.
Humby T, Cross ES, Messer L, Guerrero S, Davies W. A pharmacological mouse model suggests a new risk pathway for postpartum psychosis. Psychoneuroendocrinology. 2016;74:363–70. doi:10.1016/j.psyneuen.2016.09.019.
Gatt JM, Burton KL, Williams LM, Schofield PR. Specific and common genes implicated across major mental disorders: a review of meta-analysis studies. J Psychiatr Res. 2015;60:1–13. doi:10.1016/j.jpsychires.2014.09.014.
Howrigan DP, Simonson MA, Davies G, Harris SE, Tenesa A, Starr JM, et al. Genome-wide autozygosity is associated with lower general cognitive ability. Mol Psychiatry. 2016;21(6):837–43. doi:10.1038/mp.2015.120.
Mochizuki H, Tobo M, Itoi K. A case of ichthyosis vulgaris associated with schizophrenia-like psychosis and spike-wave stupor. Folia Psychiatr Neurol Jpn. 1980;34(3):392–3.
Acknowledgements
The authors would like to thank our patient and his family for sharing his presentation for this manuscript.
Funding
Work at Cardiff University was funded by Medical Research Council (MRC) Centre for Neuropsychiatric Genetics and Genomics (MR/L010305/1).
Availability of data and materials
The data and materials in this manuscript are not made available to any readers since they contain the patient’s personal particulars.
Author information
Authors and Affiliations
Contributions
AM, ABA, MS, and BH contributed to patient recruitment. MTA and MAB aided patient’s diagnosis, genetic and biochemical testing or treatment. WD and WE coordinated work, drafted initial manuscript, and edited manuscript. All authors contributed toward data analysis and interpretation as well as manuscript drafting. All authors read and approved the final manuscript before submission.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable. The patient’s routine medical care is described.
Consent for publication
Written informed consent was obtained from the patient’s legal guardian for publication of this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Malik, A., Amer, A.B., Salama, M. et al. X-linked ichthyosis associated with psychosis and behavioral abnormalities: a case report. J Med Case Reports 11, 267 (2017). https://doi.org/10.1186/s13256-017-1420-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-017-1420-2