Abstract
Background
Oxygen delivery to patients with chronic obstructive pulmonary disease may be challenging because of their potential hypoxic ventilatory drive. However, some oxygen delivery systems such as non-rebreathing face masks with an oxygen reservoir bag require high oxygen flow for adequate oxygenation and to avoid carbon dioxide rebreathing.
Case presentation
A 72-year-old Caucasian man with severe chronic obstructive pulmonary disease was admitted to the emergency department because of worsening dyspnea and an oxygen saturation of 81% measured by pulse oximetry. Oxygen was administered using a non-rebreathing mask with an oxygen reservoir bag attached. For fear of removing the hypoxic stimulus to respiration the oxygen flow was inappropriately limited to 4L/minute. The patient developed carbon dioxide narcosis and had to be intubated and mechanically ventilated.
Conclusions
Non-rebreathing masks with oxygen reservoir bags must be fed with an oxygen flow exceeding the patient’s minute ventilation (>6–10 L/minute.). If not, the amount of oxygen delivered will be too small to effectively increase the arterial oxygen saturation. Moreover, the risk of carbon dioxide rebreathing dramatically increases if the flow of oxygen to a non-rebreathing mask is lower than the minute ventilation, especially in patients with chronic obstructive pulmonary disease and low tidal volumes. Non-rebreathing masks (with oxygen reservoir bags) must be used cautiously by experienced medical staff and with an appropriately high oxygen flow of 10–15 L/minute. Nevertheless, arterial blood gases must be analyzed regularly for early detection of a rise in partial pressure of carbon dioxide in arterial blood in patients with chronic obstructive pulmonary disease and a hypoxic ventilatory drive. These patients are more safely managed using a nasal cannula with an oxygen flow of 1–2L/minute or a simple face mask with an oxygen flow of 5L/minute.
Similar content being viewed by others
Background
In 2011, chronic obstructive pulmonary disease (COPD) had a global prevalence of 12% and was the third leading cause of death in the USA [1]. Oxygen (O2) delivery to COPD patients with an acute disease exacerbation remains challenging. High inspired O2 concentrations should be used with caution, because COPD patients may breathe with a hypoxic drive [2]. To avoid carbon dioxide (CO2) narcosis, O2 must be provided in a controlled fashion with a target saturation of only 88–92% [3]. This recommendation was studied in a prehospital care setting of 405 patients with COPD exacerbations: When O2 was delivered via nasal prongs with a target percentage of oxygen saturation of arterial blood of 88-92%, measured by pulse oximetry (SpO2), a significantly lower in-hospital mortality (4% versus 9%, p = 0.02) was observed when compared with standard O2 delivery using a non-rebreathing mask [4]. In addition, there were fewer hypercapnic and acidotic episodes in the group with O2 delivered via a nasal cannula. Accordingly, the British Thoracic Society recommends three options for oxygen delivery to COPD patients at risk for hypercapnic respiratory failure: (i) Venturi mask 28% at 4 L/min. or Venturi mask 24% at 2-4 L/min. with a target SpO2 of 88–92%. (ii) If the SpO2 falls below 88%, O2 delivery should be changed to a nasal cannula with an O2 flow 2–6 L/min. or a simple face mask with an O2 flow of 5 L/min. (iii) Increases in partial pressure of carbon dioxide in arterial blood (PaCO2) or progressive acidotic pH require noninvasive ventilation or intubation and mechanical ventilation [5]. Non-rebreathing masks may be used in severely hypoxic patients. It is essential to set the O2 flow of non-rebreathing masks to 10–15 L/min. in order to avoid CO2 rebreathing. Because of the risk of hypercapnic respiratory failure, blood gases must be analyzed every 30–60 minutes. We report the case of a patient with COPD who developed CO2 narcosis because of inadequate use of the non-rebreathing mask.
Case presentation
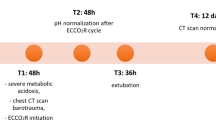
A 72-year-old, cachectic (height 170 cm, weight 50 kg, BMI 17.3 kg/m2) Caucasian man with COPD and a history of smoking had increasing shortness of breath. Pulmonary function testing performed 9 years prior showed a decreased forced expiratory volume in 1 second (FEV1) [38% of predicted, Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 3], which did not increase after albuterol inhalation. The high total lung capacity and residual volume were consistent with emphysema. On arrival of the emergency medical team at his home, our patient was dyspneic but alert. His vital signs were: blood pressure 150/100 mmHg, heart rate 103 bpm, and SpO2 81%. High-flow O2 was supplied at 10 L/min. using a non-rebreathing mask with an O2 reservoir bag (Fig. 1). The SpO2 increased to 99% within 20 minutes, and our patient was transported to the emergency department. As it was unknown whether our patient had a hypoxic respiratory drive, O2 flow was erroneously limited to 4 L/min. He got increasingly irritated, had no headache, but was no longer oriented to time, place, and person, and became unconscious, gasping for air 2 hours later. His blood pressure was 110/80 mmHg, and the heart rate was 80 bpm. Breath sounds were distant, and his tongue was cyanotic. An arterial blood gas analysis taken shortly after starting bag-valve-mask ventilation showed marked hypercapnia with respiratory acidosis (Table 1A). Noninvasive ventilation was not a possible option [6]. Our patient was intubated, mechanically ventilated, and received albuterol and ipratropium bromide by inhalation. Methylprednisolone, amoxicillin clavulante plus clarithromycin (for an infiltrate in the right paracardiac region), and low-dose theophylline were administered intravenously. Four hours later, our patient was extubated (Table 1B), and was later transferred to a medical ward. The high PaCO2 was explained by low tidal volumes and a probably hypoxic ventilatory drive (Table 1C). Because of our patient’s worsening dyspnea, he mistakenly received O2 at 2 L/min. by the same non-rebreathing mask (Fig. 1). After a few hours, intensive care unit (ICU) admission was required due to hypotension (75/50 mmHg) and bradypnea (Table 1D). He was re-intubated and mechanically ventilated for 24 hours. Norepinephrine was given to stabilize the blood pressure. An electrocardiogram (ECG) showed a sinus rhythm with right bundle branch block and right ventricular hypertrophy, and the echocardiography documented a chronic cor pulmonale with pulmonary arterial hypertension. Respiratory acidosis improved, and the patient was temporarily extubated (Table 1E). However, aspiration of a pea (removed by bronchoscopy, Fig. 2) with atelectasis of the right lung again necessitated mechanical ventilation and a tracheostomy. One month after admission, the exhausted patient died of CO2 narcosis (Table 1F). An autopsy was not performed.
Discussion
The first two episodes of CO2 narcosis (Table 1A, D) were due to near asphyxia and CO2 rebreathing because of incorrect use of the non-rebreathing mask by providing insufficient (2–4 L/min.) O2 supply. The third episode was due to probable hypoventilation and severe exhaustion (Table 1F).
Assuming a reduced tidal volume [7] of 200 mL (normal 7 mL/kg body weight = 350 mL) and a respiratory rate of 30/min., our patient’s estimated minute ventilation was 6 L/minute (respiratory minute volume [mL] = tidal volume [mL] × respiratory rate [1/min.]. The respiratory minute volume [mL] is also the sum of the alveolar ventilation [mL] + the dead space ventilation [mL]). In high-concentration reservoir masks only an O2 flow that exceeds the minute ventilation guarantees a sufficient air supply. Consequently, the reservoir bag of the non-rebreathing mask must not be allowed to deflate by more than one third during inspiration. Our patient’s respiratory dead space was 100 mL (2 mL/kg body weight), and the dead space of the mask was 50 mL. The increased dead space (150 versus 100 mL) was relevant for gas exchange; the alveolar ventilation decreased from 3 L/min. to 1.5 L/min., which substantially compromised gas exchange. Furthermore, due to insufficient washout by the low O2 flow to the face mask [8], our patient rebreathed a toxic amount of CO2. Our patient was unable to increase his low tidal volume, and an increase in the respiratory rate was insufficient, given the considerable ventilatory dead space.
Jensen et al. examined the effect of different oxygen flow rates on ventilation parameters and gas exchange in ten healthy volunteers wearing a simple oronasal face mask (Hudson®) without a reservoir bag. Lowering the oxygen supply from 5 to 0 L/min. led to an increase in minute ventilation from 4.8 to 7.5 L/min. due to an increase in tidal volume from 380 to 540 mL with almost no change in respiratory rate. The PaO2 decreased from 38 to 13 kPa, the SaO2 from 100% to 96%, while the PaCO2 remained unchanged. The authors inferred from their results that CO2 retention will occur in patients with COPD wearing a simple oronasal face mask with an O2 supply set to < 5L/minute, because these patients cannot increase their tidal volumes [8].
In severely hypoxemic patients with COPD, O2 may be delivered using a non-rebreathing mask with a target O2 flow rate of 10–15 L/min. Arterial blood gases must be analyzed regularly. Approximately 13% of patients with COPD admitted with an exacerbation of their disease will develop CO2 retention during controlled O2 therapy [2]. In this case, it is important not to stop the O2 flow completely because of the risk of rebound hypoxemia [9]. For a target SaO2 of 88–92%, an O2 supply via nasal cannula with an O2 flow rate of 1–2 L/minute is usually sufficient [5]. If not, either noninvasive or mechanical ventilation must be considered [6]. Paramedics may safely use low-inspired O2 flow (≤4 L/min.) by nasal cannula initially in patients with COPD exacerbations [10].
Conclusions
Non-rebreathing masks with O2 reservoir bags must be fed with an O2 flow exceeding the patient’s minute ventilation (>6–10 L/min.). If not, the amount of oxygen delivered will be too small to effectively increase the arterial O2 saturation. Moreover, the risk of CO2 rebreathing dramatically increases if the flow of oxygen to a non-rebreathing mask is lower than the minute ventilation, especially in patients with COPD and low tidal volumes. As a consequence, CO2 narcosis may develop. Non-rebreathing masks (with O2 reservoir bags) must be used cautiously by experienced medical staff and correctly with an appropriately high O2 flow of 10–15 L/min. [5, 11]. Nevertheless, arterial blood gases must be analyzed regularly for early detection of a rise in PaCO2 in patients with COPD and a hypoxic ventilatory drive. These patients are more safely managed using a nasal cannula with an O2 flow of 1–2L/min. or a simple face mask with an O2 flow of 5L/min. [5].
Abbreviations
- BMI:
-
Body mass index
- CO2 :
-
Carbon dioxide
- COPD:
-
Chronic obstructive pulmonary disease
- FEV1 :
-
Forced expiratory volume in 1 second
- GOLD:
-
Global Initiative for Chronic Obstructive Lung Disease
- O2 :
-
Oxygen
- PaCO2 :
-
Partial pressure of carbon dioxide in arterial blood
- PaO2 :
-
Partial pressure of oxygen in arterial blood
- SaO2 :
-
Percentage of oxygen saturation of arterial blood
- SpO2 :
-
Percentage of oxygen saturation of arterial blood, measured by pulse oximetry
References
Agusti AG, Vogelmeier C. Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2017. Available from: http://goldcopd.org. Accessed 27 Mar 2017.
Moloney ED, Kiely JL, McNicholas WT. Controlled oxygen therapy and carbon dioxide retention during exacerbations of chronic obstructive pulmonary disease. Lancet. 2001;357:526–8.
O’Driscoll R. Emergency oxygen use. BMJ. 2012;345:e6856.
Austin MA, Wills KE, Blizzard L, Walters EH, Wood-Baker R. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010;341:c5462.
O'Driscoll BR, Howard LS, Davison AG, British Thoracic S. BTS guideline for emergency oxygen use in adult patients. Thorax. 2008;63 Suppl 6:vi1–68.
Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, Simonneau G, Benito S, Gasparetto A, Lemaire F, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817–22.
Loveridge B, West P, Kryger MH, Anthonisen NR. Alteration in breathing pattern with progression of chronic obstructive pulmonary disease. Am Rev Respir Dis. 1986;134:930–4.
Jensen AG, Johnson A, Sandstedt S. Rebreathing during oxygen treatment with face mask - the effect of oxygen flow-rates on ventilation. Acta Anaesthesiol Scand. 1991;35:289–92.
Kane B, Turkington PM, Howard LS, Davison AG, Gibson GJ, O'Driscoll BR. Rebound hypoxaemia after administration of oxygen in an acute exacerbation of chronic obstructive pulmonary disease. BMJ. 2011;342:d1557.
Durrington HJ, Flubacher M, Ramsay CF, Howard LS, Harrison BD. Initial oxygen management in patients with an exacerbation of chronic obstructive pulmonary disease. QJM. 2005;98:499–504.
Kallstrom TJ, American Association for Respiratory C. AARC Clinical Practice Guideline: oxygen therapy for adults in the acute care facility--2002 revision & update. Respir Care. 2002;47:717–20.
Acknowledgements
Not applicable.
Funding
Article processing charges were defrayed by the Forschungsfonds of the Spital Limmattal.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
TH drafted the manuscript and created the figures. EA performed the bronchoscopy and had an important role in writing and reviewing the manuscript. T Hegi was in charge of the patient during his stay in the intensive care unit. He had a role in writing and reviewing the manuscript. AR critically reviewed the manuscript. MS conceived of the case report, and assisted in drafting the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Authors’ information
MS is President of the Verein Komplikationenliste. Under the auspices of the Swiss Society of General Internal Medicine the Verein Komplikationenliste uses a program for the anonymous, systematic and prospective registration of iatrogenic complications of medical interventions in hospitalized patients in Switzerland. The patient reported was part of this registry.
Ethics approval and consent to participate
Involvement of the ethics committee of the Canton of Zurich was not considered necessary, since the writing of a case report was not based on a study protocol, and is not classified as research by the Swiss Federal Act on Research on Human Beings.
Consent for publication
Written informed consent was obtained from the patient’s next-of-kin for publication of this case report and all accompanying images. A copy of the written consent is available for review by the editor of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Herren, T., Achermann, E., Hegi, T. et al. Carbon dioxide narcosis due to inappropriate oxygen delivery: a case report. J Med Case Reports 11, 204 (2017). https://doi.org/10.1186/s13256-017-1363-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-017-1363-7