Abstract
Our laboratory recently characterized a novel autism spectrum disorder (ASD)-associated de novo missense mutation in the human dopamine transporter (hDAT) gene SLC6A3 (hDAT T356M). This hDAT variant exhibits dysfunctional forward and reverse transport properties that may contribute to DA dysfunction in ASD. Here, we report that Zn2+ reverses, at least in part, the functional deficits of ASD-associated hDAT variant T356M. These data suggest that the molecular mechanism targeted by Zn2+ to restore partial function in hDAT T356M may be a novel therapeutic target to rescue functional deficits in hDAT variants associated with ASD.
Similar content being viewed by others
Findings
Introduction
The dopamine (DA) transporter (DAT) tunes DA neurotransmission by active re-uptake of DA from the synapse [1]. Our laboratory has recently characterized the first de novo mutation in the human dopamine transporter (hDAT) reported in a patient diagnosed with autism spectrum disorder (ASD), which results in a Thr to Met substitution at site 356 (hDAT T356M). We reported novel and profound functional abnormalities associated with the hDAT de novo mutation T356M, resulting in enhancement of non-vesicular, DAT-dependent DA release, referred to as anomalous DA efflux. Our data raise the possibility that anomalous DA efflux (or other disturbances in DA neurotransmission) may represent a complication relevant for behavioral abnormalities in ASD. Extracellular zinc (Zn2+) inhibits DA uptake [2]. Three amino acid side chains have been identified in DAT which coordinate zinc: H193 in extracellular loop 2 (EL2), H375 in the first helical part of extracellular loop 4 (EL4A), and E396 in the second helix of extracellular loop 4 (EL4B) [2,3]. Structural data from the DAT-homolog LeuT in the inward- and outward-facing conformation suggest that the relative orientation of H375 and E396 shifts during the transport cycle [4].
hDAT T356M displays decreased forward and reverse-transport function compared with wild-type hDAT [5]. The reduced transport capacity of the mutant was not associated with a reduction in hDAT surface expression. Amphetamine (AMPH) is a psychostimulant that targets the hDAT causing reverse transport of DA (DA efflux) [6]. hDAT T356M exhibits impaired AMPH-induced DA efflux. Here, we show that the presence of Zn2+ partially rescues both DA uptake and the AMPH-induced DA efflux impairments of hDAT T356M. Zn2+ diminishes the anomalous DA efflux property of the hDAT T356M, which might account for its ability to partially rescue transporter functions. Rescue of hDAT function by Zn2+ might reveal a new molecular mechanism to target for pharmacological intervention in patients with ASD.
Results
Zn2+ enhances [3H]DA uptake in hDAT T356M
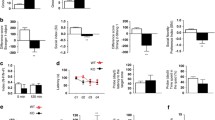
CHO cells were transiently transfected with either wild-type hDAT or hDAT T356M. Cells were incubated with 50 nM [3H]DA at 37 °C for 5 min in the presence of varying concentrations of Zn2+. Consistent with previous reports [2,3], Zn2+ decreases the DA uptake rate for wild-type hDAT (Figure 1A, filled squares). In contrast, for hDAT T356M cells, Zn2+ increases DA uptake, partially reversing the functional deficit of this variant (Figure 1A, open circles).
Zn 2+ partially reverses the hDAT T356M deficits in [ 3 H]DA uptake and amphetamine (AMPH)-mediated efflux. Methods were as previously described in Hamilton et al. [5]. (A) [3H]DA uptake counts (cpm) are plotted for hDAT and hDAT T356M over a range of Zn2+ concentrations. While Zn2+ inhibits hDAT [3H]DA uptake, Zn2+ instead increases hDAT T356M [3H]DA uptake (*p < 0.05 by one-way ANOVA followed by Dunnett’s test compared to 0 Zn2+ control; n = 4). (B) (Top) Representative AMPH-induced amperometric currents (reflecting DA efflux) are displayed in the presence or absence of 100 μM Zn2+. Arrows indicate the application of 10 μM AMPH. (Bottom) Maximal DA efflux amperometric current recorded in the presence of Zn2+ normalized to maximal current recorded in the presence of vehicle. (*p < 0.05 by paired Student’s t-test; n = 5). (C) (Top) Representative Zn2+-induced change in amperometric currents are displayed in response to 100 μM Zn2+ or vehicle control. Arrows indicate the application of 100 μM Zn2+. (Bottom) Change in amperometric current recorded in response to 100 μM Zn2+ or vehicle control (*p < 0.05 by paired Student’s t-test; n = 5).
Zn2+ enhances AMPH-induced DA efflux in hDAT T356M
To specifically measure reverse transport, DA was loaded into the cytoplasm of hDAT T356M cells by a whole-cell patch clamp pipette held in current-clamp mode. This configuration supplies intracellular DA directly to the cell independent of forward transport by hDAT and allows the cell to control its membrane voltage. The whole-cell patch pipette was filled with an internal solution containing 2 mM DA as described previously [5]. DA efflux in response to 10 μM AMPH was measured by amperometry in the presence of 100 μM Zn2+ or vehicle control [5]. Representative amperometric traces are shown in Figure 1B (top). The peak of DA efflux normalized to vehicle control is shown in Figure 1B (bottom). Zn2+ increases AMPH-induced DA efflux in hDAT T356M compared to vehicle control (Figure 1B), indicating an enhancement of reverse transport DA in the presence of Zn2+. For comparison, Zn2+ partially rescues AMPH-induced DA efflux for hDAT T356M to a level of 48% ± 12% of wild-type hDAT AMPH-induced DA efflux [5].
Zn2+ decreases baseline anomalous DA efflux in hDAT T356M
Using the whole-cell patch clamp technique coupled to amperometry, the effect of 100 μM Zn2+ on the baseline (anomalous) DA efflux of hDAT T356M was studied. The whole-cell patch pipette delivered intracellular DA into the cell in current-clamp mode. To determine the effect of Zn2+ on baseline DA efflux, the change in amperometric current was compared following the application of 100 μM Zn2+ or vehicle control. Baseline DA efflux decreased significantly in the presence of Zn2+ in comparison with that of vehicle, indicating that Zn2+ inhibits the constitutive DA efflux by hDAT T356M. Representative amperometric traces are shown in Figure 1C (top), and mean values are plotted in Figure 1C (bottom).
Discussion
Here, we explore the potential for Zn2+ in rescuing the biophysical abnormalities in the hDAT variant T356M, which we recently reported in Hamilton et al. [5]. These functional deficits in hDAT T356M may contribute to the dysfunction in DA neurotransmission associated with ASD. Zn2+ was previously shown to partially restore DA uptake function in DAT mutant Y335A, which exhibits low uptake under basal conditions [7]. Here, we demonstrate that Zn2+ reverses deficits in both forward and reverse transport in the T356M variant. Additionally, Zn2+ decreases baseline anomalous DA efflux of the hDAT T356M de novo mutation, possibly providing an explanation for the positive effects of Zn2+ on the uptake and efflux properties of this mutant transporter. This is a novel and intriguing finding in terms of ameliorating irregularities in DAT function in a de novo ASD-associated mutation.
T356 is located in transmembrane domain 7, and the hDAT Zn2+ binding site spans the spatial micro-environment between the transmembrane helices 7 and 8 and extracellular loop 2 (EL2) [8,9]. Binding of Zn2+ to DAT alters the conformational equilibrium between the inward- and outward-facing state of the DAT [8,9]. However, mutation of an intracellular tyrosine to alanine (Y335A) converts the inhibitory Zn2+ switch into an activating Zn2+ switch, whereby Zn2+ rescues functions of the Y335A mutant transporter [7,8]. Therefore, the functional impact of Zn2+ binding to mutant transporters can be different than for wild-type hDAT as we observe here for T356M (Figure 1A). Whereas Zn2+ has been suggested to promote the outward facing conformation of wild-type hDAT, the structural effect of Zn2+ binding to hDAT T356M is unknown and remains an interesting topic for future investigation.
Clinical data have previously established that mean serum Zn2+ levels are significantly lower in children diagnosed with ASD compared to unaffected children and that there exist disturbances in Zn2+ metabolism in patients diagnosed with ASD [10-12]. hDAT T356M is the first de novo DAT mutation found in a patient with ASD, and hDAT T356M functional deficits can partially be rescued by Zn2+. Whether or not Zn2+ regulation of hDAT may be directly relevant for the etiology of ASD is presently unknown. However, our work suggests that the molecular mechanism engaged by Zn2+ to partially restore function in hDAT T356M may be a novel therapeutic target to rescue, at least in part, functional deficits in hDAT variants associated with ASD.
Abbreviations
- DAT:
-
Dopamine transporter
- hDAT:
-
Human dopamine transporter
- AMPH:
-
Amphetamine
- DA:
-
Dopamine
- ASD:
-
Autism spectrum disorder
- CHO:
-
Chinese hamster ovary
References
Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, et al. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev. 2011;63(3):585–640. doi:10.1124/pr.108.000869.
Norregaard L, Frederiksen D, Nielsen EO, Gether U. Delineation of an endogenous zinc-binding site in the human dopamine transporter. EMBO J. 1998;17(15):4266–73.
Loland CJ, Norregaard L, Gether U. Defining proximity relationships in the tertiary structure of the dopamine transporter. Identification of a conserved glutamic acid as a third coordinate in the endogenous Zn(2+)-binding site. J Biol Chem. 1999;274(52):36928–34.
Krishnamurthy H, Gouaux E. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature. 2012;481(7382):469–74. doi:10.1038/nature10737.
Hamilton PJ, Campbell NG, Sharma S, Erreger K, Herborg Hansen F, Saunders C, et al. De novo mutation in the dopamine transporter gene associates dopamine dysfunction with autism spectrum disorder. Mol Psychiatry. 2013;18(12):1315–23. doi:10.1038/mp.2013.102.
Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75(6):406–33.
Kahlig KM, Lute BJ, Wei Y, Loland CJ, Gether U, Javitch JA, et al. Regulation of dopamine transporter trafficking by intracellular amphetamine. Mol Pharmacol. 2006;70(2):542–8. doi:10.1124/mol.106.023952.
Loland CJ, Norregaard L, Litman T, Gether U. Generation of an activating Zn(2+) switch in the dopamine transporter: mutation of an intracellular tyrosine constitutively alters the conformational equilibrium of the transport cycle. Proc Natl Acad Sci U S A. 2002;99(3):1683–8.
Stockner T, Montgomery TR, Kudlacek O, Weissensteiner R, Ecker GF, Freissmuth M, et al. Mutational analysis of the high-affinity zinc binding site validates a refined human dopamine transporter homology model. PLoS Comput Biol. 2013;9(2):e1002909. doi:10.1371/journal.pcbi.1002909.
Faber S, Zinn GM, Kern 2nd JC, Kingston HM. The plasma zinc/serum copper ratio as a biomarker in children with autism spectrum disorders. Biomarkers. 2009;14(3):171–80. doi:10.1080/13547500902783747.
Jackson MJ, Garrod PJ. Plasma zinc, copper, and amino acid levels in the blood of autistic children. J Autism Child Schizophr. 1978;8(2):203–8.
Li SO, Wang JL, Bjorklund G, Zhao WN, Yin CH. Serum copper and zinc levels in individuals with autism spectrum disorders. Neuroreport. 2014;25(15):1216–20. doi:10.1097/WNR.0000000000000251.
Acknowledgements
This work was supported by NSF Graduate Research Fellowship DGE0909667, NIH F31 DA 035535-01 (PJH), T32 NS007491 (NGC), DA035263 (AG), and Vanderbilt International Scholar Program (AS).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
PJH, AS, ANB, NGC, JSS, AG, HJGM, and KE designed the research. PJH, AS, ANB, and NBC performed the research. PJH, AS, ANB, NBC, NGC, JSS, AG, HJGM, and KE wrote the paper. All authors read and approved the manuscript.
Peter J Hamilton, Aparna Shekar, Heinrich JG Matthies and Kevin Erreger contributed equally to this work.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hamilton, P.J., Shekar, A., Belovich, A.N. et al. Zn2+ reverses functional deficits in a de novo dopamine transporter variant associated with autism spectrum disorder. Molecular Autism 6, 8 (2015). https://doi.org/10.1186/s13229-015-0002-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13229-015-0002-7