Abstract
Background
Allergen inhalation tests are a valuable research tool. The allergen dose producing an early asthmatic response (EAR) can be predicted from methacholine responsiveness and allergen skin test endpoint (STE). The Wright® jet nebulizer, which is both inefficient and increasingly difficult to obtain, has been used historically. We assessed the Solo® vibrating mesh nebulizer as an alternative for allergen and methacholine challenges.
Methods
Eighteen mild atopic asthmatics completed the study. Doubling concentration allergen prick skin tests were performed to determine the STE in allergen units/mL. The Wright® protocol was used to measure the methacholine provocation dose causing a 20% forced expired volume in one second (FEV1) fall (PD20) (μg) and the allergen PD20 (units). The Solo® protocol (0.5 mL nebulized to completion, tidal breathing inhalation) was used to determine both methacholine PD20 and allergen PD20. The nebulizer order was randomized and separated by ≥ 2 weeks.
Results
All data were log transformed. The allergen PD20, predicted from the methacholine PD20 and the STE, was within 2 doubling doses of the PD20 measured with the Wright® and 2.64 doubling doses of that measured with Solo®. The Wright® allergen PD20 correlated with the Wright® methacholine PD20 (r = 0.74) and the STE (r = 0.78) and more strongly with the product of the two (Wright® methacholine PD20 × STE, r = 0.91, p < 0.00001). The Solo® allergen PD20 showed similar relationships with the Solo® methacholine PD20 (r = 0.61), the STE (r = 0.75) and the product of the two (Solo® methacholine PD20 × STE, r = 0.83, p < 0.00002). The Wright® and the Solo® methacholine geometric mean PD20s were not significantly different (49.3 and 54.5 μg respectively, p = 0.62). The Wright® allergen PD20 was slightly but significantly lower than the Solo® allergen PD20 (geometric means 6.7 and 10.5 units respectively, p = 0.003).
Conclusion
The Solo® allergen PD20 showed the same relationship with methacholine responsiveness and STE as did the Wright®. The Solo® allergen PD20 was slightly but significantly higher than the Wright® allergen PD20. The Solo® vibrating mesh nebulizer was well tolerated and is an acceptable alternative for allergen challenge.
Trial registration clinicaltrials.gov: NCT03491358
Similar content being viewed by others
Background
Allergen inhalation challenge is a valuable research tool for the study of asthma pathophysiology and investigational new drug efficacy [1]. The early asthmatic response (EAR) to allergen depends on (non-allergic) airway responsiveness and the level of allergen-specific IgE [2]. It has been previously demonstrated that the concentration/dose of allergen required to produce a threshold EAR of a 20% decline in forced expired volume in one second (FEV1) can be predicted within 2–3 concentrations using the level of airway responsiveness measured by methacholine or histamine provocation and the level of allergen specific IgE assessed by the allergen skin test endpoint titration (STE) [3]. A caveat for this prediction is the requirement for methacholine and allergen to be inhaled in the same fashion using the same type of nebulizer, calibrated to the same weight loss.
Historically, the Wright® jet nebulizer (Roxon Medi-Tech, St. Leonard, QC) calibrated to run at a weight loss of 0.13 g/min with inhalation performed by two minutes of tidal breathing [4] has been used for both methacholine and allergen inhalation. The Wright® nebulizer is inefficient (approximately 75% of weight loss is evaporation [5, 6]), expensive, non-disposable, and increasingly difficult to acquire. The Aerogen Solo® vibrating mesh nebulizer, referred to as the Solo® throughout, (Aerogen Ltd, Galway Ireland) features no evaporation and has been validated for use in methacholine challenge testing [7, 8]. The current study was designed to assess the Solo® vibrating mesh nebulizer for use in the standardized allergen challenge protocol performed in AllerGen National Centres of Excellence (NCE) Clinical Investigator Collaborative (CIC) studies and to compare it to the current Wright® jet nebulizer protocol.
Methods
Participants
Eligible participants had mild atopic asthma requiring only infrequent inhaled β2 agonist, an FEV1 > 70% predicted, and a methacholine provocation dose causing a 20% fall in FEV1 (PD20) ≤ 400 μg. Participants were non-smokers with < 10 pack year cumulative smoking history. Individuals who were pregnant, lactating, who had relevant allergen exposure or respiratory tract infection within the previous 4 weeks or who had significant medical conditions were excluded. Ethics approval was received from each study site and signed informed consent was obtained prior to study entry.
Study design
Participants attended the laboratory on 5 occasions. Visit 1 visit was to assess eligibility, to obtain signed consent, to perform baseline spirometry and screening allergen skin prick tests and from these select the best allergen for inhalation testing. The selected allergen was one which was clinically relevant to the participant and which produced a large (≥ 5 mm) wheal. The STE for the selected allergen was measured at Visit 2 to allow prediction of the starting allergen concentration for inhalation in conjunction with the methacholine response [3]. At Visits 2 and 3 the methacholine PD20 and the allergen PD20 were measured respectively both with either the Wright® or the Solo® nebulizer. After a minimum 2-week washout, at Visits 4 and 5 these challenges were repeated with the other nebulizer. The order of the nebulizers was randomized.
Skin test endpoint (STE) titration
The STE was determined as previously outlined [9]. Allergens (Omega Laboratories, Montreal QC) were dispensed in protein nitrogen units/mL, biologic allergen units/mL or allergen units/mL. For conformity the allergen dose was expressed in “units”. Allergens were diluted two-fold from 1:8 to 1:1024 or beyond if required and the dilutions were labeled as the allergen concentration in units/mL. Duplicate skin prick tests were performed, the mean wheal diameter measured at 10 min, and the STE recorded as the weakest concentration (units/mL) causing a 2 mm mean wheal diameter.
Methacholine inhalation tests
The standard Wright® nebulizer methacholine test [4, 10] was done as follows. The nebulizer was calibrated to a weight loss of 0.13 g/min. Complete spirometry was initially measured in triplicate. Isotonic saline was then inhaled by tidal breathing for 2 min, and the FEV1 (truncated manoeuvre to avoid fatigue) measured at 30 and 90 s. Doubling concentrations of methacholine were then inhaled in the same manner at 5 min intervals with FEV1 repeated at 30 and 90 s The available concentrations ranged from 0.031 to 64 mg/mL; the starting concentration for an individual was selected based on previous testing if available or based on validated guidelines [10, 11]. Inhalations were stopped when the FEV1 had fallen ≥ 17% and the provocation concentration causing a 20% FEV1 fall (PC20) was interpolated from the last 2 data points [10] or extrapolated from the last data point [12]. The PC20 was converted to a PD20 (μg) based on several studies documenting that a Wright® PC20 of 16 mg/mL equates to a PD20 of 400 μg [6,7,8, 13, 14]. The accepted values for a normal (negative) methacholine challenge test are PC20 and PD20 > 16 mg/mL and > 400 μg respectively [15].
The Solo® methacholine challenge was done as previously described [8]. Doubling doses of methacholine were delivered by nebulizing 0.5 mL of methacholine to completion and inhaling by tidal breathing; this requires 90 to 180 s [8]. A concentration of 2 mg/mL × 0.5 mL × 0.4 (respiratory duty cycle [16]) exposes the individual to 400 μg. Following saline inhalation appropriate doubling concentrations up to 4 mg/mL (= 800 μg) were used. The remainder of the challenge (timing of FEV1 measurements, time between doses, calculation of the PD20, etc.) was identical to the Wright® method.
Allergen inhalation tests
Allergen inhalation tests were done as previously described using the Wright® nebulizer [17]. Spirometry was measured in triplicate. Doubling concentrations of allergen were then inhaled (2 min of tidal breathing, nebulizer calibrated to a weight loss of 0.13 g/min and starting 3 or 4 concentrations below the predicted EAR concentration [3]) at 12 min intervals until the FEV1 measured at 10 min after inhalation had fallen ≥ 15%. At an FEV1 fall between 15 and 20% the FEV1 was repeated 10 min later before giving another concentration if required. The allergen PC20 (units/mL) was converted to allergen PD20 (units) assuming a similar relationship as seen with methacholine. After PD20 measurement, participants received a single inhaled dose of salbutamol 200 μg to reverse bronchoconstriction and a single inhaled dose of fluticasone propionate 500 μg to prevent development of the late asthmatic response [18].
The Solo® allergen challenge was performed in an analogous manner. Assuming a similar relationship for dose comparison between the Solo® and the Wright® nebulizers seen with methacholine, the starting allergen concentration was 3 doubling concentrations below the starting concentration used for the Wright® (i.e. 6–7 concentrations below the Wright® prediction). The allergen, 0.5 mL, was nebulized to completion and inhaled by tidal breathing; the remainder of the challenge protocol was identical to the Wright® protocol; the result was expressed as the allergen PD20 in units.
Analysis
Statistics were done using a computerized statistics programme (Statistix 9 (Analytical Software, Tallahassee, FL, USA). PD20 and STE values were log transformed prior to analysis. The Student’s paired t test was used for comparison of means. Linear regression analysis was used for the following (all values logged):
Measured Wright® allergen PD20 vs Predicted allergen PD20.
Wright® allergen PD20 vs Wright® methacholine PD20.
Wright® allergen PD20 vs STE.
Wright® allergen PD20 vs (Wright® methacholine PD20 × STE).
Measured Solo® allergen PD20 vs Predicted allergen PD20.
Solo® allergen PD20 vs Solo® methacholine PD20.
Solo® allergen PD20 vs STE.
Solo® allergen PD20 vs (Solo® methacholine PD20 × STE).
Results
Eighteen participants, all poylsensitized but with no current allergen exposure (except house dust mite), completed the study without adverse events. Three additional enrolled participants did not complete the study; one because the FEV1 was < 70% at Visit 1, one because the methacholine PD20 was > 400 μg at Visit 2, and one because of a failure to respond to allergen (1:32 with the Solo® equating to ~ 1:4 with the Wright®) at Visit 3. Anthropometric data, baseline FEV1, baseline methacholine PD20 and allergen used for challenges are shown in Table 1.
Wright®
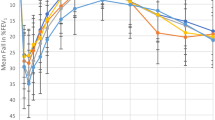
The measured allergen PD20 correlated with the predicted allergen PD20 (r = 0.91, p < 0.00001) and all predictions were within 2 (maximum 1.96) doubling doses of the measured allergen PD20 (Fig. 1). The geometric means for the measured and predicted PD20 values were 6.7 units (95% CI 2.7–15.8) and 7.0 units (95% CI 2.5–17.7) respectively (p = 0.68). The allergen PD20 (units) correlated with both the methacholine PD20 (r = 0.74) and the STE (r = 0.78,). Allergen PD20 correlated more strongly with the product of the methacholine PD20 (μg) and the STE (units/mL) (r = 0.91, p < 0.00001) (Fig. 2).
Solo®
The measured Solo® allergen PD20 correlated with the predicted allergen PD20 (r = 0.84, p = 0.000013) and was within 2 doubling does of the predicted allergen PD20 in 14 of 18 and within 2.64 doubling doses in all 18 (Fig. 3). Similar to the Wright®, the Solo® allergen PD20 correlated with both the Solo® methacholine PD20 (r = 0.61) and the STE (r = 0.75) and more strongly with the product of the 2 (r = 0.83, p = 0.00002) (Fig. 4).
Wright® Solo® comparisons
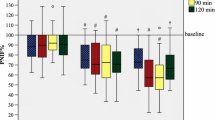
The Wright® and the Solo® methacholine PD20s were not significantly different with geometric means of 49.3 (95% CI 25.8–94.0) and 54.2 μg (95% CI 26.7–110) respectively (p = 0.62). The geometric mean Wright® allergen PD20, 6.7 units (95% CI 2.7–15.8), was slightly but significantly lower than geometric mean Solo® allergen PD20, 10.5 units (95% CI 4.4–25.1), (p = 0.003). Individual values for the Wright® and the Solo® allergen PD20s are shown in Fig. 5. There was no sequence effect (i.e. nebulizer order) nor was there any difference between the three sites.
Discussion
These data indicate that the Solo® vibrating mesh nebulizer can be successfully used for performance of allergen inhalation tests. Allergen responsiveness showed the same relationship with methacholine responsiveness and level of allergen sensitivity as was seen with the Wright® nebulizer protocol. The measured Solo® allergen PD20 was within 2.64 doubling doses of the prediction.
In 1987, an equation was developed to predict the dilution/concentration of allergen that would produce a 20% EAR [3]. This was based on the histamine PC20 (mg/mL) and the allergen skin test endpoint (dilution) producing a 2 mm wheal. Both allergen and histamine were inhaled with 2 min of tidal breathing from a Wright® nebulizer calibrated in the same manner. Methacholine was subsequently substituted for histamine in the same concentration since histamine and methacholine PC20s are identical in asthmatics [19]. In the original study, the equation successfully estimated the dilution required for an EAR within 2 doubling dilutions in 92% and 3 doubling dilutions in 100% of challenges [3]. The equation and a sample calculation are below:
For example with a methacholine PC20 of 2.2 mg/mL and an STE of 1/1024.
This prediction is routinely used as a guide for allergen challenge tests. A starting allergen dilution of 3 or occasionally 4 doubling dilutions below the prediction has proved a safe and effective method for allergen challenges performed in AllerGen NCE CIC and other studies. The purpose is to allow some test shortening when compared to methods advocating starting with the weakest allergen dilution causing a 2 mm wheal skin test response [20]. The current study validates this prediction equation when applied to the Wright® data, since all measured values were within 2 doubling doses of the prediction. Despite the slightly higher measured Solo® allergen PD20 vs the Wright® (i.e. slightly less responsive), 78% of values were within 2 doubling doses and 100% within 2.64 doubling doses. This would suggest that the 1987 prediction equation can be safely and effectively used (with modification for the units and nebulizer differences) until such time as there are enough data to develop a “Solo® specific” equation.
The major strength of this study is the experienced group of investigators at the three sites. The one weakness is the inability to assess the solute output of the jet (Wright®) nebulizer. Based on the known evaporative features [5, 6], and both breath simulation testing [6] and clinical challenge testing [7, 8, 12, 13] it is reasonable to equate a methacholine PC20 of 16 mg/mL to a methacholine PD20 of 400 μg. The current study validates this, since using this conversion the Wright® and Solo® methacholine PD20s were essentially identical. However, data are currently lacking for nebulized allergen and it is possible that allergen solutions could be handled differently by the nebulizers.
Conclusion
In summary the Solo® vibrating mesh nebulizer, proved to be a safe, effective and well tolerated device for administering inhaled allergen. This provides a valuable alternative to the Wright® jet nebulizer.
Availability of data and materials
All data are available from the corresponding author on reasonable request don.cockcroft@usask.ca.
Abbreviations
- STE:
-
skin (prick) test endpoint
- FEV1 :
-
forced expired volume in one second
- PC20 :
-
provocation concentration causing a 20% FEV1 fall
- PD20 :
-
provocation dose causing a 20% FEV1 fall
- EAR:
-
early asthmatic response
- SD:
-
standard deviation
- CI:
-
confidence interval
- NCE:
-
National Centres of Excellence
- CIC:
-
Clinical Investigator Collaborative
References
Boulet LP, Gauvreau G, Boulay ME, O’Byrne P, Cockcroft DW, Clinical Investigative Collaboration, Canadian Network of Centers of Excellence AllerGen. The allergen bronchoprovocation model: an important tool for the investigation of new asthma anti-inflammatory therapies. Allergy. 2007;62(10):1101–10.
Cockcroft DW, Ruffin RE, Frith PA, Cartier A, Juniper EF, Dolovich J, et al. Determinants of allergen-induced asthma: dose of allergen, circulating IgE antibody concentration, and bronchial responsiveness to histamine. Am Rev Respir Dis. 1979;120(5):1053–8.
Cockcroft DW, Murdock KY, Kirby J, Hargreave FE. Prediction of airway responsiveness to allergen from skin sensitivity to allergen and airway responsiveness to histamine. Am Rev Respir Dis. 1987;135(1):264–7.
Cockcroft DW, Killian DN, Mellon JJA, Hargreave FE. Bronchial reactivity to inhaled histamine: a method and clinical survey. Clin Allergy. 1977;7(3):235–43.
Cockcroft DW, Hurst TS, Gore BP. Importance of evaporative water losses during standardized nebulized inhalation provocation tests. Chest. 1989;96(3):505–8.
Coates AL, Leung K, Dell SD. Developing alternative delivery systems for methacholine challenge tests. J Aerosol Med Pulm Drug Deliv. 2014;27(1):66–70.
Blais CM, Cockcroft DW, Veilleux J, Boulay ME, Boulet LP, Gauvreau GM, et al. Methacholine challenge: comparison of airway responsiveness produced by a vibrating mesh nebulizer versus a jet nebulizer. J Aerosol Med Pulm Drug Deliv. 2018;31(2):88–93.
Davis BE, Simonson SK, Blais CM, Cockcroft DW. Methacholine challenge testing: a novel method for measuring PD20. Chest. 2017;152(6):1251–7.
Blais CM, Davis BE, Cockcroft DW. Within-tester repeatability and between-tester reproducibility of skin test endpoint titration: a quality assurance study. Ann Allergy Asthma Immunol. 2019;122(2):220–2.
Juniper EF, Cockcroft DW, Hargreave FE. Histamine and methacholine inhalation tests: tidal breathing method—laboratory procedure and standardisation. 2nd ed. Sweden: AB Draco, Lund; 1994.
Cockcroft DW, Marciniuk DD, Hurst TS, Cotton DJ, Laframboise KF, McNab BD, et al. Methacholine challenge: test-shortening procedures. Chest. 2001;120(6):1857–60.
Cockcroft DW, Davis BE. Methacholine PC20: 1-point formula. Ann Allergy Asthma Immunol. 2007;98(5):498–9.
Dell SD, Bola SS, Foty RG, Marshall LC, Nelligan KA, Coates AL. Provocative dose of methacholine causing a 20% drop in FEV1 should be used to interpret methacholine challenge tests with modern nebulizers. Ann Am Thorac Soc. 2015;12(3):357–63.
El-Gammal AI, Killian KJ, Scime TX, Beaudin S, Schlatman A, Cockcroft DW, et al. Comparison of the provocative concentration of methacholine causing a 20% fall in FEV1 between the AeroEclipse II breath-actuated nebulizer and the wright nebulizer in adult subjects with asthma. Ann Am Thorac Soc. 2015;12(7):1039–43.
Coates AL, Wanger J, Cockcroft DW, Culver BH, Bronchoprovocation Testing Task Force. ERS technical standard on bronchial challenge testing: general considerations and performance of methacholine challenge tests. Eur Respir J. 2017;49(5):1601526. https://doi.org/10.1183/13993003.01526-2016.
Blais CM, Davis BE, Graham BL, Cockcroft DW. Respiratory duty cycles in individuals with and without airway hyperresponsiveness. Chest. 2019. https://doi.org/10.1016/j.chest.2019.09.005.
Davis BE, Todd DC, Cockcroft DW. Effect of combined montelukast and desloratadine on the early asthmatic response to inhaled allergen. J Allergy Clin Immunol. 2005;116(4):768–72.
Cockcroft DW, McParland CP, O’Byrne PM, Manning P, Friend JL, Rutherford BC, et al. Beclomethasone given after the early asthmatic response inhibits the late response and the increased methacholine responsiveness and cromolyn does not. J Allergy Clin Immunol. 1993;91(6):1163–8.
Juniper EF, Frith PA, Dunnett C, Cockcroft DW, Hargreave FE. Reproducibility and comparison of responses to inhaled histamine and methacholine. Thorax. 1978;33:705–10.
Killian D, Cockcroft DW, Hargreave FE, Dolovich J. Factors in allergen-induced asthma: relevance of the intensity of the airways allergic reaction and non-specific bronchial reactivity. Clin Allergy. 1976;6(3):219–25.
Acknowledgements
The authors thank Jacquie Bramley for assistance in preparation of the manuscript and Aerogen Ltd for supplying the Solo® nebulizers.
Funding
This study was funded by a grant form AllerGen NCE. AllerGen had no role in study design, data collection analysis or interpretation, or in the writing of the manuscript. CMB received a summer student support grant from the CSACI
Author information
Authors and Affiliations
Contributions
Study design and protocol development: BED, CMB, DWC, GMG, PMO, LPB; Data collection: BED, CMB, DWC, LPB, MEB, GMG, HV, KJH, CDO; data analysis: DWC, CMB, BED; Manuscript preparation: DWC, BED, CMB; manuscript approval. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was obtained from each site and signed informed consent was obtained from each participant.
Consent for publication
We consent to publication of this paper.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Cockcroft, D.W., Davis, B.E., Blais, C.M. et al. Use of a vibrating mesh nebulizer for allergen challenge. Allergy Asthma Clin Immunol 15, 73 (2019). https://doi.org/10.1186/s13223-019-0392-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13223-019-0392-8