Abstract
Background
Reduced left ventricular ejection fraction (LVEF) ≤30% is the most powerful prognostic indicator for sudden cardiac death (SCD) in patients after myocardial infarction (MI), but there are little data about long-term changes of LVEF after revascularization and the following implantation of a cardioverter defibrillator (ICD).
Methods
We performed a retrospective analysis of 277 patients with reduced LVEF at least 1 month after MI and complete revascularization. Patients (median time post-MI 23.4 months; 74.3% after PCI, 25.7% after CABG were assigned either to group 1 (LVEF <30%) or group 2 (LVEF 30–40%). Biplane echocardiography was redone after a mean follow-up of 441 ± 220 days.
Results
LVEF increased significantly in both two groups (group 1: 26.2 ± 4.8% to 32.4 ± 8.5%; p < 0.001; group 2: 38.2 ± 2.5% to 44.4 ± 9.6%; p < 0.001). However, statistical analysis of first and second LVEF measurement by means of a LOWESS regression and with an appropriate correction of the regression towards the mean effect revealed only a moderate increase of the mean LVEF from 35 to 37% (p < 0.001) with a large interindividual variation.
Conclusions
The impact of early revascularization on LVEF appears to be low in the majority of post-MI heart failure patients. Owing to the high variability, a single measurement may not be reliable enough to justify a decision on ICD indication.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
While not consistently used across studies, the terms sudden cardiac arrest and sudden cardiac death (SCD) describe the unexpected abrupt circulatory arrest, typically owing to sustained ventricular tachycardia/ventricular fibrillation [1, 2]. These events commonly occur in patients with structural heart disease, in particular coronary heart disease (CHD) including myocardial infarction (MI).
Severely depressed left ventricular ejection fraction (LVEF) of less than 30% is the most powerful indicator for life-threatening ventricular tachyarrhythmias and SCD in patients after MI. Implantation of a cardioverter defibrillator (ICD) effectively improves survival in this high-risk population [3,4,5]. Although additional risk factors were also shown to be of predictive value [6], the estimation of SCD risk in the present study is based on the left ventricular ejection fraction. However, how the LVEF following MI changes over time is unknown. Conflicting data have been published concerning the time of ICD implantation [7,8,9,10,11]. It is not certain in how many cases LVEF improves over time and to what extent, especially in patients with stunned or hibernating myocardium [12,13,14]. Serial echocardiography is often used in clinical studies and but also in daily cardiological practice to evaluate left ventricular systolic function after MI [15, 16]. However, the reproducibility of echocardiographically determined LVEF may be poor [17, 18]. LVEF serial measurements are limited by a high interindividual as well as intraindividual variability [19,20,21,22] that is only partially controlled by 3D volumetry and optimized endocardial border detection by contrast echocardiography [23, 24].
Against this background, we systematically studied the LVEF changes in revascularized post-MI heart failure patients with an LVEF of <40% in order to give recommendations for LVEF measurements before ICD implantation to prevent SCD.
Methods
Design
This was a retrospective analysis of the prospective multi-center German PreSCD (Prevention of sudden cardiac death)-II-registry which enrolled 10,530 post-MI patients.
Patients
In the context of the registry, between November 2003 and October 2005, a total of 2046 patients at least 1 month after documented MI (median 1.2 months) were included in the monocentric substudy. All patients were hospitalized for cardiological rehabilitation in the Cardiovascular Rehabilitation Center Rüdersdorf, Germany.
Physical capacity during symptom-limited bicycle exercise ECG as well as LVEF quantified echocardiographically were documented. Out of the 2046 patients, 277 consecutive patients with LVEF ≤40% were selected for further follow-up. Two strata were prospectively defined depending on whether LVEF was <30% or 30–40%, respectively.
LVEF measurements
Biplane LVEF was determined by two-dimensional echocardiographic imaging according to Simpson [22], using the 3S probe of Vivid 7 (Vivid 7, GE Ving Med, Horten, Norway) was used. Means of three biplane planimetric measurements were rounded to multiples of 5%. Follow-up examinations were usually performed by a different physician according to the same protocol. However, follow-up LVEF were not rounded to a multiple of 5% but to integers. Follow-up observations included the complete range of LVEF values and were not restricted to patients with up to 40% LVEF (Fig. 1).
Statistics
The descriptive statistics were means ± standard deviation (SD). Scattergrams were used to visualize change in LVEF. Patients were pre-selected according to baseline LVEF and LVEF values were subject to some measurement error and the extent of biological variation amongst patients was unknown. The changes during follow-up therefore tend to regress towards the mean (RTTM). In the selected population, patients who happened to reveal LVEF values that were lower than their long-term average are over-represented as compared to patients with correct or higher LVEF values. These patients tend to regress to their long-term mean with the result that the average LVEF increase is exaggerated over the unknown real improvement for physiological reasons.
In order to determine the extent of the selection bias, LVEF change was plotted against the time interval between baseline and follow-up determination. A Locally Weighted Regression Scatter Plot Smoothing (LOWESS) regression function was superimposed on the scattergram [25]. The point where this line intercepts with the ordinate can be interpreted as estimate of the selection bias since it can be assumed that with a time interval of zero no change in true LVEF can take place and the change observed is a mixture of measurement error and selection bias only.
Results
The majority of patients were given a combination of ACE inhibitors/angiotensin receptor blockers (ARB) (85.8%), beta blockers (80.5%), statins (63.1%), aldosterone antagonists (23.8%) and diuretics (59.2%). Seven patients (2.5%) were lost to follow-up. Patients had received revascularisation by percutaneous intervention (PCI) in 74.3% and by coronary artery bypass graft surgery (CAGB) in 25.7%. Patients were scheduled for echocardiographic follow-up measurement of LVEF after mean of 441 ± 220 days.
There were 76 patients in group 1 (LVEF <30%) and 201 in group 2 (LVEF 30–40%) Baseline characteristics for the two strata and for the total group are listed in Table 1. In Group 1 80.3% of patients were males (mean age 66.1 ± 11 years), in group 2 84.6% were males (65.4 ± 10 years).
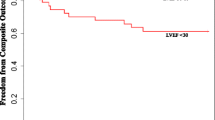
There was a significant increase of left ventricular ejection fraction for the total patient population of 6.2 ± 9.0% (35.3 ± 6.1% in group 1 vs. 41.4 ± 10.7 in group 2; p < 0.001). In both groups, the pattern of change was similar: in group 1, LVEF increased from 26.2 ± 4.8% to 32.4 ± 8.5% (p < 0.001). In group 2, there was also an improvement of LVEF from 38.2 ± 2.5 to 44.4 ± 9.6% (p < 0.001) (Fig. 2). In 167 (69%) patients, an improvement of LVEF was observed, in 33 patients (13.6%) LVEF remained constant, in 42 patients (17.4%) LVEF decreased. In 11 patients (18%) of group 1, LVEF increased to a value above 40%, in 19 patients (32%) to a value in the range of 30–40%.
In the scattergram (Fig. 3) which shows both baseline and follow-up LVEF measurement of individual patients, there was a steady trend towards higher LVEF values, but with a considerable scatter due to measurement error and individual trend variability that cannot be distinguished from one another. In consequence of the inclusion criterion of LVEF <40%, the scattergram is limited by a vertical line to the right side.
Compared to Fig. 3, in Fig. 4 the axes are rotated by 45%. Differences between follow-up and baseline LVEF measurements (ordinate) are plotted vs. averages (abscissa) analogous to Bland–Altman plots. If no patient selection had taken place, there were values in the right lower corner forming an ellipsoid scatter. The average differences are too high and were attributable to the experimental design; this demonstrates how regression towards the mean and the corresponding selection bias originate. If we restrict the analysis to patients with an average of less than 40% LVEF (n = 122), the average difference is 2.0% (95%-CI 0.55–3.5, p = 0.007). This figure estimates the average true LVEF change after correction for the selection bias.
Scatter plot: axes of Fig. 3 are rotated by 45%. Differences of follow-up and baseline LVEF measurements (ordinate) are plotted vs. averages (abscissa) analogous to Bland–Altman plots. avg average
Figure 5 contains the LOWESS line that demonstrates the time-dependence of the apparent improvement. The cutoff with the ordinate is at 7%. The LVEF increases by about 2% up to about 15 months and seems to deteriorate again after 20 months.
Likewise, the extent of change shows no dependence on the initial EF value (Fig. 6).
Discussion
According to the present analysis, apparent improvements of LVEF to a considerable extent can be attributed a statistical phenomenon and are not true effects. Second, LVEF may show considerable intraindividual variation during follow-up. Owing to such high variability, a single measurement may not be reliable enough to justify a decision on ICD indication.
The increase in LVEF in the study population was moderate at 6%. This is in line with the expectation based on a pathophysiological viewpoint, that true left ventricular function it is highly improbable to recover to normal in patients with a damaged post-MI ventricle. This applies to patients with severely reduced LV function as well as patients with a moderate reduction. Although complete revascularization is one of the most important interventions for improving left ventricular function and reducing ischemic-driven ventricular tachyarrhythmias after MI, the recovery of impaired LVEF even after complete revascularization in post-myocardial patients usually is moderate at best. Recovery of systolic function in patients with ischemic cardiomyopathy can be expected only in stunned and hibernating myocardial segments [19]. In our data, after accounting for statistical artefacts, analysis of the time dependence of recovery reveals a slight increase by about 2% up to more than 1 year followed by a steady state and a slight decrease after almost 2 years, most likely due to progression of CHD.
The second important result is that the raw LVEF measurements suggest more marked changes in the preselected individuals. The change in mean values in subgroup populations is strongly affected by regression towards the mean (RTTM). Regression towards the mean reflects a statistical effect describing the relationship between two linked measurements [17,18,19,20,21]: if the initial value is above or below the mean, the later value is likely to be closer to the mean than the initial value. Studies of treatment effects in clinical trials and longitudinal follow-up investigations of disproportionate variables usually analyze whether there is a correlation between the change in the variable and its initial value, but the results are biased by the RTTM effect [26,27,28,29]. RTTM appears to be a selection phenomenon, emphasizing the need to include control groups in order to adjust for the bias caused by RTTM. Our study comprised a predefined subgroup of patients with LVEF of ≤40%. A second measurement allowed the complete range of possible LVEF to be established. Consequently, asymmetrically truncated subgroups were compared with a homogeneous Gaussian distribution of ejection fraction values. This entailed an overestimation of the rise in LVEF, which can only be avoided by excluding all patients with LVEF >40% in the second measurement. After taking RTTM into consideration, the improvement of LVEF was less impressive. Most importantly, it was not significant for clinical decisions, even though major proportion of patients had a mild increase of LVEF as measured after revascularization. Additionally, assessed improvement of LVEF is a combination of interindividual and intraindividual variability, RTTM and actual change of LV function. Variability should be taken into account when the ejection fraction is used as a parameter of improved or worsened cardiac function. Even in comparison of homogenous groups, individual changes of LVEF should be interpreted as significant only when they exceed the total variability of echocardiographic imaging [17, 24].
In normally distributed variables with homogeneous sample sizes, conventional comparison tests are appropriate to assess changes of values. However, the usual statistical methods for subgroup analyses should be avoided because of preselection bias and other measurements like score tests based on truncated data, regression based t test, likelihood tests or Wald´s test should be preferred.
Limitations
Our study has a number of potential limitations. First, our patients displayed a wide range of time interval between MI and first assessment LVEF. Although the median was 1.2 months, there were several patients with a considerably longer interval, which at least in the infarctional area reflects a stable myocardial scar without remodeling capacity. Second, the initial values of left ventricular ejection fractions were rounded up or down to 5% steps, which include a maximum error of 2%. A third potential limitation stems from different observers in respect of the initial LVEF measurement. The second LVEF was assessed by only one investigator to limit interindividual variability as much as possible.
Conclusions
Even after complete revascularization, left ventricular dysfunction in post-myocardial heart failure patients remains largely unchanged. Change in mean values in subgroup populations are influenced by regression toward the mean. However, since physicians have no other sources of information at hand, they are bound to interpret the observed changes as real improvements and thus may tend to draw excessively optimistic conclusions with regard to the clinical course of their patients. While the real (as opposed to the apparent) recovery of LVEF after early post-MI revascularization turned out to be rather moderate, a single LVEF measurement is probably not reliable enough to allow a definite decision on ICD implantation. In consequence of the considerable instability of LVEF determinations that was observed, LV function should be assessed repeatedly before making treatment decisions with long-term effects such as ICD implantation for primary prevention of SCD.
Abbreviations
- CABG:
-
coronary artery bypass graft operation
- ICD:
-
implantable cardioverter-defibrillator
- LOWESS:
-
locally weighted regression scatter plot smoothing
- LVEF:
-
left ventricular ejection fraction
- MI:
-
myocardial infarction
- PCI:
-
percutaneous coronary intervention
- PreSCD II:
-
prevention of sudden cardiac death ii
- RTTM:
-
regression toward the mean
- SCD:
-
sudden cardiac death
- SD:
-
standard deviation
References
Kong MH, Fonarow GC, Peterson ED, Curtis AB, Hernandez AF, Sanders GD, Thomas KL, Hayes DL, Al-Khatib SM. Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol. 2011;57(7):794–801.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200.
Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–83.
Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013;61(3):e6–75.
Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–37.
Buxton AE, Lee KL, Hafley GE, Pires LA, Fisher JD, Gold MR, Josephson ME, Lehmann MH, Prystowsky EN. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. J Am Coll Cardiol. 2007;50(12):1150–7.
Goldenberg I, Moss AJ, McNitt S, Zareba W, Hall WJ, Andrews ML, Wilber DJ, Klein HU. Time dependence of defibrillator benefit after coronary revascularization in the Multicenter Automatic Defibrillator Implantation Trial (MADIT)-II. J Am Coll Cardiol. 2006;47(9):1811–7.
Wilber DJ, Zareba W, Hall WJ, Brown MW, Lin AC, Andrews ML, Burke M, Moss AJ. Time dependence of mortality risk and defibrillator benefit after myocardial infarction. Circulation. 2004;109(9):1082–4.
Bigger JT Jr. Prophylactic use of implanted cardiac defibrillators in patients at high risk for ventricular arrhythmias after coronary-artery bypass graft surgery. Coronary artery bypass graft (CABG) patch trial investigators. N Engl J Med. 1997;337(22):1569–75.
Solomon SD, Zelenkofske S, McMurray JJ, Finn PV, Velazquez E, Ertl G, Harsanyi A, Rouleau JL, Maggioni A, Kober L, et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005;352(25):2581–8.
Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, Fain E, Gent M, Connolly SJ. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351(24):2481–8.
Slart RH, Bax JJ, van Veldhuisen DJ, van der Wall EE, Dierckx RA, de Boer J, Jager PL. Prediction of functional recovery after revascularization in patients with coronary artery disease and left ventricular dysfunction by gated FDG-PET. J Nucl Cardiol. 2006;13(2):210–9.
Schinkel AF, Poldermans D, Rizzello V, Vanoverschelde JL, Elhendy A, Boersma E, Roelandt JR, Bax JJ. Why do patients with ischemic cardiomyopathy and a substantial amount of viable myocardium not always recover in function after revascularization? J Thorac Cardiovasc Surg. 2004;127(2):385–90.
Haas F, Jennen L, Heinzmann U, Augustin N, Wottke M, Schwaiger M, Lange R. Ischemically compromised myocardium displays different time-courses of functional recovery: correlation with morphological alterations? Eur J Cardiothorac Surg. 2001;20(2):290–8.
Otterstad JE, St John Sutton M, Froland G, Skjaerpe T, Graving B, Holmes I. Are changes in left ventricular volume as measured with the biplane Simpson’s method predominantly related to changes in its area or long axis in the prognostic evaluation of remodelling following a myocardial infarction? Eur J Echocardiogr. 2001;2(2):118–25.
St John Sutton. M, Otterstat JE, Plappert T, Parker A, Sekarski D, Keane MG, Poole-Wilson P, Lubsen K. Quantitation of left ventricular volumes and ejection fraction in post-infarction patients from biplane and single plane two-dimensional echocardiograms. A prospective longitudinal study of 371 patients. Eur Heart J. 1998;19(5):808–16.
Kuecherer HF, Kee LL, Modin G, Cheitlin MD, Schiller NB. Echocardiography in serial evaluation of left ventricular systolic and diastolic function: importance of image acquisition, quantitation, and physiologic variability in clinical and investigational applications. J Am Soc Echocardiogr. 1991;4(3):203–14.
Hole T, Otterstad JE, St John Sutton M, Froland G, Holme I, Skjaerpe T. Differences between echocardiographic measurements of left ventricular dimensions and function by local investigators and a core laboratory in a 2-year follow-up study of patients with an acute myocardial infarction. Eur J Echocardiogr. 2002;3(4):263–70.
Jhang JS, Diamond JA, Phillips RA. Interobserver Variability of Left Ventricular Measurements in a Population of Predominantly Obese Hypertensives Using Simultaneously Acquired and Displayed M-Mode and 2-D Cine Echocardiography. Echocardiography. 1997;14(1):9–14.
Bax JJ, Visser FC, Poldermans D, Elhendy A, Cornel JH, Boersma E, van Lingen A, Fioretti PM, Visser CA. Time course of functional recovery of stunned and hibernating segments after surgical revascularization. Circulation. 2001;104(12 Suppl 1):I314–8.
Fast J, Jacobs S. Limits of reproducibility of cross-sectional echocardiographic measurement of left ventricular ejection fraction. Int J Cardiol. 1990;28(1):67–72.
Otterstad JE, Froeland G, St John Sutton M, Holme I. Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur Heart J. 1997;18(3):507–13.
Jenkins C, Bricknell K, Chan J, Hanekom L, Marwick TH. Comparison of two- and three-dimensional echocardiography with sequential magnetic resonance imaging for evaluating left ventricular volume and ejection fraction over time in patients with healed myocardial infarction. Am J Cardiol. 2007;99(3):300–6.
Olszewski R, Timperley J, Szmigielski C, Monaghan M, Nihoyannopoulos P, Senior R, Becher H. The clinical applications of contrast echocardiography. Eur J Echocardiogr. 2007;8(3):S13–23.
Cleveland W. Robust Locally Weighted Regression and Smoothing Scatterplots. J Am Stat Assoc. 1979;74:829–36.
Browne SM, Halligan PW, Wade DT, Taggart DP. Cognitive performance after cardiac operation: implications of regression toward the mean. J Thorac Cardiovasc Surg. 1999;117(3):481–5.
Morton V, Torgerson DJ. Regression to the mean: treatment effect without the intervention. J Eval Clin Pract. 2005;11(1):59–65.
Yudkin PL, Stratton IM. How to deal with regression to the mean in intervention studies. Lancet. 1996;347(8996):241–3.
Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol. 2005;34(1):215–20.
Authors’ contributions
HV and KW developed the conception and design of the study, and analysed, interpreted the data and revised the manuscript critically for important intellectual content. RR read all echocardiographs, interpreted the data and wrote the first version of the manuscript. KW developed the conception and performed the statistical analysis, interpreted the data, and revised the manuscript critically for important intellectual content. KB contributed to the conception of the study, data collection and revised the manuscript critically for important intellectual content. AS contributed to the conception of the study and to the interpretation of results and revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Acknowledgements
We gratefully thank all the study participants. We thank Mrs. Kirsten Stolze for the valuable technical assistance.
Competing interests
All authors declare that they have no competing interests.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Consent to publish
Consent to publish has been obtained from all patients in the informed consent form before initiation of the study.
Ethics approval and consent to participate
For this registry, the ethics committee of the Physicians Chamber Brandenburg (which was competent for the application of the principal investigator KB) approved the study materials. Before documentation, all patients provided informed consent that their data could be extracted from the patient files and analysed with respect to the research topic. The study did not involve any study related procedures, and diagnostics or therapy of patients was not changed by study participation.
Funding
The study was supported by a grant from Boston Scientific. RR has received travel and accommodation expenses from Boston Scientific.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Reibis, R., Salzwedel, A., Bonaventura, K. et al. Improvement of left ventricular ejection fraction in revascularized postmyocardial patients: indication for statistical fallacy. BMC Res Notes 10, 244 (2017). https://doi.org/10.1186/s13104-017-2562-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-017-2562-4