Abstract
Background
The regular intake of fiber generates numerous health benefits. However, the efficacy depends on the duration of consumption and the ingested dose. Studies investigating the optimal dose are of interest to enable the inclusion of fiber in the routine treatment of diabetic patients.
Objective
We aimed to evaluate the effects of different doses of β-glucan (BG—isolated from Saccharomyces cerevisiae), in comparison to n-3 supplement, on the inflammatory and metabolic parameters of Wistar rats induced to diabetes by streptozotocin.
Methods
Forty animals were randomly divided into six groups receiving 0 mg/kg, 10 mg/kg, 20 mg/kg, or 40 mg/kg BG daily for 4 weeks or fish oil derivative [1000 mg/kg of omega-3 fatty acids (n-3)] for the same period. One additional group was composed of healthy controls. Serum metabolic and immunological parameters were evaluated by colorimetric and ELISA assays respectively. Histopathological analysis of the liver, small intestine and pancreas were also conducted. Significant changes due to BG intake were set into regression models with second-degree fit in order to estimate the optimal BG dose to achieve health benefits.
Results
The animals that ingested BG had lower food and water intake (p < 0.05) than the negative control group (0 mg/kg). However, consumption was still elevated in comparison to healthy controls. Blood glucose and serum levels of total cholesterol, LDL-c, and TG (p < 0.05) reduced in comparison to diabetic animals without treatment (better or similar to n-3 group depending on dose), but did not reach normal levels (in comparison to healthy controls). HDL-c was not different (p > 0.05) among all groups. These reductions were already seen with the lowest dose of 10 mg/kg. On average, the serum levels of the hepatic enzymes ALT and AST were 40% and 60% lower in the BG groups in comparison to diabetic animals without treatment (better results than n-3 group). The group receiving 40 mg/kg reached similar values of healthy controls for ALT; whereas the same result occurred from the dose of 10 mg/kg for AST. The ideal dose, estimated from the mean of all metabolic parameters was approximately 30 mg/kg/day. Regarding the immunological profile, TNF-α significantly decreased in the BG groups compared to controls (p < 0.05), reaching better values than n-3 group and similar to healthy controls. No significant differences were found between the groups in IL-1β or IL-10 (p > 0.05). No histological changes were found in the pancreas, liver, or intestine due to treatment among diabetic animals.
Conclusions
BG significantly reduced blood glucose as well as serum total cholesterol, LDL-c and TG. There was a hepatoprotective effect due to the reduction in ALT and AST and a reduction in TNF-α, indicating a modulation of the immune response. In general, BG effects were better than n-3 supplement (or at least comparable) depending on the dose.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is a chronic disease characterized by the autoimmune or idiopathic destruction of pancreatic cells [1] and/or insulin resistance [2]. Diabetic individuals have several abnormalities in the metabolism of macronutrients [3], resulting in hyperglycemia and predisposition to the development of several comorbidities, such as atherosclerosis, arterial hypertension, stroke, and acute myocardial infarction [4]. These associated complications compromise the quality of life of the affected individuals, harming their emotional, physical, and social well-being [5]. Additionally, this disease puts a great financial burden to the health systems worldwide [6].
Conventional treatment of DM involves changes in lifestyle with an emphasis on food and nutrition education and regular physical activity [7], in addition sometimes to oral medicine [8] and/or insulin therapy [9]. Variations in blood glucose are a major challenge for patients with DM, especially type 1 DM [10]. Functional and nutraceutical foods have been investigated as adjuvants in the control of this disease [11]. In this context, prebiotics comprise substrates that are selectively utilized by host microorganisms conferring a health benefit. This broader definition includes even non-carbohydrate substances [12]. Fermentable soluble fibers modifyes intestinal microflora, promoting increase of Lactobacillus and Bifidobacterium, and decrease Bacteroides and Clostridium [13]. Besides, omega-3 poliunsatured free fatty acids (PUFA) supplementation decrease Faecalibacterium, and increase Bacteroidetes and butyrate-producing bacteria belonging to the Lachnospiraceae family [14, 15]. Evidence from systematic reviews evaluating randomized clinical trials indicate that probiotics and/or prebiotics (and symbiotics–combining both) present antidiabetic effects by interfering with the composition of the gut microbial environment, reducing intestinal endotoxin concentrations and decreasing energy absorption [16]. The consumption of up to 3 g/day of marine-based n-3 PUFAs is generally regarded as safe (GRAS) by US Food and Drug Administration (FDA) [15]. Yeast BG supplementation derived from S. cerevisiae has been approved for use in food supplements by the FDA and received GRAS status in 2008 (goverment revenue number [GRN]: 000239) at a maximum dose of 200 mg per serving [17], with the daily dose ranging from 100–500 mg [18].
Soluble fiber, including β-glucan (BG), has received attention due to its hypoglycemic [19,20,21] and hypocholesterolemic effects [22, 23], with consequent reduction in insulin resistance [24], hepatoprotection [19], and immunostimulation [25]. These effects can help decrease DM comorbidities [26] by forming a protective intestinal barrier, delaying the absorption of lipids and free cholesterol [27]. This barrier acts as one of the main defense mechanisms of the body and produces immunoregulatory signals [28].
The regular intake of fiber generates numerous health benefits [29]. However, the greater efficacy of BG depends on the time of consumption and the ingested dose [30]. Thus, studies that investigate the optimal intake dose are of public health interest to produce data that will enable cost savings, promoting the inclusion of fiber in the routine treatment of DM patients [31] and reducing the risk of toxicity in comparison to other treatments [32].
Most studies investigate the effects of cereal fibers. The present study aimed to evaluate the effects of the ingestion of different doses of BG (isolated from Saccharomyces cerevisiae), in comparison to n-3 supplement, on the metabolic and inflammatory profile of rats with streptozotocin-induced DM.
Materials and methods
Animals
The present study was approved by the Ethics Committee on Animal Use (CEUA) under protocol number 082/17. A total of 40 male rats of the Wistar breed (Rattus norvegicus albinus) were used. The animals were 11 weeks old, with a weight of 278.4 grams (± 19 g). These animals were subjected to quarantine (38 days) and acclimated for 7 days. Then the animals were randomly distributed into five groups (N = 7/group) and kept in collective boxes. One additional group was composed of healthy controls (n = 5). The animals were treated in a climate-controlled room with a constant temperature of 23 ± 2°C and a light-dark cycle of 12/12 h. Commercial food and water were provided ad libitum throughout the experiment.
Experimental induction of diabetes
DM was induced by the intraperitoneal administration of 70 mg/kg of streptozotocin (STZ) (Sigma, ST. Louis, MO, USA) dissolved in ice-cold citrate buffer (pH 4.5) (4 °C) [33]. Induction was done at the end of the afternoon, and after 48 h, the animals were fasted for 8 h. Blood glucose was measured by incision of the tail tip with previous topic anesthesia by 1 mg/kg lidocaine ointment using the Accu-Check Active device (© 2016 Roche Diabetes Care, lot 06061982, Germany). Animals with blood glucose above 250 mg/dL were considered diabetic [34].
Oral administration of β-glucan and fish oil
The doses of BG were given through gavage and diluted in 0.3 mL of saline solution daily (Table 1). The BG used was obtained from the extract of the cell wall of Saccharomyces cerevisiae [Macrogard (Açucareira Quatá S/A—Divisão Biorigin, Lençois Paulista, SP, Brazil; Composition: β-glucan—minimum 60.0%; raw protein—maximum. 8.0%; pH (2% solution) 4.0–7.0; ash—maximum 10.0 g/100 g].
Commercially acquired fish oil capsules were broken and poured into an amber glass daily. The dose was also administered by oral gavage as listed in Table 1. According to the data reported by the manufacturer, the fish-derived oil used had 0.58 g eicosapentaenoic acid (EPA) and 0.37 g docosahexaenoic acid (DHA) for each 3 grams of the product.
Collecting material for analysis
At the end of the 28-day experimental period, the animals were fasted for 8 h and euthanized through exsanguination by cardiac puncture after anesthesia containing 50 mg/kg of sodium thiopental intraperitoneally. The blood samples were stored in sterile siliconized tubes (Vacuette®, Centerlab, Belo Horizonte, MG, Brazil), vacuum-sealed with clot activator (micronized silica particles), and plasma collection was performed using 4% EDTA (anticoagulant). Next, the tubes were centrifuged at 4000 rpm for 20 min. The liquid contents were poured into 2 mL Eppendorf tubes and stored in an ultrafreezer at − 80 °C until the time of analysis. Liver, small intestine, and pancreas were collected and kept in 10% formalin solution for 48 h (Fig. 1).
Histopathological analysis of the liver, small intestine, and pancreas
After 48 h in 10% buffered formalin, the samples were conditioned in 70% ethanol. The fragments were processed in the Histotechnic (DM-70/12D OMA, São Paulo, SP, Brazil) and embedded in paraffin for cutting in a rotary microtome. The sections were cut to 4 µm thick. They were stained with hematoxylin and eosin for analysis of morphological features under light microscopy. Histological slides were evaluated by an experienced veterinary pathologist blind to the treatments. The pancreas were analyzed histologically for the number of cells present in the islets of Langerhans, and these were classified according to the following score: normal (−) (number of islet cells greater than 30) (+) mild lesion (number of islet cells 20–30); (++) moderate lesion (number of islet cells 10–20) (+++) severe lesion (number of islet cells less than 10) [35]. The liver and small intestine (duodenum, jejunum, and ileum) were analyzed histologically to identify the occurrence of lesions or any other type of microscopic alteration. Histological images were captured using coupled camera to a light microscope (CX31 binocular microscope, Olympus Optical do Brasil Ltda, São Paulo, SP, Brazil).
Metabolic and immunological analyzes
The serum concentrations of triglycerides (TG), total cholesterol (TC), and HDLc were determined using a colorimetric assay according (Lab Test®, Lagoa Santa, Minas Gerais, Brazil). The level of LDLc was calculated using the Friedewald equation [36], where LDLc = TC − HDLc − TG/5. For these analyses, serum and reagent were pipetted together and then incubated in a water bath at 37 °C for 10 min. The reading was done at a wavelength of 505 nm for TG and TC at 500 nm for HDLc in an Epoch Biotek ® spectrophotometer (Biotek®, Winooski, USA). Calculations were done with the formulas described below:
The liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were obtained by plasma analysis in a colorimetric assay (Bioliquid®, Pinhais, Paraná, Brazil). The reagent (1 mL) was incubated for 3 min at 37 ℃. Next, the plasma samples were added, and the reading was taken at 340 nm after 1 min. The concentration was determined according to the following formula:
The serum concentrations of interleukin (IL)-1β, IL-10, and tumor necrosis factor alpha (TNF-α) were determined by enzyme-linked immunosorbent assay with commercial kits (Invitrogen®, Thermo Fisher Scientific, Vienna, Austria). Serological samples were diluted (1:5) and pipetted with the reagent. These were incubated for 120 min at room temperature (21°~ 25° C) in a 3-dimensional homogenizer KJMR-V® (Global Equipment, Global Trade Technology, São Paulo, Brazil). The readings were taken at 450 nm in the Epoch Biotek® spectrophotometer (Winooski, VT, USA).
Statistical analysis
The data were compared by analysis of variance (ANOVA) followed by the Student–Newman–Keuls post hoc test (p < 0.05) in the statistical software Prism 5.0 (GraphPad Prism, CA, USA). Data are expressed as the mean ± standard deviation. For the regression model, the second-degree fit was performed using Excel software (Microsoft Excel, 2013).
Results
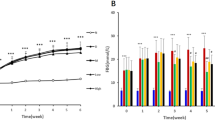
The water and food intake (Fig. 2) were higher in the vehicle (negative control group - 0 mg BG) and 10 mg/kg BG groups than the other treatments (p < 0.05), demonstrating the classic symptom of polydipsia and polyphagia, especially in the 0 mg group. However, consumption was still elevated in comparison to healthy controls (p < 0.05). There was a significant reduction in blood glucose (approximately 27%) at all nonzero BG doses (p < 0.05) compared with the 0 mg and omega-3 groups (Fig. 3). There was a significant reduction in total cholesterol (Fig. 3) above 10 mg/kg BG doses (approximately 23%), as well as in TG (Fig. 3) (32% reduction) and LDL-c (Fig. 3) (approximately 30%) (p < 0.05). On the other hand, no significant differences were seen in HDL-c (Fig. 3). In general, all BG presented similar results compared to omega-3 (p > 0.05), except for blood glucose levels in which BG presented better results (Fig. 3). Although several parameters significantly improved, the reached values were still higher than the healthy group. HDL-c was not different (p > 0.05) among all groups, including the healthy controls.
Daily water and food intake of diabetic Wistar rats induced by intraperitoneal injection of streptozotocin (70 mg/kg) and treated with different doses of β-glucan from Saccharomyces cerevisiae for 28 days. Different lowercase letters indicate significant differences by the Student–Newman–Keuls test at 5% probability (p < 0.05)
Metabolic parameters of diabetic Wistar rats induced by intraperitoneal injection of streptozotocin (70 mg/kg) and treated with different doses of β-glucan from Saccharomyces cerevisiae for 28 days. Different lowercase letters indicate significant differences by the Student–Newman–Keuls test at 5% probability (p < 0.05)
Liver enzymes ALT and AST (Fig. 3) were both lower (up to 40% and 60%, respectively) in BG groups vs. the 0 mg group. There was a significant reduction in ALT in the omega-3 group, similar to the 20 mg BG group (p < 0.05). The group receiving 40 mg/kg reached similar values of healthy controls for ALT; whereas the same result occured from the dose of 10 mg/kg for AST. No significant differences were found between the groups in serum IL-1β, IL-10, or IL-1β/IL-10 ratio (Fig. 4b), but a significant reduction in TNF-α was observed when compared to 0 mg (p < 0.05), reaching healthy control values.
Inflammatory parameters of diabetic Wistar rats induced by intraperitoneal injection of streptozotocin (70 mg/kg) and treated with different doses of β-glucan from Saccharomyces cerevisiae for 28 days. Different lowercase letters indicate significant differences by the Student–Newman–Keuls test at 5% probability (p < 0.05)
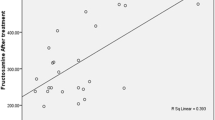
In order to estimate the best dose of BG for each parameter (blood glucose, TC, LDL-c, TG, ALT, and AST) were fit into a second-degree linear model (Fig. 5). The optimal dose was estimated by the mean of all parameters, and the value found was approximately 30 mg/kg/day. No significant changes were found in histological samples of the liver or duodenum, jejunum, or ileum submucosa, regardless of the treatment. According to the established criteria, there was a significant reduction in the number of pancreatic islet cells due to the induction of diabetes (Table 2), but no differences were found among treated or 0 mg groups (p > 0.05). Healthy controls present only normal scores, differing from all other diabetic groups.
Discussion
The present study demonstrated the beneficial effects of the consumption of BGs on blood glucose, reducing clinical signs of polyphagia and polydipsia. There were also positive effects on serum total cholesterol, LDL-c, and TG, in addition to a hepatoprotective effect. Water and food intake were significantly lower in the groups receiving BG regardless of the dose. This reduction may be associated with the ability of BG to promote satiety [37]. BGs increase central YY peptide secretion [38] and delay gastric emptying by increasing viscosity and water retention in the intestine [39] (reducing intestinal peristalsis, i.e., postprandial contractility is extended) aiding in glucose homeostasis [40] and reducing the appetite. The decrease in water and food intake with omega-3 supplementation also occurs due to its ability to modify the expression of neuropeptides related to appetite in the hypothalamic axis [41].
In the present study, the results of BG and n-3 were quite equivalent, with even better results for BG on reducing blood glucose levels depending on the dose. BG is able to reduce the absorption of glucose due to its ability to form a barrier (gel/viscose) in the intestine, causing a delay in the absorption of carbohydrates [42]. In addition, the capacity of yeast BG to aid in the decrease in glucose transporters SGLT1 and GLUT2 in the small intestine has been reported [43]. This same mechanism was also demonstrated for oat BG [44].
In obese and type 2 diabetes, the composition of the gut microbiota is associated to excessive ingestion of high-fat diets (HFD) and low-grade inflammation. Taking together, these factors play important roles for development of obesity and other chronic diseases [45]. Briefly, HFD generates microbiota shift increasing the expression of fat translocase, scavenger receptor CD36 and the scavenger receptor class B type 1 (SR-BI). SR-BI binds and incorporate lipids and lipopolysaccharides (LPS) to chilomicrons. After epithelial translocation, LPS is transferred to lipoproteins (such as HDL) and is directed to adipocytes. In this site, LPS contributes to M2 to M1 phenotype macrophage polarization and adipocyte hypertrophy [45]. Tryptophan-derived metabolites produced by the gut microbiota also controls the expression of the miR-181 family in white adipocytes that regulates energy expenditure and insulin sensitivity [46]. In the present study diabetes was induced by streptozotocin β-cell destruction, which approximates to type 1 diabetes. Even in this situation, we found positive effects of BG ingestion.
Microbial fermentation of prebiotics generates short-chain fatty acids (SCFA), such as acetate and butyrate, that have been shown to protect against oxidative and mitochondrial stress [47], enhance gut barrier, increase glucangon-like peptide 1 and 2 (GLP-1 and GLP-2) secretion [48], which delay gastric emptying and induce satiety [49]. Besides, incretins reduce hepatic expression of inflammatory and oxidative stress markers during obesity and diabetes [50]. GLP-1 stimulates insulin and reduces glucagon secretions from pancreatic α cells, reducing liver glucose output [51] and improving peripheral uptake of glucose [52]. Besides, SCFA inhibits lipolysis and reduces inflammation, enhancing energy metabolism regulation [16]. A recent study showed that SCFAs enhanced the viability of islets and β-cells, prevented STZ-induced cell apoptosis, viability reduction, mitochondrial dysfunction, and the overproduction of reactive oxygen species (ROS) and nitric oxide (NO) [47]. Mechanisms responsible for the efficacy of dietary n-3 PUFAs include reduction in IFN-γ, IL-17, IL-6, and TNF-α corroborating our findings (reduction in TNF-α) [53]. Indeed, n-3 PUFAs preventes lymphocyte infiltration into regenerating pancreatic islets, and elevates the expression of the β cell markers (Pdx1) and paired box 4 (Pax4) [53] what resembles our present findings on increased glucose control in animals ingesting n-3 supplement against control group.
Decreased glucose was also found in diabetic rats receiving doses of 6 mg and 12 mg of BG derived from fungus. The authors found significant reductions of 17% and 52% in blood glucose, respectively [54]. A significant reduction in blood glucose of 32% was also reported in a previous study of our group using a dose of 30 mg/kg BG for 28 days in streptozotocin-induced diabetic animals [19]. No significant differences were found in blood glucose levels with the use of omega-3 in the present study, corroborating previous studies in type 1 diabetic patients [55, 56]. Indeed, side effects of the use of omega-3 in glucose homeostasis have been reported, caused by increasing need for insulin [57], because polyunsaturated fatty acids can induce changes in the fluidity of cell membranes [58], decreasing the affinity of insulin for its receptors [57].
The biochemical parameters TC, TG, and LDLc also decreased significantly with the ingestion of BG. These hypoglycemic and hypolipidemic effects are also related to the ability of BG to form a gel barrier in the intestine, delaying the absorption of carbohydrates and lipids in enterocytes and consequently reducing cholesterol [59]. With the formation of the gel, there is an increase in the fecal bulk viscosity that prolongs gastric emptying [60], increasing the water layer with a consequent decrease in the uptake of cholesterol in the intestine [61] and greater elimination in the feces [62]. Among the main mechanisms of cholesterol reduction is the decreased absorption of bile salts due to the ability of BG to adsorb these salts, reducing their resorption and return to the liver [63]. Hepatic cholesterol reduction regulates the synthesis of the LDLc receptor. This fact generates increased uptake of the LDL-c by the liver, as well as negatively modulate the synthesis of 3′-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMG coA reductase), the enzyme responsible for cholesterol synthesis [63], through fermentation and production of SCFAs. Hypolipidemic effect of omega-3 was demonstrated in the present study. This effect seems to be related to increased EPA and DHA in the hepatic membrane [64]. In addition, it has been suggested that EPA can act as a second messenger, also reducingn HMG-coA reductase [65], the same effect attributed to BG. Shinozaki et al. [66] found that a dose of 1800 mg/day of EPA significantly reduced TC, TG, and LDLc after 6–24 months. Lobato et al. [19] demonstrated a 32% reduction in TG concentration and 41% reduction in ALT, demonstrating a hepatoprotective effect of BG. In addition, another study from our group showed a significant reduction in glucose, cholesterol, and TG levels in diabetic animals [59]. The reduction in blood glucose is directly related to the decrease in ALT due to the inhibition of the participation of this enzyme in the gluconeogenesis pathway [67].
In this study, BG did not affect HDLc. Previous meta-analysis was not able to determine whether dietary fiber intake was associated with HDLc metabolism [68]. However, it can be inferred that fiber intake reduced LDLc without reducing HDL-c, which is a benefit [26]. This process may have occurred due to the high level of cholesterol in the bloodstream (due to diabetes) and through processes triggered by epinephrine and hydrolysis enzymes, generating proportional synthesis of HDLc to carry free cholesterol to be metabolized in the liver [69]. It is important that HDLc was not decreased by BG because, given its association with reverse cholesterol transport, it can suppress the accumulation of cholesterol in peripheral tissues [70], also aiding in its systemic decrease. These results are consistent with previous research [71].
BG significantly reduced TNF-α blood levels in the present study, corroborating previous results [72] probably because of an increase in the intestinal barrier. These results were similar to omega-3 , in which EPA and DHA supplementation increases adiponectin and reduces TNF-α [73]. It is important to highlight the safety of BG for human and animal consumption. Yeast BG at different concentrations showed no adverse inflammatory, hematological or toxicological effects in mice [74]. Several studies report the safety of oral BGs consumption regardless of the source (oat, mushroom, or yeast) or used doses [75, 76].
Although we have some limitations regarding microbiota sequencing and lack of molecular markers quantification, the present study results may be speculated into potential translation into humans. A randomised, double-blind, placebo-controlled clinical trial testing BG from Saccharomyces cerevisiae was performed in overweight and obese subjects (3 g/day) for 12 weeks. Results indicated that daily supplementation was useful for improving body weight and waist circumference, without adverse effects [77]. Meta-analysis evaluating the effect of oat BG intake on glycaemic control of diabetic patients (using only randomized controlled trials) indicated that BG ingestion significantly lowered concentrations in fasting plasma glucose and glycosylated hemoglobin (HbA1c) [78]. Another meta-analysis also showed that beta-glucan extracted from oats were effective in decreasing fasting glucose and fasting insulin of T2D and tented to lower HbA1c [79]. Consistent with our results, another meta-analysis of clinical trials showed that β-glucan has a lowering effect on LDLc, non-HDLc and apoB [26]. Although oat and yeast β-glucans have some chemical differences, a previous study showed that as long as the purity of β-glucan is high, there is no difference among the sources of β-glucans [43, 80].
The broader definition of prebiotics including non-carbohydrate sources [12] opened space for other substances that could join this concept. Several substances such as Polydextrose (PDX), Xylo-oligosaccharides (XOS), Pectic-oligosaccharides (POS), Gluco-oligosaccharides, Malto-oligosaccharides, Isomaltooligosaccharides (IMO), Soya-oligosaccharides (SOS), Fenugreek, Gold-based nanomaterials, selenium compounds, and nanoceria can be considered [13, 81] candidates. In this sense, even omega-3 can be considered a candidate if we consider this definition.
Our results highlight the improvement of important metabolic parameters such as blood glucose levels and liprotein profile in a dose-dependent manner after consuming yeast BG. These outcomes add some new information regarding potential preventive and therapeutic care for type 1 diabetes, which often presents difficulties in control clinical set. The future of prebiotic research will probably include more studies focusing on the specificity of prebiotics for intestinal bacteria [13]. Clinical trials investigating the role of yeast BG in both type 1 and type 2 diabetic patients are necessary in order to establish optimal clinical protocols for general care. Dietary fiber ingestion of 100–500 mg/day has been reported as safe [18]. BGs may be consumed in different formulations; such as breakfast cereals or baked goods [22].
Conclusion
The consumption of Saccharomyces cerevisiae BGs demonstrated promising effects in the treatment of DM by decreasing metabolic parameters (blood glucose, total cholesterol, LDLc, and TG) in addition to its hepatoprotective effect through the reduction in ALT and AST. It significantly reduced blood levels of TNF-α. The optimal estimated dose for the observed benefits in diabetic rats was around 30 mg/kg/day. In general, BG effects were better than n-3 supplement (or at least comparable) depending on the dose.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. The Lancet. 2014;383:69–82.
Hardy OT, Czech MP, Corvera S. What causes the insulin resistance underlying obesity? Curr Opin Endocrinol Diabetes Obesity. 2012;19:81–7.
Al-Maskari AY, Al-Maskari MY, Al-Sudairy S. Oral manifestations and complications of diabetes mellitus: a review. Sultan Qaboos Univ Med J. 2011;11:179–86.
de Ferranti SD, de Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, et al. Type 1 diabetes mellitus and cardiovascular disease. Circulation. 2014;130:1110–30.
Semenkovich K, Brown ME, Svrakic DM, Lustman PJ. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. 2015;75:577–87.
Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81.
Levesque C. Therapeutic lifestyle changes for diabetes mellitus. Nurs Clin North Am. 2017;52:679–92.
Tanabe M, Motonaga R, Terawaki Y, Nomiyama T, Yanase T. Prescription of oral hypoglycemic agents for patients with type 2 diabetes mellitus: a retrospective cohort study using a Japanese hospital database. J Diabetes Investigat. 2017;8:227–34.
Hatz K, Elisabeth Minder A, Lehmann R, Drescher T, Gerendas B, Schmidt-Erfurth U, et al. The prevalence of retinopathy in patients with type 1 diabetes treated with education-based intensified insulin therapy and its association with parameters of glucose control. Diabetes Res Clin Pract. 2019;148:234–9.
Frid A, Tura A, Pacini G, Ridderstråle M. Effect of oral pre-meal administration of betaglucans on glycaemic control and variability in subjects with type 1 diabetes. Nutrients. 2017;9:1004.
Alkhatib A, Tsang C, Tiss A, Bahorun T, Arefanian H, Barake R, et al. Functional foods and lifestyle approaches for diabetes prevention and management. Nutrients. 2017;9:1310.
Gibson GR, Hutkins RW, Sanders ME, Prescott SL, Reimer RA, Gibson GR, et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Med Biochem Comm. 2017;14:491–502.
Wang S, Xiao Y, Tian F, Zhao J, Zhang H, Zhai Q, et al. Rational use of prebiotics for gut microbiota alterations: Specific bacterial phylotypes and related mechanisms. J Funct Foods. 2020;66:103838.
Costantini L, Molinari R, Farinon B, Merendino N. Impact of omega-3 fatty acids on the gut microbiota. Int J Mol Sci. 2017;18:2645.
Parolini C. Effects of fish n-3 PUFAs on intestinal microbiota and immune system. Marine Drugs. 2019;17:374.
Kim YA, Keogh JB, Clifton PM. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr Res Rev. 2018;31:35–51.
Thompson IJ, Oyston PCF, Williamson DE. Potential of the β-glucans to enhance innate resistance to biological agents. Expert Rev Anti-Infect Therapy. 2010;8:339–52.
Vetvicka V, Vannucci L, Sima P, Richter J. Beta glucan: supplement or drug? From laboratory to clinical trials. Molecules. 2019;24:1251.
Vieira Lobato R, De Oliveira Silva V, Francelino Andrade E, Ribeiro Orlando D, Gilberto Zangeronimo M, Vicente de Sousa R, et al. Metabolic effects of Β-Glucans (Saccharomyces cerevisiae) Per Os administration in rats with streptozotocin-induced diabetes. Nutrición Hospitalaria. 2015;32:256–64.
de O. Silva V, Lobato R, Andrade E, Orlando D, Borges B, Zangeronimo M, et al. Effects of β-Glucans ingestion on alveolar bone loss, intestinal morphology, systemic inflammatory profile, and pancreatic β-Cell function in rats with periodontitis and diabetes. Nutrients. 2017;9:1016.
Andrade E, Lima A, Nunes I, Orlando D, Gondim P, Zangeronimo M, et al. Exercise and beta-glucan consumption (Saccharomyces cerevisiae) improve the metabolic profile and reduce the atherogenic index in type 2 diabetic rats (HFD/STZ). Nutrients. 2016;8:792.
Francelino Andrade E, Vieira Lobato R, Vasques Araújo T, Gilberto Zangerônimo M, Vicente Sousa R, José Pereira L. Effect of beta-glucans in the control of blood glucose levels of diabetic patients: a systematic review. Nutr Hosp. 2014;31:170–7.
de Araújo TV, Andrade EF, Lobato RV, Orlando DR, Gomes NF, de Sousa RV, et al. Effects of beta-glucans ingestion (Saccharomyces cerevisiae) on metabolism of rats receiving high-fat diet. J Animal Physiol Animal Nutrit. 2017;101:349–58.
Tosh SM. Review of human studies investigating the post-prandial blood-glucose lowering ability of oat and barley food products. Eur J Clin Nutr. 2013;67:310–7.
Kim SY, Song HJ, Lee YY, Cho K-H, Roh YK. Biomedical issues of dietary fiber beta-glucan. J Korean Med Sci. 2006;21:781–9.
Ho HVTT, Sievenpiper JL, Zurbau A, Blanco Mejia S, Jovanovski E, Au-Yeung F, et al. The effect of oat β-glucan on LDL-cholesterol, non-HDL-cholesterol and apoB for CVD risk reduction: a systematic review and meta-analysis of randomised-controlled trials. Br J Nutr. 2016;116:1369–82.
Whitehead A, Beck EJ, Tosh S, Wolever TM. Cholesterol-lowering effects of oat β-glucan: a meta-analysis of randomized controlled trials. Am J Clin Nutrit. 2014;100:1413–21.
Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013;342:447–53.
Verspreet J, Damen B, Broekaert WF, Verbeke K, Delcour JA, Courtin CM. A critical look at prebiotics within the dietary fiber concept. Annu Rev Food Sci Technol. 2016;7:167–90.
Cugnet-Anceau C, Nazare J-A, Biorklund M, Le Coquil E, Sassolas A, Sothier M, et al. A controlled study of consumption of β-glucan-enriched soups for 2 months by type 2 diabetic free-living subjects. Br J Nutr. 2010;103:422.
Silva FM, Kramer CK, de Almeida JC, Steemburgo T, Gross JL, Azevedo MJ. Fiber intake and glycemic control in patients with type 2 diabetes mellitus: a systematic review with meta-analysis of randomized controlled trials. Nutr Rev. 2013;71:790–801.
Bowers GJ, Patchen ML, MacVittie TJ, Hirsch EF, Fink MP. A comparative evaluation of particulate and soluble glucan in an endotoxin model. Int J Immunopharmacol. 1986;8:313–21.
de la Garza-Rodea AS, Knaän-Shanzer S, den Hartigh JD, Verhaegen APL, van Bekkum DW. Anomer-equilibrated streptozotocin solution for the induction of experimental diabetes in mice (Mus musculus). J Am Assoc Lab Animal Sci. 2010;49:40–4.
Coskun O, Kanter M, Korkmaz A, Oter S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and ?-Cell damage in rat pancreas. Pharmacol Res. 2005;51:117–23.
Sharma AK, Bharti S, Kumar R, Krishnamurthy B, Bhatia J, Kumari S, et al. Syzygium cumini ameliorates insulin resistance and β-cell dysfunction via modulation of PPAR, dyslipidemia, oxidative stress, and TNF-α in type 2 diabetic rats. J Pharmacol Sci. 2012;119:205–13.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
Rebello CJ, Burton J, Heiman M, Greenway FL. Gastrointestinal microbiome modulator improves glucose tolerance in overweight and obese subjects: a randomized controlled pilot trial. J Diabetes Complicat. 2015;29:1272–6.
Vitaglione P, Lumaga RB, Stanzione A, Scalfi L, Fogliano V. β-Glucan-enriched bread reduces energy intake and modifies plasma ghrelin and peptide YY concentrations in the short term. Appetite. 2009;53:338–44.
Johansen HN, Knudsen KE, Sandström B, Skjøth F. Effects of varying content of soluble dietary fibre from wheat flour and oat milling fractions on gastric emptying in pigs. Br J Nutr. 1996;75:339–51.
Müller M, Canfora EE, Blaak EE. Gastrointestinal Transit Time, Glucose Homeostasis and Metabolic Health: modulation by Dietary Fibers. Nutrients. 2018;10:275.
Ma S, Ge Y, Gai X, Xue M, Li N, Kang J, et al. Transgenic n-3 PUFAs enrichment leads to weight loss via modulating neuropeptides in hypothalamus. Neurosci Lett. 2016;611:28–32.
Bashir KMI, Choi JS. Clinical and physiological perspectives of β-Glucans: the past, present, and future. Int J Mol Sci. 2017;18:1906.
Cao Y, Sun Y, Zou S, Li M, Xu X. Orally administered Baker’s Yeast β-glucan promotes glucose and lipid homeostasis in the livers of obesity and diabetes model mice. J Agric Food Chem. 2017;65:9665–74.
Abbasi NN, Purslow PP, Tosh SM, Bakovic M. Oat β-glucan depresses SGLT1- and GLUT2-mediated glucose transport in intestinal epithelial cells (IEC-6). Nutr Res. 2016;36:541–52.
Hersoug LG, Møller P, Loft S. Gut microbiota-derived lipopolysaccharide uptake and trafficking to adipose tissue: implications for inflammation and obesity. Obesity Rev. 2016;17:297–312.
Virtue AT, McCright SJ, Wright JM, Jimenez MT, Mowel WK, Kotzin JJ, et al. The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci Translat Med. 2019;11:1892.
Hu S, Kuwabara R, de Haan BJ, Smink AM, de Vos P. Acetate and butyrate improve β-cell metabolism and mitochondrial respiration under oxidative stress. Int J Mol Sci. 2020;21:1542.
Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the g-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–71.
Hellström PM, Näslund E, Edholm T, Schmidt PT, Kristensen J, Theodorsson E, et al. GLP-1 suppresses gastrointestinal motility and inhibits the migrating motor complex in healthy subjects and patients with irritable bowel syndrome. Neurogastroenterol Motil. 2008;20:649–59.
Cani PD, Possemiers S, Van De Wiele T, Guiot Y, Everard A, Rottier O, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–103.
Hare KJ, Knop FK, Asmar M, Madsbad S, Deacon CF, Holst JJ, et al. Preserved inhibitory potency of GLP-1 on glucagon secretion in type 2 diabetes mellitus. J Clin Endocrinol Metabol. 2009;94:4679–87.
Stahel P, Xiao C, Nahmias A, Lewis GF. Role of the gut in diabetic dyslipidemia. Front Endocrinol. 2020;11:116.
Bi X, Li F, Liu S, Jin Y, Zhang X, Yang T, et al. ω-3 polyunsaturated fatty acids ameliorate type 1 diabetes and autoimmunity. J Clin Investigat. 2017;127:1757–71.
Miranda-Nantes CCBO, Fonseca EAI, Zaia CTBV, Dekker RFH, Khaper N, Castro IA, et al. Hypoglycemic and Hypocholesterolemic effects of Botryosphaeran from Botryosphaeria rhodina MAMB-05 in diabetes-induced and hyperlipidemia conditions in rats. Mycobiology. 2011;39:187–93.
De Caterina R, Madonna R, Bertolotto A, Schmidt EB. n-3 fatty acids in the treatment of diabetic patients: biological rationale and clinical data. Diabetes Care. 2007;30:1012–26.
Poreba M, Mostowik M, Siniarski A, Golebiowska-Wiatrak R, Malinowski KP, Haberka M, et al. Treatment with high-dose n-3 PUFAs has no effect on platelet function, coagulation, metabolic status or inflammation in patients with atherosclerosis and type 2 diabetes. Cardiovasc Diabetol. 2017;16:50.
Stacpoole PW, Alig J, Ammon L, Crockett SE. Dose-response effects of dietary marine oil on carbohydrate and lipid metabolism in normal subjects and patients with hypertriglyceridemia. Metabolism Clin Exp. 1989;38:946–56.
Wang X, Chan CB. n-3 polyunsaturated fatty acids and insulin secretion. J Endocrinol. 2015;224:R97–106.
Silva de O V, Lobato RV, Andrade EF, de Macedo CG, Napimoga JTC, Napimoga MH, et al. β-Glucans (Saccharomyces cereviseae) reduce glucose levels and attenuate alveolar bone loss in diabetic rats with periodontal disease. Plos ONE. 2015;10:e0134742.
Jenkins DJ, Kendall CW, Axelsen M, Augustin LS, Vuksan V. Viscous and nonviscous fibres, nonabsorbable and low glycaemic index carbohydrates, blood lipids and coronary heart disease. Curr Opin Lipidol. 2000;11:49–56.
Gee JM, Blackburn NA, Johnson IT. The influence of guar gum on intestinal cholesterol transport in the rat. Br J Nutrit. 1983;50:215–24.
El Khoury D, Cuda C, Luhovyy BL, Anderson GH. Beta glucan: health benefits in obesity and metabolic syndrome. J Nutr Metabol. 2012;2012:851362.
Chen J, Huang X-F. The effects of diets enriched in beta-glucans on blood lipoprotein concentrations. J Clin Lipidol. 2009;3:154–8.
Kim HK, Choi H. Dietary alpha-linolenic acid lowers postprandial lipid levels with increase of eicosapentaenoic and docosahexaenoic acid contents in rat hepatic membrane. Lipids. 2001;36:1331–6.
Das UN. Beneficial effect(s) of n-3 fatty acids in cardiovascular diseases: but, why and how? Prostaglandins Leukotrienes Essential Fatty Acids (PLEFA). 2000;63:351–62.
Shinozaki K, Kambayashi J, Kawasaki T, Uemura Y, Sakon M, Shiba E, et al. The long-term effect of eicosapentaenoic acid on serum levels of lipoprotein (a) and lipids in patients with vascular disease. J Atherosclerosis Thrombosis. 1996;2:107–9.
Wang B, Smyl C, Chen C-Y, Li X-Y, Huang W, Zhang H-M, et al. Suppression of postprandial blood glucose fluctuations by a low-carbohydrate, high-protein, and high-omega-3 diet via inhibition of gluconeogenesis. Int J Mol Sci. 2018;19:1823.
Yanai H, Katsuyama H, Hamasaki H, Abe S, Tada N, Sako A. Effects of carbohydrate and dietary fiber intake, glycemic index and glycemic load on HDL metabolism in Asian populations. J Clin Med Res. 2014;6:321–6.
Wang Y, Xu D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis. 2017;16:132.
Zhou Q, Wu J, Tang J, Wang J-J, Lu C-H, Wang P-X. Beneficial effect of higher dietary fiber intake on plasma HDL-C and TC/HDL-C ratio among Chinese Rural-to-urban migrant workers. Int J Environ Res Public Health. 2015;12:4726–38.
Drozdowski LA, Reimer RA, Temelli F, Bell RC, Vasanthan T, Thomson ABR. β-Glucan extracts inhibit the in vitro intestinal uptake of long-chain fatty acids and cholesterol and down-regulate genes involved in lipogenesis and lipid transport in rats. J Nutr Biochem. 2010;21:695–701.
Cao Y, Sun Y, Zou S, Duan B, Sun M, Xu X. Yeast β-Glucan suppresses the chronic inflammation and improves the microenvironment in adipose tissues of ob/ob mice. J Agric Food Chem. 2018;66:621–9.
Becic T, Studenik C. Effects of omega-3 supplementation on adipocytokines in prediabetes and type 2 diabetes mellitus: systematic review and meta-analysis of randomized controlled trials. Diabetes Metabol J. 2018;42:101.
Delaney B, Carlson T, Zheng GH, Hess R, Knutson N, Frazer S, et al. Repeated dose oral toxicological evaluation of concentrated barley beta-glucan in CD-1 mice including a recovery phase. Food Chem Toxicol. 2003;41:1089–102.
Chen SN, Chang CS, Chen S, Soni M. Subchronic toxicity and genotoxicity studies of Antrodia mushroom β-glucan preparation. Regul Toxicol Pharmacol. 2018;92:429–38.
Túrmina J, Carraro E, Alves da Cunha M, Dekker R, Barbosa A, dos Santos F, et al. toxicological assessment of β-(1à6)-glucan (Lasiodiplodan) in mice during a 28-day feeding study by gavage. Molecules. 2012;17:14298–309.
Santas J, Lázaro E, Cuñé J. Effect of a polysaccharide-rich hydrolysate from Saccharomyces cerevisiae (LipiGo®) in body weight loss: randomised, double-blind, placebo-controlled clinical trial in overweight and obese adults. J Sci Food Agric. 2017;97:4250–7.
Shen XL, Zhao T, Zhou Y, Shi X, Zou Y, Zhao G. Effect of oat β-glucan intake on glycaemic control and insulin sensitivity of diabetic patients: a meta-analysis of randomized controlled trials. Nutrients. 2016;8:39.
He LX, Zhao J, Huang YS, Li Y. The difference between oats and beta-glucan extract intake in the management of HbA1c, fasting glucose and insulin sensitivity: a meta-analysis of randomized controlled trials. Food Funct. 2016;7:1413–28.
Korolenko TA, Bgatova NP, Ovsyukova MV, Shintyapina A, Vetvicka V. Hypolipidemic effects of β-glucans, mannans, and fucoidans: mechanism of action and their prospects for clinical application. Molecules. 2020;25:1819.
Bubnov R, Babenko L, Lazarenko L, Kryvtsova M, Shcherbakov O, Zholobak N, et al. Can tailored nanoceria act as a prebiotic? Report on improved lipid profile and gut microbiota in obese mice. EPMA J. 2019;10:317–35.
Acknowledgements
The authors acknowledge the Department of Veterinary Medicine of the Universidade Federal de Lavras–UFLA for their assistance in Animal Laboratory and analysis.
Funding
This study was supported by the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico and Tecnológico—CNPq), the Research and Support Foundation of the State of Minas Gerais (Fundação de Amparo and Pesquisa do Estado de Minas Gerais—FAPEMIG) and the Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Nível Superior—CAPES).
Author information
Authors and Affiliations
Contributions
JSG: Animal experiments, Data curation, Writing-Original draft preparation. BM: Animal experiments. MSV and IK: Laboratory analysis. LJP: Conceptualization, Writing-Reviewing and Editing Supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics Committee on Animal Use (CEUA) of the Universidade Federal de Lavras—UFLA under protocol number 082/17.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
de Sales Guilarducci, J., Marcelino, B.A.R., Konig, I.F.M. et al. Therapeutic effects of different doses of prebiotic (isolated from Saccharomyces cerevisiae) in comparison to n-3 supplement on glycemic control, lipid profiles and immunological response in diabetic rats. Diabetol Metab Syndr 12, 69 (2020). https://doi.org/10.1186/s13098-020-00576-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-020-00576-6