Abstract
Background
The relationship between environmentally transmitted tick parasites, Ixodes spp., and their main reproductive host, deer, is generally thought to be positive. However, measuring host abundance and density directly can be challenging and indirect methods are often used. The observed relationship between the parasite and host may be affected by sampling scale and season, which could lead to different inferences being made. Here, we aimed to test the effect of sampling scale and season on the relationship between density of deer and the density of questing Ixodes ricinus nymphs.
Methods
The density of deer (primarily Dama dama) was estimated using line transect distance sampling of deer dung quantified in different seasons (winter and summer) and measured at three different nested scales (site, transect and observation level). Questing nymph density was measured using blanket drag methods and estimates were calculated at the same scales as deer density estimates. General linear models were used to evaluate the relationship between questing nymphs, deer density and other environmental variables at each sampling scale and each season deer density was measured at.
Results
While a positive relationship between deer density and questing nymph density was detected at the site and transect scale, no relationship was apparent at the observation level. This was likely due to increased variation and reduced precision of deer dung counts at the finest sampling scale. Seasonal changes in deer populations were observed likely reflecting seasonal shifts in habitat usage. The summer estimates of deer density explained questing nymph density whereas winter estimates did not.
Conclusions
Our results show that the scale of sampling can affect the detectability of the positive association between host and vector species. Furthermore, such associations can be obscured if hosts exhibit seasonal changes in habitat use. Thus, both sampling scale and season are important to consider when investigating the relationship between host and vector species.

Similar content being viewed by others
Background
The distribution and abundance of parasites is expected to be highly dependent on host abundance, density and space use [1, 2]. In the Northern hemisphere Ixodes ricinus are vectors for multiple pathogens important for animal and human health such as Borrelia burgdorferi (sensu lato), the tick-borne encephalitis virus complex, Babesia spp. and Anaplasma phagocytophilum [3, 4]. The relationship between I. ricinus and their primary reproductive hosts, deer, is generally thought to be positive [5]. However, complexities due to multiple tick-host species and life stages of the vector may affect the presence or detectability of the relationship [6]. Furthermore, the challenge of collecting direct observational data on hosts means that information on host density and space use is rarely available and may need to be inferred from indirect observations [7].
Ixodes ricinus have three active life stages (larva, nymph and adult). Nymphs are considered epidemiologically most important for transmission of the emerging zoonotic pathogen B. burgdorferi (s.l.) [8, 9]. Understanding factors associated with increased nymph abundance is helpful to quantify disease risk and manage tick populations [10, 11]. Deer, as large mammals, are able to host a large number of adult ticks thereby providing opportunities for reproduction and expanding the size of the tick population [5, 12, 13]. However, there is no direct relationship between the reproductive success of adult ticks and the abundance of questing nymphs [14]. The abundance of nymphs is a result of both adult reproductive success and factors associated with larval survival. The main factors affecting larval survival are the availability of tick hosts to provide blood meals and environmental conditions which influence off-host survival [9, 11]. A range of host species including small mammals and ground foraging birds play an important role providing blood meals for larvae [15, 16]. Therefore, these other hosts and environmental factors can also influence the presence and strength of the relationship between the density of reproduction hosts (e.g. deer) and questing nymph density.
Previous studies investigating the relationship between deer and questing ticks, including nymphs, have used varying methods and scales to assess the role of deer (see Table 1). They either examined the effect of deer presence or absence (e.g. exclusion or culling [22, 26]) or measured deer density, using either direct (e.g. aerial surveys and census counts [32]) or indirect methods (e.g. surveys of deer signs or dung pellet counts [11, 33]). Sampling scale varied from local (1 m2–1 km2) to regional (1 km2–100 km2) with the relationship, if detected, varying in strength [13, 20, 22]. Generally, the effect of sampling scale is unclear. Measuring deer density at a large scale will show the overall distribution of deer over a large area. However, the spatial distribution of deer within that area will not be captured [36] and variation of other factors, such as different host species [37, 38] or even clustering of tick infestations [39], may influence whether the relationship is detectable at the smaller scale. Both from a theoretical and applied perspective, it is useful to understand at what scale the association between deer and ticks is most distinct.
The season at which deer density was estimated may also affect the observed relationship with tick density, as deer movement and habitat use may vary seasonally [34, 40]. Measuring deer density during spring and summer coincides with the tick questing period [41], but deer density sampled during winter may provide more accurate estimates due to lower vegetation density and easier detectability of dung piles [42]. However, if deer movement and habitat use changes seasonally, deer density measured in the winter may not be relevant to nymph density, even if winter estimates are more accurate. This is because deer will only encounter questing ticks in the summer and, if deer migrate seasonally, they will only provide blood meals and affect tick populations in their summer habitat. Therefore, to test for an association between deer density and nymph density, the sampling season for estimating deer density must also be taken into account.
Using a naturally fragmented landscape with discrete habitat patches we aimed to quantify the relationship between the density of deer (primarily fallow deer, Dama dama) and questing nymph density of I. ricinus across three hierarchical spatial scales: (i) observation level as the finest scale; (ii) transect level as a medium scale; and (iii) site level as the broadest scale. Secondly, using estimates of deer density measured in different seasons (winter and summer), we aimed to test the effect of deer sampling season on the observed relationship between deer density and questing nymph density.
Methods
Study area
This study was conducted in Loch Lomond and the Trossachs National Park, Scotland, UK (56° 5′ N, 4° 36′ W). Twelve islands on Loch Lomond and seven mainland sites around Loch Lomond were used for this study (study area = 120 km2; see Additional file 1: Figure S1). The sites were predominantly woodland and between 0.03–1.15 km2 in area, for further information see Millins et al. [43]. Fallow deer (D. dama) are known to commonly occur in the study area, whereas roe deer (Capreolus capreolus) and sika deer (Cervus nippon) were observed only once in a 2008 survey and are therefore expected to be rare in this area or present at low densities [43, 44]. Red deer (Cervus elaphus) are not present on the islands and have not been observed in the lowland mainland sites [43]. We therefore expected fallow deer to be the dominant deer species in the study area, although other species may be present at lower densities. Small mammal and bird communities on the islands are similar to those of surrounding woodlands [43, 45]. Livestock are present in areas surrounding mainland sites, and on one island site (sheep on Inchtavannach, TA, pers. obs.).
At each site, line transects were placed in north-south orientation. The western-most transect was placed randomly within the first 200 m of the site, and subsequent transects were placed at 200 m intervals ensuring even coverage of all sites (total transect length of 26.6 km). Twenty observation points were marked along the transects at equal intervals. The different scales investigated were: (i) observation level with estimates recorded at each sampling point; (ii) transect level with estimates calculated or averaged for each transect within each site; and (iii) site level with estimates calculated or averaged for each site.
Density of questing nymphs
The abundance of questing nymphs was estimated by collecting ticks during the peak questing period (May-July) in 2016. The blanket drag method provides an index of relative abundance of questing nymphs in the environment [10, 21]. Twenty 10-m2 blanket drags were conducted for each site corresponding to the observation points. Sampling was conducted once per site over a period of one to three days. A 1-m2 blanket was dragged across the vegetation for 10 m, within 5 m parallel to the line transect to avoid previously disturbed vegetation. Tick nymphs collected on the blanket were then counted and stored in 70% ethanol. Sampling was carried out when the vegetation was dry and between 9:00 and 16:00 h.
At the beginning and end of each drag, ground temperature and humidity were recorded (Hygro-Thermometer, ETI, Worthing, UK). Vegetation height, density and type were recorded at 3 intervals along the 10 m transect using a sward stick placed vertically in the vegetation and averaged [31]. Woodland type was determined as either mature oak and birch woodland (deciduous) or managed coniferous plantation (coniferous) [43].
Estimated density of deer
To estimate the density of deer, two line transect surveys of deer dung using distance sampling were conducted in January-March (winter) and May-July (summer) 2016, using a previously established methodology [42]. A single observer walked each line transect which was marked at each 50 m interval using the GPS position (eTrex 10, Garmin, Olathe, Kansas, USA) and biodegradable flagging tape. Each observation of deer dung and the distance to the transect from the centre of the pellet group was recorded. Deer dung was identified to species level (see Additional file 2: Table S1), all analyses used the total deer dung observations. The dung was marked with biodegradable tape to prevent double counts of the same observation at subsequent surveys. During the second survey, vegetation type, height and density were measured, as described above, to account for the effect of vegetation growth on detection probability of dung during the summer.
At the observation level, deer density could not be estimated, we therefore used the number of deer dung observations along the transect within 100 m2 of each blanket drag. The density of deer was estimated at both the transect level and the site level using Distance software (version 7.3) [46], using a defecation rate of 21.4 pellet groups per deer per day, as reported for fallow deer [47], and an estimated decay rate measured for this study [48] (see Additional file 3: Table S2, Figure S2). Deer density was estimated for both the winter and summer and the results from both surveys were combined to create an average deer density (see Additional file 2: Table S1). For site level, seasonal variation (winter, summer) and vegetation density (high and low) in the summer were accounted for by stratifying data and using different probability of detection functions to estimate deer density at each site. At the transect level, data were not sufficient to stratify by vegetation density so were combined to estimate average deer density on each transect.
Statistical analyses
All analyses were carried out using R software version 3.6.2 [49] and the lme4 package [50]. Three separate general linear models were used to test the effect of sampling scale on the observed relationship between nymph density and estimated deer density for each level: (i) observation; (ii) transect; and (iii) site.
For the observation level model, a generalized linear mixed effect model (GLMM) was used with log-transformed number of nymphs as the response variable. Deer density measured as the number of deer dung observations was included as a fixed effect and a nested random effect of site and transect was included.
For the transect level model, a GLMM was used with log transformed mean number of nymphs per 10 m2 as the response variable and site as a random effect. Estimated deer density calculated for each transect was included as a fixed effect.
For the site level model, a general linear model (GLM) was used with mean number of nymphs per 10 m2 as the response variable. Estimated deer density calculated for each site was included as a fixed effect. All three models also included temperature, humidity, vegetation type, vegetation height and density, woodland type, proximity to the mainland and location of site (island or mainland) as fixed effects. Two-way interaction terms between vegetation height and density, and temperature and humidity were included in each model.
To investigate the effect of host estimates from different seasons, a GLM was used with the mean number of questing nymphs per 10 m2 as the response variable, and estimated deer density in winter and summer as main effects. To investigate the effect of sampling season on deer density estimates, a GLM was used with estimated deer density as the response variable. Location of site (island or mainland) and season (winter or summer) and their interaction were included.
All models were simplified in a step-down approach, dropping the least significant term and comparing nested models using log likelihood ratio tests (LRT). All model residuals were checked for normality. To account for zeroes in the log-transformation of the response variable, a positive constant was added.
Results
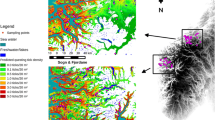
The mean number of questing nymphs measured was 0.79 nymphs per m2, ranging from 0.05 to 2.55 per m2. There was no difference between location of sites (mainland or island; Fig. 1c). Estimates were within the same range as elsewhere in Scotland (e.g. 0.1–1.6 nymphs per m2 [11]). The mean estimated deer density measured in the study area was 21.9 deer per km2, ranging from 3.1 to 53.4 deer per km2 (Fig. 1a; see Additional file 4: Table S3), similar to previous estimates of deer on the islands (24.6 deer per km2 [43]). Only 5% of the observed dung was identified as being from roe or sika deer, the remaining 95% were attributed to fallow deer (n = 2790/2962, see Additional file 2: Table S1). This supports our expectation that the fallow deer were the dominant deer species in the system.
Maps of the study area showing each site shaded to the measured density of deer at site level (a), deer density (per km2) at transect level (b), and questing nymph density (per 10 m2) at each sampling location (c). Inset map shows the location of the study area in Scotland, UK. Made in QGIS 2.14.20 [62]
Scale effect
At the observation level, the mean number of dung observations was 2.1, ranging from 0 to 13.5 observations. At this scale, the number of deer dung observations was not a significant predictor of the number of questing nymphs (LRT: χ2 = 0.645, df = 1, P = 0.422; Fig. 2c). The density of questing nymphs however was negatively associated with temperature (LRT: χ2 = 10.46, df = 1, P = 0.001). This model explained 57.2% of the variance of which 2.8% was explained by the fixed effect of temperature (\(R^{ 2}_{\text{c}}\)= 0.572, \(R^{ 2}_{\text{m}}\) = 0.028), the rest of which was accounted for by the nested random effect.
At the transect level, the mean estimated density of deer was 22.35 deer per km2, ranging from 0 to 70.54 deer per km2 (Fig. 1b; see Additional file 5: Table S4). At this scale, deer density was the only significant predictor of questing nymph density (LRT: χ2 = 7.84, df = 1, P = 0.005) with a positive predicted increase of 0.26 questing nymphs per increase in deer per km2 (Fig 2b). This model explained 74.2% of the variance with 9.8% explained by the fixed effect, deer density (\(R^{ 2}_{\text{c}}\) = 0.742, \(R^{ 2}_{\text{m}}\) = 0.098).
At the site level, a positive association between nymph and deer density was detected, with a predicted increase of 0.29 questing nymphs per increase in deer per km2 (LRT: χ2 = 152.09, df = 1, P < 0.02; Fig. 2a). The interaction between temperature and humidity (LRT: χ2 = 119.7, df = 1, P < 0.01) was significant with nymph density predicted to increase with higher temperatures and lower humidity. Height and density of vegetation (LRT: χ2 = − 1.12, df = 2, P = 0.049) also had a marginal effect, predicting tick density to be higher at lower vegetation density and height. The model fixed effects explained 82.8% of the variance (R2 = 0.83; Table 2).
Season effect
The winter survey estimates of deer density were not a significant predictor of questing nymph density (LRT: χ2 = 6.65, df = 1, P = 0.522; Fig. 3a). In contrast, the summer survey estimates were a strong positive predictor of questing nymph density (LRT: χ2 = 128.7, df = 1, P < 0.005; Fig. 3b; Table 3).
Estimates of deer density at site level were lower during the summer compared to the winter (Additional file 4: Table S3; LRT: χ2 = 9.71, df = 1, P = 0.0012), location of site (island or mainland) was not significant (LRT: χ2 = 385.6, df = 1, P = 0.14; Fig. 4a). The winter survey estimated a higher deer density at site level (mean = 27.29 deer per km2, SE = ± 13.51), than the summer survey (mean = 12.91 deer per km2, SE = ± 13.51). This difference was also detected at the transect level estimates (LRT: χ2 = 1633.3, df = 1, P < 0.001; Fig. 4b) and observation level deer dung counts (LRT: χ2 = 761.2, df = 1, P < 0.0001; Fig. 4c).
Discussion
When investigating the relationship between deer density and questing nymph density, studies have used varying sampling scales but the effect of sampling scale on the observed relationship is generally not considered. Furthermore, although host habitat use during summer is most relevant to seasonally active tick populations, vegetation growth can affect detection of dung used to estimate deer density and may bias estimates, thus winter estimates may be more accurate. In this study, we detected a positive relationship between fallow deer density and questing nymph density at the broadest spatial sampling scale (site level) and the middle scale (transect level), but not at the finest sampling scale used (observation level). While a positive effect between deer and tick density was detected in summer, this relationship was not detectable using winter estimates of deer density.
The finest sampling scale used counts of deer dung observations and questing nymphs on 10 m2 blanket drags and no relationship was observed. The majority of the variance explained was by the nested random effect suggesting that there is a high degree of variation between transects and sites which is not explained by any of the measured variables. This may explain why an association between deer and nymphs was not detected at this scale. It is also important to consider the methods used, as the counts of deer dung cover a smaller area and do not account for the probability of detection, defecation or decay rates [42]. Although more robust methods to measure deer density, such as distance sampling of deer dung, require more resources, such approaches are important to consider when planning field studies on the effect of deer density on tick populations [51]. Additionally, in cases where resources allow, additional complementary methods could be used to improve accuracy of estimates (e.g. camera trapping and dung counts [52]).
At the middle and broadest sampling scales in the current study (transect and site level), estimated deer density was a significant predictor of questing nymph density [5, 10, 26]. Deer density estimates were similar at both of these scales. However, the deer density estimates at the middle scale revealed variation in habitat usage within sites that was not visible when calculating estimates at the broadest scale. Highlighting the potential usefulness of using a smaller scale to understand spatial variation in deer density [53]. Identifying areas of higher deer use, which may lead to increased tick abundance, could be applied to target vector control strategies to specific areas [54]. Habitat usage of deer may vary on a local scale for a number of reasons such as availability of resources, anti-predator behaviour and reproductive behaviour [55, 56]. Therefore, understanding and accounting for this potential variation is important for measuring deer density.
When investigating the relationship between deer and nymphs, it may also be important to consider the species of deer present [44, 57]. Deer species may differ in habitat use, affecting encounters with ticks [21], or may differ in their role to host different tick life stages [58, 59]. Although it is not known whether different deer species may affect tick populations differently, they have been shown to vary in their association with tick-borne pathogens [60]. In the present study, fallow deer were the main deer species present. Roe and sika deer, if present, are expected to be at low densities in the study area [43] and therefore considered to make a minimal contribution as tick hosts ticks in this system. However, deer population structures may vary and, if they affect tick populations differently, may need to be considered separately in their role as a primary reproduction host.
Ticks are seasonally active, and the summer estimates of deer density made during the tick questing period (May-October [9]) significantly predicted the density of questing nymphs, whereas winter estimates did not. Seasonal differences in estimated deer density were observed between winter and summer which may be explained by seasonal movement of deer [40, 56]. Winter may be more favourable for accurately estimating deer density [42] but was less relevant for explaining questing nymph density because deer movement occurs seasonally. Encounter rate of the host and vector is reduced due to low or no tick activity in areas of deer use during the winter. It is therefore important to consider deer seasonal ranges, timing of deer movement and how this may interact with tick activity [34]. Furthermore, these patterns may shift with predicted climate change as the timing of tick activity and deer movement may shift due to changes in environmental conditions [31, 58].
Patterns of habitat use in fallow deer have been shown to be variable depending on habitat availability and season supporting these conclusions [56, 61]. We observed lower deer density estimates on mainland sites during summer, but only a small seasonal effect was observed on the islands. Vegetation height was accounted for in the analyses, but growth of vegetation during the summer may have affected the detectability of dung [42], explaining reduced observations of dung in the summer. Therefore, although summer vegetation may have had an effect, a seasonal change in deer space use is consistent with our findings [56].
In addition to understanding the spatial and seasonal effect, it is also important to consider longer temporal effects. The characteristics of the I. ricinus life-cycle means that a stronger relationship between deer and nymphs may be observed if a time lag is taken into consideration. As deer are important for feeding adult ticks, the length of time between successful adult feeding on deer and questing nymph activity may be important to quantify [9]. If deer density and space use are consistent over time, this time lag may not have a significant effect. However, as deer density is likely to vary with factors such as resource availability [56], there may be further complexity in the association between deer density and questing nymph density. If deer density changes, either naturally or by human intervention, the effect on ticks is more likely to be seen in larvae before it is observed in nymphs [23, 27]. Measuring how the relationship between deer density and larval and nymph density changes over time may improve understanding of the effect of these mechanisms.
Conclusions
This study provides empirical evidence that spatial scale and season affects the detectability and strength of the relationship between deer density and nymph density. While a positive relationship was found at two broader sampling scales, it was not detected at the finer spatial scale. The intermediate scale used in this study (i.e. transect level) detected within-site variation not measured at the broadest scale (i.e. site level). This study also highlights that winter estimates of deer density were not a useful predictor of questing nymph abundance, while summer estimates were. To optimise study design, it is also important to consider the effect of seasonal changes in host and vector distribution. Improving understanding of tick population drivers, including the role of different deer species and quantifying the time lag between host abundance and density of different tick life stages, will facilitate vector management strategies in a changing climate.
Availability of data and materials
Data supporting the conclusions of this article are included within the article. The datasets used and/or analysed during the present study are available from the corresponding author on reasonable request.
Abbreviations
- GLMM:
-
Generalized liner mixed effects model
- GLM:
-
General linear model
- LRT:
-
Likelihood ratio test
- SE:
-
Standard error
References
Cable J, Ellison AR, Pascoe EL, Barber I, Boag B, Morgan ER, et al. Global change, parasite transmission and disease control: lessons from ecology. Philos Trans R Soc B Biol Sci. 2017;372:20160088.
Kilpatrick AM, Randolph SE. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet. 2012;380:1946–55.
Dantas-Torres F, Chomel BB, Otranto D. Ticks and tick-borne diseases: a One Health perspective. Trends Parasitol. 2012;28:437–46.
Rizzoli A, Silaghi C, Obiegala A, Rudolf I, Hubálek Z, Földvári G, et al. Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: new hazards and relevance for public health. Front Public Health. 2014;2:1–26.
Hofmeester TR, Sprong H, Jansen PA, Prins HHT, Van Wieren SE. Deer presence rather than abundance determines the population density of the sheep tick, Ixodes ricinus, in Dutch forests. Parasit Vectors. 2017;10:433.
Johnson PTJ, Preston DL, Hoverman JT, LaFonte BE. Host and parasite diversity jointly control disease risk in complex communities. Proc Natl Acad Sci. 2013;110:16916–21.
Royle JA, Nichols JD, Kéry M, Ranta E, Kery M. Modelling occurrence and abundance of species when detection is imperfect. Oikos. 2013;110:353–9.
Wilhelmsson P, Lindblom P, Fryland L, Nyman D, Jaenson TG, Forsberg P, et al. Ixodes ricinus ticks removed from humans in northern Europe: seasonal pattern of infestation, attachment sites and duration of feeding. Parasit Vectors. 2013;6:362.
Földvári G. Life cycle and ecology of Ixodes ricinus: the roots of public health importance. In: Takken A, Sprong H, van Wieren SE, Braks AHM, editors. Ecology and prevention of Lyme borreliosis, vol. 4. Wageningen: Wageningen Academic Publishers; 2016. p. 31–40.
James MC, Bowman AS, Forbes KJ, Lewis F, Mcleod JE, Gilbert L. Environmental determinants of Ixodes ricinus ticks and the incidence of Borrelia burgdorferi sensu lato, the agent of Lyme borreliosis, in Scotland. Parasitology. 2013;140:237–46.
Millins C, Gilbert L, Johnson P, James M, Kilbride E, Birtles R, et al. Heterogeneity in the abundance and distribution of Ixodes ricinus and Borrelia burgdorferi (sensu lato) in Scotland: implications for risk prediction. Parasit Vectors. 2016;9:595.
Lane RS, Piesman J, Burgdorfer W. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu Rev Entomol. 1991;36:587–609.
Rand PW, Lubelczyk C, Lavigne GR, Elias S, Holman MS, Lacombe EH, et al. Deer density and the abundance of Ixodes scapularis (Acari: Ixodidae). J Med Entomol. 2003;40:179–84.
Kilpatrick AM, Dobson ADM, Levi T, Salkeld DJ, Swei A, Ginsberg HS, et al. Lyme disease ecology in a changing world: consensus, uncertainty and critical gaps for improving control. Philos Trans R Soc B Biol Sci. 2017;372:20160117.
van Duijvendijk G, Gort G, Takken W. Rodents as hosts for Ixodes ricinus and Borrelia afzelii. In: Takken A, Sprong H, van Wieren SE, Braks AHM, editors. Ecology and prevention of Lyme borreliosis, vol. 4. Wageningen: Wageningen Academic Publishers; 2016. p. 63–73.
Heylen D, Matthysen E, Fonville M, Sprong H. Songbirds as general transmitters but selective amplifiers of Borrelia burgdorferi sensu lato genotypes in Ixodes rinicus ticks. Environ Microbiol. 2014;16:2859–68.
Wilson ML, Telford SR III, Piesman J, Spielman A. Reduced abundance of immature Ixodes dammini (Acari, Ixodidae) following elimination of deer. J Med Entomol. 1988;25:224–8.
Daniels TJ, Fish D, Schwartz I. Reduced abundance of Ixodes scapularis (Acari: Ixodidae) and Lyme disease risk by deer exclusion. J Med Entomol. 1993;30:1043–9.
Rand PW, Lubelczyk C, Holman MS, Lacombe EH, Smith RP. Abundance of Ixodes scapularis (Acari: Ixodidae) after the complete removal of deer from an isolated offshore island, endemic for Lyme Disease. J Med Entomol. 2004;41:779–84.
Perkins SE, Cattadori IM, Tagliapietra V, Rizzoli AP, Hudson PJ. Localized deer absence leads to tick amplification. Ecology. 2006;87:1981–6.
Ruiz-Fons F, Gilbert L. The role of deer as vehicles to move ticks, Ixodes ricinus, between contrasting habitats. Int J Parasitol. 2010;40:1013–20.
Gilbert L, Maffey GL, Ramsay SL, Hester AJ. The effect of deer management on the abundance of Ixodes ricinus in Scotland. Ecol Appl. 2012;22:658–67.
Deblinger RD, Wilson ML, Rimmer DW, Spielman A. Reduced abundance of immature Ixodes dammini (Acari: Ixodidae) following incremental removal of deer. J Med Entomol. 1993;30:144–50.
Stafford KC, Denicola AJ, Kilpatrick HJ. Reduced abundance of Ixodes scapularis (Acari: Ixodidae) and the tick parasitoid Ixodiphagus hookeri (Hymenoptera: Encyrtidae) with reduction of white-tailed deer. J Med Entomol. 2003;40:642–52.
Jordan RA, Schulze TL, Jahn MB. Effects of reduced deer density on the abundance of Ixodes scapularis (Acari: Ixodidae) and Lyme disease incidence in a Northern New Jersey endemic area. J Med Entomol. 2007;44:752–7.
Kilpatrick HJ, Labonte AM, Stafford KC. The relationship between deer density, tick abundance, and human cases of Lyme disease in a residential community. J Med Entomol. 2014;51:777–84.
Wilson ML, Adlera GH, Spielman A. Correlation between abundance of deer and that of the deer tick, Ixodes dammini (Acari: Ixodidae). Ann Entomol Soc Am. 1985;78:172–6.
Wilson ML, Ducey AM, Litwin TS, Gavin TA, Spielman A. Microgeographic distribution of immature Ixodes dammini ticks correlated with that of deer. Med Vet Entomol. 1990;4:151–9.
Jordan RA, Schulze TL. Deer browsing and the distribution of Ixodes scapularis (Acari : Ixodidae) in central New Jersey forests. Environ Entomol. 2005;34:801–6.
Ostfeld RS, Canham CD, Oggenfuss K, Winchcombe RJ, Keesing F. Climate, deer, rodents, and acorns as determinants of variation in Lyme-disease risk. PLoS Biol. 2006;4:e145.
Gilbert L. Altitudinal patterns of tick and host abundance: a potential role for climate change in regulating tick-borne diseases? Oecologia. 2010;162:217–25.
Tagliapietra V, Rosà R, Arnoldi D, Jamecci F, Capelli G, Montarsi F, et al. Saturation deficit and deer density affect questing activity and local abundance of Ixodes ricinus (Acari, Ixodidae) in Italy. Vet Parasitol. 2011;183:114–24.
Cagnacci F, Bolzoni L, Rosà R, Rizzoli A. Effect of deer density on tick infestation of rodents and the hazard of tick-borne encephalitis II: population and infection models. Int J Parasitol. 2012;42:373–81.
Qviller L, Risnes-Olsen N, Bærum KM, Meisingset EL, Loe LE, Ytrehus B, et al. Landscape level variation in tick abundance relative to seasonal migration in red deer. PLoS One. 2013;8:e71299.
Werden L, Barker IK, Bowman J, Gonzales EK, Leighton PA, Lindsay LR, et al. Geography, deer, and host biodiversity shape the pattern of Lyme disease emergence in the Thousand Islands Archipelago of Ontario, Canada. PLoS ONE. 2014;9:e85640.
Kie JG, Terry Bowyer R, Nicholson MC, Boroski BB, Loft ER. Landscape heterogeneity at differing scales: effects on spatial distribution of mule deer. Ecology. 2002;83:530–44.
Jaenson TGT, Jaenson DGE, Eisen L, Petersson E, Lindgren E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasit Vectors. 2012;5:8.
Boyard C, Vourch G, Barnouin J. The relationships between Ixodes ricinus and small mammal species at the woodland-pasture interface. Exp Appl Acarol. 2008;44:61–76.
Kitron U, Jones CJ, Bouseman JK, Nelson JA, Baumgartner DL. Spatial analysis of the distribution of Ixodes dammini (Acari: Ixodidae) on white-tailed deer in Ogle County. Illinois. J Med Entomol. 1992;29:259–66.
Cagnacci F, Focardi S, Heurich M, Stache A, Hewison AJM, Morellet N, et al. Partial migration in roe deer: migratory and resident tactics are end points of a behavioural gradient determined by ecological factors. Oikos. 2011;120:1790–802.
Mannelli A, Bertolotti L, Gern L, Gray J. Ecology of Borrelia burgdorferi sensu lato in Europe: transmission dynamics in multi-host systems, influence of molecular processes and effects of climate change. FEMS Microbiol Rev. 2012;36:837–61.
Marques FFC, Buckland ST, Goffin D, Dixon CE, Borchers DL, Mayle BA, et al. Estimating deer abundance from line transect surveys of dung: sika deer in southern Scotland. J Appl Ecol. 2001;38:349–63.
Millins C, Dickinson ER, Isakovic P, Gilbert L, Wojciechowska A, Paterson V, et al. Landscape structure affects the prevalence and distribution of a tick-borne zoonotic pathogen. Parasit Vectors. 2018;11:621.
Ward AI. Expanding ranges of wild and feral deer in Great Britain. Mamm Rev. 2005;35:165–73.
Paterson V. Population dynamics of rodents and their parasite communities in a naturally fragmented landscape. PhD Thesis, University of Glasgow, Glasgow, UK; 2012.
Thomas L, Buckland ST, Rexstad EA, Laake JL, Strindberg S, Hedley SL, et al. Distance software: design and analysis of distance sampling surveys for estimating population size. J Appl Ecol. 2010;47:5–14.
Mayle B, Peace A, Gill R. How many deer? A guide to estimating deer population size. Forestry Commission field book. Edinburgh: Forestry Commission, 1999.
Laing SE, Buckland ST, Burns RW, Lambie D, Amphlett A. Dung and nest surveys: estimating decay rates. J Appl Ecol. 2003;40:1102–11.
R Core Development Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015.
Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2014;67:1.
Marcon A, Battocchio D, Apollonio M, Grignolio S. Assessing precision and requirements of three methods to estimate roe deer density. PLoS ONE. 2019;14:10.
Pfeffer SE, Spitzer R, Allen AM, Hofmeester TR, Ericsson G, Widemo F, et al. Pictures or pellets? Comparing camera trapping and dung counts as methods for estimating population densities of ungulates. Remote Sens Ecol Conserv. 2018;4:173–83.
Cargnelutti B, Reby D, Desneux L, Angibault J-M, Joachim J, Hewison AJM. Space use by roe deer in a fragmented landscape some preliminary results. Rev Ecol. 2002;57:29–37.
Piesman J, Eisen L. Prevention of tick-borne diseases. Annu Rev Entomol. 2008;53:323–43.
Bongi P, Ciuti S, Grignolio S, Del Frate M, Simi S, Gandelli D, et al. Anti-predator behaviour, space use and habitat selection in female roe deer during the fawning season in a wolf area. J Zool. 2008;276:242–51.
Thirgood SJ. The effects of sex, season and habitat availability on patterns of habitat use by fallow deer (Dama dama). J Zool. 1995;235:645–59.
Johnson BK, Kern JW, Wisdom MJ, Findholt SL, Kie JG. Resource selection and spatial separation of mule deer and elk during spring. J Wildl Manage. 2000;64:685.
Gray JS, Dautel H, Estrada-Peña A, Kahl O, Lindgren E. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip Perspect Infect Dis. 2009;2009:593232.
Mysterud A, Hatlegjerde IL, Sørensen OJ. Attachment site selection of life stages of Ixodes ricinus ticks on a main large host in Europe, the red deer (Cervus elaphus). Parasit Vectors. 2014;7:510.
Jaenson TGT, Petersson EH, Jaenson DGE, Kindberg J, Pettersson JHO, Hjertqvist M, et al. The importance of wildlife in the ecology and epidemiology of the TBE virus in Sweden: incidence of human TBE correlates with abundance of deer and hares. Parasit Vectors. 2018;11:477.
Singh NJ, Börger L, Dettki H, Bunnefeld N, Ericsson G. From migration to nomadism: Movement variability in a northern ungulate across its latitudinal range. Ecol Appl. 2012;22:2007–20.
QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2017. http://qgis.osgeo.org.
Acknowledgements
We thank the Scottish Centre for Ecology and the Natural Environment for an accommodation bursary (to ED) and field support throughout this study; Natasha Turner, Marissa Wong and Mairi Hilton for field assistance; Angus Lothian for field assistance and providing comments on the manuscript. We would also thank two anonymous reviewers for their constructive comments.
Funding
We are very grateful to the Glasgow Natural History Society who partially funded the study through the Blodwen Lloyd Bins Bequest.
Author information
Authors and Affiliations
Contributions
ED, RB and CM conceived the study design. ED conducted the data collection and statistical analysis. ED wrote the manuscript. All authors contributed to drafting the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Figure S1.
A map showing the study area of Loch Lomond, which is located in the south-west of Scotland as seen in the insert map. Dark grey areas denote each of the 12 island sites and 7 mainland sites used in the study, with their corresponding labels. The map was created in ArcGIS.
Additional file 2: Table S1.
Models estimating the density of fallow deer, with different key functions and series expansion terms, were tested [1]. The model outputs for the estimated deer density at the two scales (site and transect) and for summer, winter, and combined estimates. Site level estimates were post-stratified by each site, and transect level estimates were post-stratified by each transect at each site. Survey effort and the number of observations for each survey is reported. Estimated deer dung decay rates (winter = 85.60 ± 3.46 days, summer = 80.37 ± 3.13 days, combined = 80.20 ± 4.47 days) and defecation rate of 21.4 pellet groups per deer per day [2], were used. Fallow deer were used based on knowledge of the deer in the area [3] and identification of the dung [4]. Roe deer and Sika deer were recorded once in the study area in 2008 (Jimmy Irvine, Scottish Natural Heritage personal communication [3]), 5.8% of dung was visually identified as not being fallow deer (n = 172/2962). Defecation rates for these species are similar [1, 5], therefore would not lead to bias in estimated density if included. No observations of red deer dung were recorded. The Akaike’s information criterion (AIC) was compared and the model with the lowest difference (∆AIC) was selected. ƒ(0) is the probability detection function of the perpendicular distances. Pooled estimates of the density of individuals (D) in the study area are shown, upper and lower 95% confidence limits (LCL and UCL) and the percentage coefficient of variation (%CV).
Additional file 3: Table S2.
To measure the length of time to dung decay, a representative sample of fresh dung pellets were marked in December 2015 at the beginning of the first survey (n = 119), and for the second survey fresh dung pellets were marked in March 2016 prior to the survey (n = 658). At the end of both surveys, these sites were returned to and the age and proportion of surviving pellets that could be relocated was recorded. The mean time to decay was estimated using the proportion of pellets surviving the period of time from the beginning of the respective survey to the end which was then modelled as a function of age using logistic regression model as outlined by Laing et al. [1]. Decay rates were first calculated independently for each survey, as well as calculating overall decay rate by fitting a single model combining all data. Figure S2. Logistic regression predicting the time for dung to decay with both surveys combined.
Additional file 4: Table S3.
Estimated deer density (deer per km2) at each site during the winter and summer surveys, and the combined estimate of deer density, with estimated percentage coefficient of variation (%CV).
Additional file 5: Table S4.
Estimated deer density (deer per km2) at each transect at each site during the winter and summer surveys, and the combined estimate of deer density, with estimated percentage coefficient of variation (%CV).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dickinson, E.R., Millins, C. & Biek, R. Sampling scale and season influence the observed relationship between the density of deer and questing Ixodes ricinus nymphs. Parasites Vectors 13, 493 (2020). https://doi.org/10.1186/s13071-020-04369-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-020-04369-8