Abstract
Background
Trachoma is the leading infectious cause of blindness globally. The WHO has recommended the SAFE (Surgery, Antibiotics, Facial cleanliness and Environmental improvements) strategy to eliminate trachoma as a public health problem. The F and E arms of the strategy will likely be important for sustained disease reductions, yet more evidence is needed detailing relationships between hygiene, sanitation and trachoma in areas with differing endemicity. This study addressed whether the regional differences in water, sanitation, and hygiene (WASH) variables were associated with the spatial distribution of trachomatous inflammation-follicular (TF) among children aged 1 to 9 years in the Amhara National Regional State of Ethiopia.
Methods
Data from 152 multi-stage cluster random trachoma surveys were used to understand the degree of clustering of trachoma on two spatial scales (district and village) in Amhara using a geographical information system and the Getis-Ord Gi* (d) statistic for local clustering. Trained and certified graders examined children for the clinical signs of trachoma using the WHO simplified system. Socio-demographic, community, and geoclimatic factors thought to promote the clustering of the disease were included as covariates in a logistic regression model.
Results
The mean district prevalence of TF among children aged 1 to 9 years in Amhara was 25.1% (standard deviation = 16.2%). The spatial distribution of TF was found to exhibit global spatial dependency with neighboring evaluation units at both district and village level. Specific clusters of high TF were identified at both the district and the village scale of analysis using weighted estimates of the prevalence of the disease. Increased prevalence of children without nasal and ocular discharge as well as increased prevalence of households with access to a water source within 30 minutes were statistically significantly negatively associated with clusters of high TF prevalence.
Conclusions
Water access and facial cleanliness were important factors in the clustering of trachoma within this hyperendemic region. Intensified promotion of structural and behavioral interventions to increase WASH coverage may be necessary to eliminate trachoma as a public health problem in Amhara and perhaps other hyper-endemic settings.
Similar content being viewed by others
Background
Trachoma is caused by repeated ocular infections with the bacterium Chlamydia trachomatis and is the leading infectious cause of blindness globally [1, 2]. Since 1998, the World Health Organization (WHO) and the Global Alliance to Eliminate Blinding Trachoma by 2020 (GET 2020) have relied on a multifaceted strategy to progress towards elimination of trachoma as a public health problem. This strategy, known by the acronym SAFE, relies on surgery to correct trachomatous trichiasis, antibiotics to treat infection, the promotion of facial cleanliness, and environmental improvements to interrupt transmission and reduce the suitability of the habitat for the physical vector Musca sorbens [3, 4].
Amhara National Regional State, Ethiopia is home to approximately 20 million people and many administrative districts (known locally as woredas) are endemic for trachoma [5]. Based on baseline prevalence data, the SAFE strategy was scaled up throughout all of Amhara starting in 2007 [6]. Population-based trachoma impact surveys, conducted following approximately 3 to 5 years of SAFE, demonstrated a decrease in trachomatous inflammation-follicular (TF) among children aged 1 to 9 years [5, 7]. However, this was not the case in all districts [8]. Moreover, TF prevalence appeared to be spatially heterogeneous post-SAFE, with progress observed in some districts while others remained hyperendemic. This analysis used cross-sectional survey data to describe the spatial clustering of TF among children aged 1 to 9 years in Amhara at two important aggregate spatial scales, the district and the village (known locally as gotts). Further analysis aimed to identify the environmental, household and behavioral factors associated with a high burden of TF at each scale.
Methods
Survey methods
Multi-level cluster-random surveys were conducted with consistent methodology in all 152 districts of Amhara between 2011 and 2016. The survey methods used for sampling, grader training, electronic data capture, and many variable definitions have been described elsewhere [5, 8,9,10,11]. Briefly, within each district, the village was the primary sampling unit and a compact segment of approximately 30 to 40 households within the village was the secondary sampling unit [5, 12]. The cumulative number of previous rounds of Mass Drug Administration (MDA) of antibiotics within each district prior to its impact survey (as reported annually to the International Trachoma Initiative) was applied to each village surveyed within the applicable district (range: 3–11 rounds) [13]. The presence of a health care facility and the presence of a paved road in the village were directly observed by the survey team.
The surveys captured household data related to hygiene and sanitation by interviewing an adult household member, most often the head female, in Amharic. Definitions for household variables not discussed here are consistent with previously published trachoma impact survey data as well as the WHO Joint Monitoring Programme guidelines where applicable [8, 14]. Data collected pertaining to latrine use were not available for all surveys and were excluded from the analysis. A dichotomous variable represented the highest education any adult in the household had completed as any formal education or none. Household crowding was defined as greater than 5 persons per household regardless of the physical size of the household [15].
After interviewing the head of household, recorders enumerated all living household members along with their age and sex. Conjunctival examinations were then performed on present household members to estimate the prevalence of all 5 signs of trachoma based on the WHO simplified trachoma grading system [16]. During this examination process, facial cleanliness among children aged 1–9 years was directly observed by the grader and was defined as the absence of any nasal and ocular discharge on the observed child’s face.
Data analysis
Survey weights were calculated using the inverse of the 2-stage joint sampling probability [8].
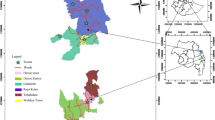
Estimates for prevalence were produced using svy commands in STATA version 14.0 (STATA Corporation, College Station TX, USA). Weighted estimates of district and village level TF prevalence among children aged 1 to 9 years were mapped using an Adindan projection for Universal Transverse Mercator zone 37 North in ArcMap version 10.4.1 (ESRI, Redlands, CA, USA). Village point location data was calculated in decimal degrees by averaging the latitude and longitude values of all participating households within the village. Likewise, for each district the geographic centroid was calculated based on the GPS locations of selected villages.
We performed a series of spatial analyses, beginning with global assessments of spatial autocorrelation and continuing to tests of local variation in the observed autocorrelation to assess potential spatial influence between neighboring evaluation units (districts or villages) [17]. A global Moran’s I (implemented via ArcMap) suggested an overall pattern of positive spatial autocorrelation in TF prevalence and identified the average spatial scale of clustering. Specifically, the global analysis suggested that spatial neighbors defined via a 25-km fixed distance band yielded the peak in overall spatial autocorrelation between villages (where the average nearest neighbor distance between villages was 4.84 km, standard deviation 3.26 km).
Next, utilizing GeoDa version 1.8 (Luc Anselin, Chicago, IL, USA), we defined connectivity maps at the district scale, where we found a neighborhood consisting of the 8 nearest neighbors best captured the observed spatial relationships. We defined a spatial weights matrix corresponding to this neighborhood definition and used this throughout the remainder of our analyses.
Moving from global to local estimates, we next used the Getis-Ord Gi* (d) (via ArcMap) local statistic to identify high prevalence clusters (“hotspots”) among the 152 districts and 1558 villages in the analysis. At the two spatial scales, we calculated the Getis-Ord Gi* (d) statistic using the survey-weighted prevalence estimates for each feature, and the spatial weights matrix for each spatial scale defined above. To reduce the potential for false positive results, we used a false discovery rate adjustment to account for multiple testing [17]. Hotspots were identified with 90% confidence for districts and 95% confidence for villages and coded as a new dichotomous variable that was used as the outcome in logistic regression models. The significance value (P-value) associated with the local Getis-Ord Gi* (d) test statistics served as a measure of the unusualness of potential local hotspots of prevalence. The significance threshold for district hotspots was adjusted to 0.1 in order for the number of clusters to be large enough to perform a standard maximum likelihood based logistic regression. This allowed us to perform similar analysis on the districts and villages to enhance methodological comparison between spatial scales. All districts and villages identified with significantly low local prevalence (coldspots) were grouped into a non-hotspot category to serve as the comparator group.
For variables collected as part of the survey, the weighted estimates corresponding to the spatial scale being analyzed, district and village were used as the covariates of interest. To explore the potential impact of climate variables, we imported raster surfaces for average annual temperature, average annual precipitation, and altitude from BioClim Global Climate Datasets [18]. We summarized data for each district by extracting the average point values from that district, and the geographic centroid for each village served as the location for the village-level extracted geoclimatic variables.
To complete our analyses, two separate regression analyses were performed to assess the factors contributing to residence in a hotspot at the district and village levels. Variables were considered significant to incorporate in the model selection process if the P-value was < 0.05. Collinearity was assessed prior to model selection using a condition index greater than 30 and a variable decomposition factor greater than 0.5 as threshold values [19]. All water, sanitation, and hygiene (WASH) variables were included in the model until covariates unrelated to WASH were assessed for inclusion in the model. Manual backwards stepwise selection assessed the model’s performance after exclusion of different sets of independent variables from the model. Model fit was assessed with the Akaikeʼs information criterion (AIC) where the best-fit model was selected as exhibiting the lowest AIC. The final model fit was again examined with different combinations of the exposure variables to select a high-performance model. The final models were assessed for discriminatory performance using a receiver operator curve (ROC) and the final model’s fit to the data was examined using the Hosmer-Lemeshow test.
Results
Between 2011 and 2016, field teams surveyed 1558 villages in all 152 districts of Amhara, enumerating 282,400 individuals of whom 202,312 (71.6%) were examined for clinical signs of trachoma. Among all individuals enumerated, 75,144 were children aged 1 to 9 years and 69,236 (92.1%) of these children were examined for clinical signs of trachoma.
Cluster analysis performed using the Getis-Ord Gi* (d) identified 12 districts (7.9%) as statistically significant hotspots of TF prevalence among children aged 1 to 9 years (Fig. 1). Access to water in less than 30 minutes, access to an improved water source, and mean number of household items owned were negatively associated with district-level hotspots in univariate analysis (Table 1). Furthermore, the percent of villages with a paved road and the average annual precipitation in the districts both had significant negative univariate associations with hotspots. When comparing the distribution of clean face prevalence estimates among districts, the mean clean face prevalence was lower for the TF hotspot districts than for non-hotspot districts (Fig. 2). In the logistic model, clean face was identified to be a statistically significant (odds ratio (OR): 0.91, 95% CI: 0.86–0.96) individual level (negative) predictor. The best-fit district-level multivariate model (model 6, Table 2) included the predictors: clean face (adjusted odds ratio (AOR): 0.90; 95% CI: 0.83–0.97; per 1% prevalence increase), access to water within 30 minutes (AOR: 0.95, 95% CI: 0.91–0.99; per 1% increase), household access to an improved water source (AOR: 0.96; 95% CI: 0.92–, 1.00; per 1% increase) and presence of a health facility in the village (AOR: 0.99; 95% CI: 0.94–1.10; per 1% increase), (AIC = 48.87). This model had good fit (Hosmer-Lemeshow Test = 2.28, P = 0.97) and exhibited good discriminatory power from the ROC curve generated for the model, area under the curve, AUC = 0.94.
Of the 1558 surveyed villages, 325 (20.9%) were identified as having an unusually high prevalence of TF among children aged 1 to 9 years compared to the global average (Fig. 3). The selected multivariate model for explanatory factors in village hotspots (model 20, Table 3) identified clean face, mean count of household items, previous rounds of MDA within the district, average annual precipitation, and average annual temperature as significant explanatory variables. Although not significant, access to water in less than 30 minutes, formal education among household interviewees, and the presence of a paved road were retained in the model as they increased model performance. The model had good fit (Hosmer-Lemeshow Test = 9.68, P = 0.29) and exhibited good discriminatory power from the ROC curve generated for the model (AUC = 0.78).
Discussion
After SAFE interventions, the distribution of TF among children aged 1 to 9 years in Amhara remained spatially clustered at both the district and the village spatial scales. Hotspots of TF clustering overlapped at both spatial scales in the northeast part of the region. Household access to water was a statistically significant predictor of TF clustering at the district level, and directly observed clean face among children aged 1 to 9 years was a significant predictor at both spatial scales. This suggests that hygiene and water availability could contribute in important ways to the spatial pattern of trachoma throughout Amhara. Along with continuing to provide annual MDA, a continued focus on F and E interventions are likely needed to sustainably eliminate trachoma as a public health problem in the most affected areas of the region.
Starting in 2007, the Trachoma Control Programme in Amhara has scaled up the SAFE strategy to all districts in the region. At scale, the Programme annually delivers approximately 16 million doses of antibiotics, provides health education on face washing and hygiene to approximately 3400 villages and 8000 schools, and assists in the construction of approximately 380,000 latrines [8]. Accordingly, previous reports have demonstrated that increases in household latrines have been observed regionally [8]. However, recently published reports have demonstrated that much of the region still has hyperendemic levels of TF and a considerable burden of infection with Chlamydia trachomatis, and thus many more years of SAFE interventions are likely needed [8, 11]. This report better details the pattern and clustering of TF in a post-SAFE setting from a large programme at scale. The identified hotspots may represent areas of the region where the SAFE strategy has yet to be effective. These data will allow the programme to focus on these hotspots to ensure that high MDA coverage is being achieved, and to ensure that F and E efforts are intensified. It is likely that these areas will be the last in the region to eliminate trachoma as a public health problem [13].
The prevalence of children with a clean face was a robust correlate of residence in a trachoma hotspot. Mechanistically, nasal and ocular discharge on children’s faces can serve as a reservoir of infection and can lead to disease transmission [20, 21]. Previous research has shown that poor facial cleanliness including ocular and nasal discharge is consistently associated with both trachoma clinical signs and infection in children [22, 23]. However, of the few published randomized trials focused on facial cleanliness, only one demonstrated a reduction in severe inflammatory trachoma after a face washing intervention [24, 25]. It has been demonstrated over the years that graders in the field can reliably grade clean face, but it has also been shown that clean face does not always predict whether a face has been recently washed [21, 26, 27]. This current study illustrates that once this indicator was aggregated to the more programmatically relevant village or district level in Amhara, clean face was a consistent correlate of TF burden. Although the role of clean face as a trachoma indicator will continue to be debated, transmission reduction through improving hygiene should remain a key component of the SAFE strategy. Promoting knowledge about hygiene behavior is likely not sufficient, rather distinct F and E interventions must be designed with a grounding in behavior change theory, and adapted to each community with local support to create lasting behavioral change [28, 29]. Geographically targeting hotspots would allow programmes to more efficiently deploy these resource intensive projects.
Beyond clean face, several other WASH variables were associated with TF hotspots in these analyses. Increased access to water within 30 minutes was significantly negatively associated with residence in a trachoma hotspot district when controlling for other variables in the model, supporting some prior research [15, 30, 31] but not all [22, 32], that water access is an important predictor of trachoma. The survey question did not enable the respondent to relate whether the water supply was consistently available. Directly observed household water quantity and its allocation for hygiene may be a better health indicator in future studies [33]. Access to an improved water source was not a significant predictor of residence in a hotspot district or hotspot village. It may be possible that the type of water source used for hygiene behaviors is not as important as having access to water, or as important as how that water is used in the household [33, 34]. Household access to a latrine was not a significant predictor of hotspots at the geographical scales considered in our analysis, although previous work has shown links between the presence of sanitation and trachoma [22]. In a previous report from this region, a significantly lower odds of trachoma were found in communities with ≥ 80% community latrine use [35]. These results may have differed from ours due to the different latrine definitions used (presence vs use) and the fact that our analysis focused solely on trachoma hotspots. In a 13-country cross-sectional analysis, high community coverage of improved sanitation was associated with lower TF. However, in a sensitivity analysis with any latrine coverage as the exposure, a definition more comparable to ours, a statistically significant association was not observed [36]. The observation of the presence or absence of a latrine without accounting for usage may obscure the exposure/disease relationship and should be further accounted for in future research linking sanitation and trachoma [37, 38].
The quantitative analysis of spatial patterns is a valuable tool to comprehend the complex interrelationships inherent within human populations. Regional differences in psychosocial, contextual, and technological barriers may inhibit habituation of hygiene behaviors and the ability to create a sustained impact on trachoma. The identification of clusters is dependent on the spatial scale analyzed. The differences in the observed percentage of villages compared to the observed percentage of district hotspots could be due to a stronger influence of factors promoting clustering at finer geographical resolution, or the spatial effects might be masked as a result of the modifiable areal unit problem when observing the hotspot results from the district analysis [39]. Surveys were designed to provide district-level estimates, therefore there may have been more variability in the village-level analysis which may have led to different results at different spatial scales. We observed, for example both hotspot and non-hotspot villages within the same district. Although impact surveys are designed to account for this village-level variability, it is possible that high-prevalence subpopulations could be missed by district-level surveys causing problems for control programmes. Interpreting individual village data from district-level surveys should be done with caution however, as it is expected that even districts reaching elimination may contain villages with elevated levels of C. trachomatis infection as part of a naturally occurring distribution [40].
This study used cross-sectional data and therefore causal links between SAFE interventions and trachoma prevalence could not be determined. Since pre-SAFE baseline surveys were not conducted at the district level, it was not possible to conduct longitudinal analyses. Future studies using repeat impact survey data will be needed to better understand the contributions of these interventions. All multivariate models at the village-level showed that more rounds of MDA were associated with higher likelihood of a village being a TF hotspot. Many of those hotspot villages were located in districts which were among the first enrolled in the SAFE programme, and which had high levels of TF initially [7]. The high trachoma prevalence in these districts would have warranted more MDA rounds as per the WHO guidelines and thus this result may have been due to reverse causality. This analysis also depended on the outcome TF, a clinical sign which has been shown to not correspond well to C. trachomatis infection in a post-SAFE setting [41]. Despite this, study teams did include certified trachoma graders who underwent the same training and were required to pass a field reliability exam before each survey round. Lastly, the examination of relationships between WASH variables and TF was a secondary aim of these surveys, and the large size of the dataset may have allowed for detecting statistically significant relationships despite small effect sizes. Although cross-sectional modeling such as this is a common practice in the trachoma literature, there are more robust ways to test these relationships such as a WASH trial that is currently underway in some of the most challenging districts of the region [42].
Conclusions
The Trachoma Programme in Amhara has made progress by reducing the prevalence of the disease in many areas since fully implementing the SAFE strategy; however, the distribution of trachoma was not spatially random. This study used data from 69,236 children aged 1 to 9 years across the entire region of Amhara to evaluate spatial relationships at a large-scale, between districts, and then further compared the results to a finer-scale, village-level analysis. Important WASH variables were correlates of trachoma hotspots in this region including facial cleanliness and water access. Spatial analysis is a powerful tool to identify geographical areas in greatest need of intervention and can help to reduce operational costs by targeting the most appropriate interventions locally. Directing F and E interventions to areas with the most severe trachoma will likely accelerate the elimination of trachoma as a public health problem.
Availability of data and materials
The complete dataset analyzed in this paper is not publicly available in its entirety due to ongoing analyses by other authors covering separate but related topics. Datasets required to reproduce analyses and results presented in this study are available from the corresponding author upon reasonable request.
Abbreviations
- AIC:
-
Akaike information criterion
- AOR:
-
adjusted odds ratio
- AUC:
-
area under the curve
- CI:
-
confidence interval
- GET2020:
-
Global Alliance to Eliminate Blinding Trachoma by 2020
- GPS:
-
global positioning system
- IRB:
-
internal review board
- OR:
-
odds ratio
- MDA:
-
mass drug administration
- ROC:
-
receiver operating characteristics
- SAFE:
-
surgery, antibiotics, facial cleanliness, and environmental improvement
- TF:
-
trachomatous inflammation-follicular
- WASH:
-
water sanitation and hygiene
- WHO:
-
World Health Organization
References
Mariotti SP, Pascolini D, Rose-Nussbaumer J. Trachoma: global magnitude of a preventable cause of blindness. Br J Ophthalmol. 2009;93:563–8.
Gambhir M, Basanez MG, Burton MJ, Solomon AW, Bailey RL, Holland MJ, et al. The development of an age-structured model for trachoma transmission dynamics, pathogenesis and control. PLoS Negl Trop Dis. 2009;3:e462.
Emerson P, Bailey R, Olaimatu M, Walraven G, Lindsay S. Transmission ecology of the fly Musca sorbens, a putative vector of trachoma. Trans R Soc Trop Med Hyg. 2000;94:28–32.
WHO. Report of the 3rd global scientific meeting on trachoma, Baltimore, MD. Geneva: World Health Organization; 2010.
King JD, Teferi T, Cromwell EA, Zerihun M, Ngondi JM, Damte M, et al. Prevalence of trachoma at sub-district level in Ethiopia: determining when to stop mass azithromycin distribution. PLoS Negl Trop Dis. 2014;8:e2732.
Emerson PM, Ngondi J, Biru E, Graves PM, Ejigsemahu Y, Gebre T, et al. Integrating an NTD with one of “the big three”: combined malaria and trachoma survey in Amhara Region of Ethiopia. PLoS Negl Trop Dis. 2008;2:e197.
Ngondi J, Gebre T, Shargie EB, Adamu L, Ejigsemahu Y, Teferi T, et al. Evaluation of three years of the SAFE strategy (Surgery, Antibiotics, Facial cleanliness and Environmental improvement) for trachoma control in five districts of Ethiopia hyperendemic for trachoma. Trans R Soc Trop Med Hyg. 2009;103:1001–10.
Stewart AEP, Zerihun M, Gessese D, Melak B, Sata E, Nute AW, et al. Progress to eliminate trachoma as a public health problem in Amhara National Regional State, Ethiopia: results of 152 population-based surveys. Am J Trop Med Hyg. 2019. https://doi.org/10.4269/ajtmh.19-0450.
King JD, Buolamwini J, Cromwell EA, Panfel A, Teferi T, Zerihun M, et al. A novel electronic data collection system for large-scale surveys of neglected tropical diseases. PLoS ONE. 2013;8:e74570.
Oswald WE, Stewart AE, Kramer MR, Endeshaw T, Zerihun M, Melak B, et al. Association of community sanitation usage with soil-transmitted helminth infections among school-aged children in Amhara Region, Ethiopia. Parasites Vectors. 2017;10:91.
Nash SD, Stewart AEP, Zerihun M, Sata E, Gessese D, Melaku B, et al. Ocular Chlamydia trachomatis infection under the SAFE strategy in Amhara, Ethiopia, 2011–2015. Clin Infect Dis. 2018;67:1840–6.
Turner AG, Magnani RJ, Shuaib M. A not quite as quick but much cleaner alternative to the expanded programme on immunization (EPI) cluster survey design. Int J Epidemiol. 1996;25:198–203.
Jimenez V, Gelderblom HC, Mann Flueckiger R, Emerson PM, Haddad D. Mass drug administration for trachoma: how long is not long enough? PLoS Negl Trop Dis. 2015;9:e0003610.
WHO/UNICEF. Improved and unimproved water sources and sanitation facilities. Joint monitoring programme for water supply, sanitation and hygiene (JMP). Geneva: World Health Organization; 2016.
Bero B, Macleod C, Alemayehu W, Gadisa S, Abajobir A, Adamu Y, et al. Prevalence of and risk factors for trachoma in Oromia Regional State of Ethiopia: results of 79 population-based prevalence surveys conducted with the global trachoma mapping project. Ophthalmic Epidemiol. 2016;23:392–405.
Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bull World Health Organ. 1987;65:477–83.
Getis A, Ord JK. The analysis of spatial association by use of distance statistics. Geogr Anal. 1992;24:189–206.
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–78.
David K, Mitchel K. Logistic regression: a self learning text. New York: Springer-Verlag Inc; 1994.
Gower EW, Solomon AW, Burton MJ, Aguirre A, Munoz B, Bailey R, et al. Chlamydial positivity of nasal discharge at baseline is associated with ocular chlamydial positivity 2 months following azithromycin treatment. Investig Ophthalmol Vis Sci. 2006;47:4767–71.
King JD, Ngondi J, Kasten J, Diallo MO, Zhu H, Cromwell EA, et al. Randomised trial of face-washing to develop a standard definition of a clean face for monitoring trachoma control programmes. Trans R Soc Trop Med Hyg. 2011;105:7–16.
Stocks ME, Ogden S, Haddad D, Addiss DG, McGuire C, Freeman MC. Effect of water, sanitation, and hygiene on the prevention of trachoma: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001605.
Taylor HR, Burton MJ, Haddad D, West S, Wright H. Trachoma. Lancet. 2014;384:2142–52.
West S, Munoz B, Lynch M, Kayongoya A, Chilangwa Z, Mmbaga B, et al. Impact of face-washing on trachoma in Kongwa, Tanzania. Lancet. 1995;345:155–8.
Ejere HO, Alhassan MB, Rabiu M. Face washing promotion for preventing active trachoma. Cochrane Database Syst Rev. 2015;4:CD003659.
West SK, Congdon N, Katala S, Mele L. Facial cleanliness and risk of trachoma in families. Arch Ophthalmol. 1991;109:855–7.
West SK, Ansah D, Munoz B, Funga N, Mkocha H. The, “F” in SAFE: reliability of assessing clean faces for trachoma control in the field. PLoS Negl Trop Dis. 2017;11:e0006019.
Delea MG, Solomon H, Solomon AW, Freeman MC. Interventions to maximize facial cleanliness and achieve environmental improvement for trachoma elimination: a review of the grey literature. PLoS Negl Trop Dis. 2018;12:e0006178.
Dodson S, Heggen A, Solomon AW, Sarah V, Woods G, Wohlgemuth L. Behavioural change interventions for sustained trachoma elimination. Bull World Health Organ. 2018;96:723–5.
Hsieh YH, Bobo LD, Quinn TO, West SK. Risk factors for trachoma: 6-year follow-up of children aged 1 and 2 years. Am J Epidemiol. 2000;152:204–11.
Muhammad N, Mohammed A, Isiyaku S, Adamu MD, Gwom A, Rabiu MM. Mapping trachoma in 25 local government areas of Sokoto and Kebbi states, northwestern Nigeria. Br J Ophthalmol. 2014;98:432–7.
Ngondi J, Gebre T, Shargie EB, Adamu L, Teferi T, Zerihun M, et al. Estimation of effects of community intervention with antibiotics, facial cleanliness, and environmental improvement (A, F, E) in five districts of Ethiopia hyperendemic for trachoma. Br J Ophthalmol. 2010;94:278–81.
Stelmach RD, Clasen T. Household water quantity and health: a systematic review. Int J Environ Res Public Health. 2015;12:5954–74.
Polack S, Kuper H, Solomon AW, Massae PA, Abuelo C, Cameron E, et al. The relationship between prevalence of active trachoma, water availability and its use in a Tanzanian village. Trans R Soc Trop Med Hyg. 2006;100:1075–83.
Oswald WE, Stewart AE, Kramer MR, Endeshaw T, Zerihun M, Melak B, et al. Active trachoma and community use of sanitation, Ethiopia. Bull World Health Organ. 2017;95:250–60.
Garn JV, Boisson S, Willis R, Bakhtiari A, Al-Khatib T, Amer K, et al. Sanitation and water supply coverage thresholds associated with active trachoma: modeling cross-sectional data from 13 countries. PLoS Negl Trop Dis. 2018;12:e0006110.
Bracken P, Kvarnström E, Ysunza A, Kärrman E, Finnson A, Saywell D. Making sustainable choices—the development and use of sustainability oriented criteria in sanitary decision making. In: Third international conference on ecological sanitation, Durban, South Africa, 2005. Waterfund.go.ke
Beyene A, Hailu T, Faris K, Kloos H. Current state and trends of access to sanitation in Ethiopia and the need to revise indicators to monitor progress in the post-2015 era. BMC Public Health. 2015;15:451.
Waller L, Gotway C. Applied spatial statistics for public health data. Hoboken: Wiley; 2004.
Lietman TM, Gebre T, Abdou A, Alemayehu W, Emerson P, Blumberg S, et al. The distribution of the prevalence of ocular chlamydial infection in communities where trachoma is disappearing. Epidemics. 2015;11:85–91.
Ramadhani AM, Derrick T, Macleod D, Holland MJ, Burton MJ. The relationship between active trachoma and ocular Chlamydia trachomatis infection before and after mass antibiotic treatment. PLoS Negl Trop Dis. 2016;10:e0005080.
Aiemjoy K, Aragie S, Gebresillasie S, Fry DM, Dagnew A, Hailu D, et al. Defining diarrhea: a population-based validation study of caregiver-reported stool consistency in the Amhara Region of Ethiopia. Am J Trop Med Hyg. 2018;98:1013–20.
Acknowledgements
We are grateful to all study participants who volunteered their time to participate in the surveys. We also thank the dedicated field teams, drivers and supervisors for their work on these surveys.
Funding
Funding for the survey in summer 2013 was provided by USAID’s ENVISION project, managed by RTI International. The Trachoma Control Programme is a collaboration between the Amhara National Regional State Health Bureau and the Lions-Carter Center Sightfirst Initiative.
Author information
Authors and Affiliations
Contributions
Conception and design: FMA, AWN, ES, AEPS, EKC, ZT and SDN. Acquisition of data; MZ, ES, AEPS, DG, BM, TA, GA, EKC, MC, BG, ZT and SDN. Data curation and management: FMA, TA, AWN and SDN. Analysis and interpretation: FMA, AWN, ES, AEPS, TA, EKC, LAW, ZT and SDN. Manuscript writing: FMA, AWN, AEPS, EKC, LAW and SDN. Manuscript revision: FMA, AWN, MZ, ES, AEPS, DG, BM, TA, GA, EKC, MC, BG, LAW, ZT and SDN. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was reviewed by the Institutional Review Board of Emory University (Study IRB #079-2006) and the Amhara National Regional State Health Bureau. Consideration was given to the low literacy rate in the study region, and it was determined that a verbal informed consent should be obtained from study participants prior to data collection. Following the guidelines outlined by the Declaration of Helsinki, informed consent for individuals and parents of children participating in the study were recorded.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Altherr, F.M., Nute, A.W., Zerihun, M. et al. Associations between Water, Sanitation and Hygiene (WASH) and trachoma clustering at aggregate spatial scales, Amhara, Ethiopia. Parasites Vectors 12, 540 (2019). https://doi.org/10.1186/s13071-019-3790-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-019-3790-3