Abstract
Background
The present study evaluated the therapeutic effectiveness of moxidectin 1.0% (w/v) and imidacloprid 10% (w/v) (Advocate® spot-on solution for cats, Bayer Animal Health) against natural infections with the eyeworm Thelazia callipaeda in cats. This study was conducted as a GCP, negative-controlled, blinded and randomised field study in privately owned cats living in an area in southern Italy where T. callipaeda is enzootic.
Methods
The study was carried out in 30 cats (19 females and 11 males, aged from 8 months to 5 years, weighing 1.2–5.2 kg) of different breeds, naturally infected by T. callipaeda. At study inclusion (Day 0), animals were physically examined and the infection level was assessed by examination of both eyes for clinical score and live adult T. callipaeda count. Each cat was weighed and randomly assigned to one of the treatment groups (G1: Advocate, G2: untreated control). Clinical assessments and T. callipaeda adult counts were performed on Day 14. At the study completion visit on Day 28, clinical assessments and counts of T. callipaeda adults and larvae were performed. All cats were daily observed by their owners and general health conditions were recorded during the entire period of the study.
Results
The primary effectiveness variable was the percentage of animals in G1 group (Advocate) showing a complete elimination (parasitological cure) of adult eye worms at Day 14 and Day 28 . The effectiveness of the treatment in the G1 group was 93.3 and 100% at Day 14 and Day 28 , respectively, when compared to group G2. Total worm count reduction from both eyes for Advocate was 96.3% on Day 14 and 100% on Day 28. Clinical data were confirmed by the examination of conjunctival pouch flushing. An overall reduction in the number of cats with lacrimation and conjunctivitis was observed following treatment despite the fact that in a few cats treated with Advocate clinical signs persisted due to the chronic nature of the disease.
Conclusions
Based on the results of the present trial, a single dose of Advocate was found to be safe and highly effective in the treatment of natural T. callipaeda infection in cats.
Similar content being viewed by others
Background
Thelazia callipaeda is a spiruroid nematode residing in the conjunctival pouches of dogs, cats, wild carnivores and humans. Since its first description in dogs from northern Italy [1], reports of thelaziosis have increased showing a wide distribution throughout European countries and its capability to provoke ocular manifestations such as conjunctivitis, keratitis, corneal opacity and ulcers [2,3,4] due to the mechanical action of the adult parasite on the conjunctival and corneal epithelium. Due to its original distribution in East Asian countries, this worm used to be regarded as the “oriental eye-worm”, but after more recent descriptions in animals and humans throughout European countries such as Italy [5], Spain [6, 7], Croatia [8], Greece [9], Serbia [10], Bulgaria and Hungary [11], this term is no longer appropriate. The increase of infections in both animals and humans is related to the presence of the T. callipaeda vector, Phortica variegata, a drosophilid of the subfamily Steganinae which, in Europe, acts as intermediate host of the eyeworm [12, 13].
Phortica variegata live in forested and meadow areas where several competent wild hosts (e.g. red foxes, wolves, beech martens and wild cats [14]) perpetuate the infection, being reservoirs of the infection to dogs and cats [15]. Among available treatment procedures, physical removal of nematodes from conjunctival pouches consisting in a saline rinse (effective for adult and immature nematodes) and mechanical adult nematode collection by fine forceps or swabs has been described [16]. For canine thelaziosis, many treatments have been proposed, including topic instillation of organophosphates [17] or moxidectin [18]; however, these had major local side effects.
In 2016, Otranto et al. [19] published results on the effectiveness of moxidectin 2.5% w/v and imidacloprid 10% w/v (Advocate® spot-on solution for dogs, Bayer Animal Health) in thelaziosis showing a high effectiveness: a single application of this drug was sufficient to safely eliminate 100% of nematodes in infected dogs within seven days after a single administration. Moreover, the latter formulation acts systemically after topical application and consequently ocular irritation as local side effect while treating eye worm infections will not occur. In cats, the administration of milbemycin oxime at 2 mg/kg was demonstrated to have a high effectiveness in the treatment of T. callipaeda infections [20], but no registered products are currently available for the treatment of thelaziosis in this species.
The aim of this study was to evaluate the effectiveness of moxidectin 1.0% w/v and imidacloprid 10% w/v (Advocate spot-on solution for cats, Bayer Animal Health) against natural T. callipaeda infection in cats.
Results
At baseline all cats were infected by T. callipaeda. The average number of adult T. callipaeda specimens observed in G1 and G2 was 2.13 and 1.87, respectively. The number of parasites and the number of cats with clinical manifestations (lacrimation, conjunctivitis and ocular discharge; Table 1) in each eye was homogenous among groups (F(1,28) = 0.767, P = 0.389 and Fisher’s exact test, P = 0.500, for the number of parasites and for lacrimation, conjunctivitis and ocular discharge, respectively). Keratitis and ulcers were absent in all the cats. The number and percentage of cats positive for T. callipaeda infection at each study day are reported in Table 2. Effectiveness in G1 (Advocate) was 93.3% on Day 14 and 100% on Day 28. The total number and mean of live T. callipaeda adults retrieved and counted on both eyes is reported in Table 2. The reduction in the number of worms counted in both eyes was 96.3% on Day 14 and 100% on Day 28 for the treated group. A natural mild decrease was observed for the untreated group showing a worm reduction of 3.6% on Day 14 and 7.1% on Day 28. The comparison of worm reduction between groups showed a significant difference at all post-treatment visits when tested by ANOVA (F(1,28) = 67.600, P < 0.0001). Among ocular signs associated with T. callipaeda presence, only lacrimation, conjunctivitis and discharge were recorded in the included cats; details of observed symptoms are reported in Table 1. None of the cats exhibited keratitis and ulcers except one cat in the untreated group that showed both symptoms at the study closure visit (Day 28). A significant difference between treated and control groups were observed for lacrimation on Day 28 (Fisher’s exact test, P = 0.028) and for conjunctivitis on Day 28 (Fisher’s exact test, P = 0.020). No treatment related adverse effects were recorded.
Discussion
A single spot on application of Advocate, containing moxidectin 1.0% (w/v) and imidacloprid 10% (w/v), was highly effective in the treatment of feline thelaziosis. Only one cat out of 15 treated was found positive two weeks after treatment and no worms were detected four weeks after treatment administration. No side effects were observed during the whole study period. As was previously demonstrated for dog patients [21], this commercial formulation showed to be safe and effective for the treatment of T. callipaeda infection in feline patients. In this study a low prevalence of ocular clinical signs was observed, confirming that the disease has a high variability in the clinical presentation and that the occurrence of subclinical asymptomatic infections is common. Even if some clinical signs showed a significant reduction (lacrimation and conjunctivitis) in treated cats, the observation of the persistency of clinical signs in a few treated animals, also following parasitological cure is due to the nature of the disease that frequently has a chronic course. Concerning the “One Health” approach, as reported elsewhere [15], cases of human thelaziosis are reported in areas where the infection is highly prevalent in the animal population. Cats are hosts that can often reach high densities in peridomestic habitats; therefore, a safe and effective drug being approved for the treatment of feline thelaziosis represents an important asset in T. callipaeda control.
Conclusions
The results of the present study demonstrate that Advocate is safe and highly effective in the treatment of T. callipaeda infection in cats after single administration of the recommended dose rate.
Methods
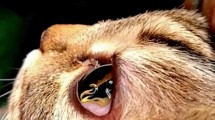
The study was conducted as a Good Clinical Practice (GCP), negative controlled, blinded and randomized field study in privately owned cats living in a T. callipaeda enzootic area of the Basilicata region (southern Italy), following approval by the Italian Ministry of Health (DGSAF- 0028002-06/12/2016-DGSAF-MDS-P). Study animals were located in areas enzootic for thelaziosis where the presence of the vector and of the diseases in dogs and cats was already proven [20, 22]. A sample size of 30 cats, 15 per group, was estimated to detect a difference between proportions (i.e. % of non-infected cats) in treated animals versus untreated, assuming a prevalence of infected cats in the untreated group of 73.3% (Motta et al. [20]) and a confidence level of 95% [software nQuery+nTerim 3.0 (StatSols, Statistical Solutions© Ltd. 2014). Thirty cats (19 females and 11 males) aged from 8 months to 5 years, of different breeds, in good health conditions and with at least one live adult T. callipaeda nematode in one eye (Fig. 1) were included in the study following the collection of the owner informed consent form. Details are provided in Table 3.
At inclusion (D0) animals were physically examined, weighed and inspected at both eyes, including a thorough examination underneath the third eyelid to retrieve and count live adult of T. callipaeda. Clinical manifestations suggestive of eye worm infection such as lacrimation, conjunctivitis, ocular discharge, keratitis, and ulcers, were recorded and classified as absent, mild, moderate or severe.
Allocation to study groups (G1: Advocate, G2: untreated) was done in accordance with a random treatment allocation plan. In order to avoid bias due to the contact between treated and untreated cats, animals of the same household were allocated to the same study group. Advocate was administered on the basis of the body weight, following the instructions reported on the label of the commercial packaging to provide a minimum spot-on dose of 1.0 mg/kg moxidectin and 10 mg/kg imidacloprid.
On Day 14 and Day 28 (study closure) cats were physically examined and the infection was evaluated by inspection of both eyes, including conjunctival pouches and third eyelids for live adult T. callipaeda count and clinical scores.
At Day 28, the final visit, the conjunctival pouch of both eyes of each animal was flushed twice with 2.5 ml of 0.9% saline solution to collect samples for the evaluation of the presence of T. callipaeda adults and larvae. The instilled liquid was recollected by placing a Petri dish right under the eye and transferred into a tube that was centrifuged for 5 min at 700× g. The supernatant was aspirated and the sediment (1 ml solution) assessed by microscopic examination (40×) for the presence of parasite stages. Nematodes were counted and morphologically identified according to Skrjiabin et al. [23] and Otranto et al. [24].
From Day 0 to Day 28 all cats were observed daily by their owners to assess and record potential abnormalities of the general health and, in case of their occurrence, the veterinarian was responsible for examining the cat and recording results of the clinical examination.
The primary variable for the effectiveness evaluation was the number of cats showing a complete elimination of adult eye worms on Day 14 and Day 28 by comparison of G1 (treated cats) and G2 (untreated cats). As secondary descriptive parameters, worm count, presence and/or severity of ocular clinical signs were calculated and compared between the two groups on Day 14 and Day 28. Effectiveness (%) in the treatment of T. callipaeda infection was calculated for each time point using the following formula:
Effectiveness was claimed if a significant difference between groups G1 and G2 was demonstrated by Fisher’s exact test calculated on contingency tables for parasitological cure with 5% significance level of probability of rejecting the null hypothesis.
Worm count and ocular clinical signs reductions in the treated group were compared at time points (t) Day 14 and Day 28 with the untreated group as follows:
where Ct0 was the baseline count before treatment and Ct was the count at time t after treatment for each secondary variable under investigation. The significance of the worm count reduction in treated cats was analyzed by ANOVA with standard statistical assumption. Statistical analysis was planned and conducted in compliance with current guidelines [25]. Statistical calculations and randomization were performed with: SPSS® statistical package for Windows, v.23.0 and nQuery + nTerim 3.0 (StatSols).
Abbreviations
- GCP:
-
Good Clinical Practice
- G1:
-
Study group of cats treated with imidacloprid 10% and moxidectin 1.0% spot-on
- G2:
-
Study group of untreated cats
- Day 0:
-
Day of inclusion of selected cats
- Day 14:
-
14th day of the study
- Day 28:
-
28th day of the study, closure
References
Rossi L, Bertaglia PP. Presence of Thelazia callipaeda Railliet & Henry, 1910, in Piedmont, Italy. Parassitologia. 1989;31:167–72.
Shen J, Gasser RB, Chu D, Wang Z, Yuan X, Cantacessi C, et al. Human thelaziosis - a neglected parasitic disease of the eye. J Parasitol. 2006;92:872–5.
Malacrida F, Hegglin D, Bacciarini L, Otranto D, Nägeli F, Nägeli C, et al. Emergence of canine ocular thelaziosis caused by Thelazia callipaeda in southern Switzerland. Vet Parasitol. 2008;157:321–7.
Otranto D, Dantas-Torres F, Brianti E, Traversa D, Petrić D, Genchi C, et al. Vector-borne helminths of dogs and humans in Europe. Parasit Vectors. 2013;6:16.
Otranto D, Dutto M. Human thelaziasis, Europe. Emerg Infect Dis. 2008;14:647–9.
Fuentes I, Montes I, Saugar JM, Latrofa S, Gárate T, Otranto D. Thelaziosis in humans, a zoonotic infection, Spain, 2011. Emerg Infect Dis. 2012;18:2073–5.
Marino V, Gálvez R, Colella V, Sarquis J, Checa R, Montoya A, et al. Detection of Thelazia callipaeda in Phortica variegata and spread of canine thelaziosis to new areas in Spain. Parasit Vectors. 2018;11:195.
Paradžik MT, Samardžić K, Živičnjak T, Martinković F, Janjetović Ž, Miletić-Medved M. Thelazia callipaeda - first human case of thelaziosis in Croatia. Wien Klin Wochenschr. 2016;128:221–3.
Papadopoulos E, Komnenou A, Thomas A, Ioannidou E, Colella V, Otranto D. Spreading of Thelazia callipaeda in Greece. Transbound Emerg Dis. 2018;65:248–52.
Tasić-Otašević S, Gabrielli S, Trenkić-Božinović M, Petrović A, Gajić B, Colella V, et al. Eyeworm infections in dogs and in a human patient in Serbia: a One Health approach is needed. Comp Immunol Microbiol Infect Dis. 2016;45:20–2.
Colella V, Kirkova Z, Fok É, Mihalca AD, Tasić-Otašević S, Hodžić A, et al. Increase in eyeworm infections in eastern Europe. Emerg Infect Dis. 2016;22:1513–5.
Otranto D, Cantacessi C, Testini G, Lia RP. Phortica variegata as an intermediate host of Thelazia callipaeda under natural conditions: evidence for pathogen transmission by a male arthropod vector. Int J Parasitol. 2006;36:1167–73.
Máca J, Otranto D. Drosophilidae feeding on animals and the inherent mystery of their parasitism. Parasit Vectors. 2014;7:516.
Otranto D, Dantas-Torres F, Mallia E, DiGeronimo PM, Brianti E, Testini G, et al. Thelazia callipaeda (Spirurida, Thelaziidae) in wild animals: report of new host species and ecological implications. Vet Parasitol. 2009;166:262–7.
Otranto D, Cantacessi C, Dantas-Torres F, Brianti E, Pfeffer M, Genchi C, et al. The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part II: Helminths and arthropods. Vet Parasitol. 2015;213:24–37.
Otranto D, Lia RP, Buono V, Traversa D, Giangaspero A. Biology of Thelazia callipaeda (Spirurida, Thelaziidae) eyeworms in naturally infected definitive hosts. Parasitology. 2004;129:627–33.
Rossi L, Peruccio C. Thelaziosi oculare nel cane: aspetti clinici e terapeutici. Veterinaria. 1989;2:47–50.
Lia RP, Traversa D, Agostini A, Otranto D. Field efficacy of moxidectin 1 per cent against Thelazia callipaeda in naturally infected dogs. Vet Rec. 2004;154:143–5.
Otranto D, Colella V, Crescenzo G, Solari Basano F, Nazzari R, Capelli G, et al. Efficacy of moxidectin 2.5% and imidacloprid 10% in the treatment of ocular thelaziosis by Thelazia callipaeda in naturally infected dogs. Vet Parasitol. 2016;227:118–21.
Motta B, Schnyder M, Basano FS, Nägeli F, Nägeli C, Schiessl B, et al. Therapeutic efficacy of milbemycin oxime/praziquantel oral formulation (Milbemax®) against Thelazia callipaeda in naturally infested dogs and cats. Parasit Vectors. 2012;5:85.
Bianciardi P, Otranto D. Treatment of dog thelaziosis caused by Thelazia callipaeda (Spirurida, Thelaziidae) using a topical formulation of imidacloprid 10% and moxidectin 2.5%. Vet Parasitol. 2005;129:89–93.
Otranto D, Ferroglio E, Lia RP, Traversa D, Rossi L. Current status and epidemiological observation of Thelazia callipaeda (Spirurida, Thelaziidae) in dogs, cats and foxes in Italy: a “coincidence” or a parasitic disease of the Old Continent? Vet Parasitol. 2003;116:315–25.
Skrjabin KI, Sobolev AA, Ivashkin VM. Essentials of Nematodology, Vol XVI: Spirurata of Animals and Man and the Diseases Caused by Them. Part 4 Thelazioidea. Moskow: Izdatel’sto Akademii Nauk SSSR; 1967.
Otranto D, Lia RP, Traversa D, Giannetto S. Thelazia callipaeda (Spirurida, Thelaziidae) of carnivores and humans: morphological study by light and scanning electron microscopy. Parassitologia. 2003;45:125–33.
Agency of European Medicines. Guideline on Statistical Principles for Clinical Trials for Veterinary Medicinal Products (Pharmaceuticals). 2012 (EMA/CVMP/EWP/81976/2010). https://www.ema.europa.eu/documents/scientific-guideline/guideline-statistical-principles-clinical-trials-veterinary-medicinal-products-pharmaceuticals_en.pdf. Accesse`d 21 Dec 2018.
Acknowledgements
The authors thank Viviana Tarallo (Dipartimento di Medicina Veterinaria, Università degli Studi di Bari) for her work with the administrative procedures of the study; Egidio Mallia of the veterinary services of the Parco Regionale di Gallipoli Cognato Piccole Dolomiti Lucane (Matera) for his support during the field studies; and Roland Schaper (Bayer Animal Health GmbH, Leverkusen, Germany) for his useful advice.
Funding
This study was funded by Bayer Animal Health.
Availability of data and materials
Data supporting the conclusions of this article are included within the article. Raw data of this study are available at the Department of Veterinary Medicine, University of Bari.
Author information
Authors and Affiliations
Contributions
DO, FSB, MGP and GP conceived and designed the study. RPL and DO carried out the field activities. GC carried out the statistical analyses. RN monitored the study. FSB, LF and DO drafted the first version of the manuscript; the other authors critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Italian Ministry of Health (DGSAF- 0028002-06/12/2016-DGSAF-MDS-P).
Consent for publication
Not applicable.
Competing interests
MGP and GP are employees of Bayer Animal Health GmbH, which funded the study. DO, FSB, RPL, LF and GC declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Otranto, D., Solari Basano, F., Pombi, M. et al. Effectiveness of the spot-on combination of moxidectin and imidacloprid (Advocate®) in the treatment of ocular thelaziosis by Thelazia callipaeda in naturally infected cats. Parasites Vectors 12, 25 (2019). https://doi.org/10.1186/s13071-018-3262-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-018-3262-1