Abstract
Background
Tick-borne rickettsial pathogens are emerging worldwide and pose an increased health risk to both humans and animals. A plethora of rickettsial species has been identified in ticks recovered from human and animal patients. However, the detection of rickettsial DNA in ticks does not necessarily mean that these ticks can act as vectors for these pathogens. Here, we used artificial feeding of ticks to confirm transmission of Rickettsia massiliae and Rickettsia raoultii by Rhipicephalus sanguineus (sensu lato) and Dermacentor reticulatus ticks, respectively. The speed of transmission was also determined.
Methods
An artificial feeding system based on silicone membranes were used to feed adult R. sanguineus (s.l.) and D. reticulatus ticks. Blood samples from in vitro feeding units were analysed for the presence of rickettsial DNA using PCR and reverse line blot hybridisation.
Results
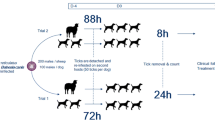
The attachment rate of R. sanguineus (s.l.) ticks were 40.4% at 8 h post-application, increasing to 70.2% at 72 h. Rickettsia massiliae was detected in blood samples collected 8 h after the R. sanguineus (s.l.) ticks were placed into the in vitro feeding units. D. reticulatus ticks were pre-fed on sheep and subsequently transferred to the in vitro feeding system. The attachment rate was 29.1 % at 24 h post-application, increasing to 43.6 % at 96 h. Rickettsia raoultii was detected in blood collected 24 h after D. reticulatus was placed into the feeding units.
Conclusions
Rhipicephalus sanguineus (s.l.) and D. reticulatus ticks are vectors of R. massiliae and R. raoultii, respectively. The transmission of R. massiliae as early as 8 h after tick attachment emphasises the importance of removing ticks as soon as possible to minimise transmission. This study highlights the relevance of in vitro feeding systems to provide insight into the vectorial capacity of ticks and the dynamics of tick-borne pathogen transmission.
Similar content being viewed by others
Background
Spotted fever group (SFG) rickettsiae are obligate intracellular Gram-negative bacteria belonging to the genus Rickettsia and are recognised agents of emerging infectious diseases in humans [1]. In Europe, Rickettsia raoultii was recently identified as an SFG Rickettsia causing tick-borne lymphadenopathy (TIBOLA), Dermacentor-borne necrosis erythema and lymphadenopathy (DEBONEL) and scalp eschar neck lymphadenopathy (SENLAT) [2,3,4,5]. Symptoms may include an inoculation eschar, cervical lymphadenopathy, high fever, malaise, and headaches [6]. Rickettsia raoultii is transmitted by, and isolated from, Dermacentor marginatus, D. reticulatus, D. nuttalli and D. silvarum ticks [3, 7,8,9].
The risk of human and animal exposure to pathogens transmitted by D. reticulatus is increasing in Europe, as this tick expands to new geographical areas [10,11,12,13,14]. In recent case reports from several clinical centres in China, R. raoultii was found infecting patients who presented with a febrile illness or a painful rash [9, 15]. In western Siberia, a recent study of 273 patients who were suffering from suspected tick-borne diseases was screened for rickettsial DNA, and ten of the patients tested positive; in further sequencing, three of the ten patients tested explicitly positive for R. raoultii [16]. These findings indicate that R. raoultii is emerging in a large part of the Eurasian continent.
Rickettsia massiliae is an SFG Rickettsia species prevalent worldwide, transmitted by and isolated from Rhipicephalus sanguineus (s.l.) [17,18,19,20,21,22]. Rickettsia massiliae is identified as an agent causing human disease [17, 23, 24] that can present as a febrile illness with rash [17, 23] or inoculation eschar and neck lymphadenopathy [24]. Also, there is an indication that R. massiliae may cause disease in dogs [25]; DNA of R. massiliae has been detected in blood from a dog with a splenic disease [26].
Moreover, in recent studies, R. raoultii DNA has been detected in questing Ixodes ricinus and Dermacentor silvarum ticks [9, 27, 28]. Further studies are required to determine whether these tick species can transmit R. raoultii.
To gain a better understanding of the transmission dynamics of SFG rickettsiae, we used an in vitro feeding system wherein adult D. reticulatus and R. sanguineus (s.l.) ticks were fed through silicone membranes over a blood reservoir. To monitor the transmission of R. raoultii and R. massiliae, blood samples were collected at regular intervals and analysed by PCR combined with the reverse line blot (RLB) hybridisation assay. The speed of transmission was also determined.
Methods
Ticks
All ticks used in this study originated from tick colonies maintained for several generations in the acaridarium established at the Utrecht Centre for Tick-Borne Diseases (UCTD). Dermacentor reticulatus ticks were kept at 90% relative humidity; developing stages were kept at 25 °C and non-developing stages were stored at 12 °C. The colony of D. reticulatus originated from the vegetation near Rozenburg in the south-western part of the Netherlands [29]. Rhipicephalus sanguineus (s.l.) ticks originated from dogs in Greece and were kept at 20 °C and 90% relative humidity.
To verify the presence of R. raoultii and R. massiliae in ticks derived from the respective tick colonies, PCR followed by sequencing (Microsynth, Balgach, Switzerland) of the ompA, ompB, sca4, gltA and 16S rRNA genes, together with the 23S–5S IGS region, was carried out as described previously [30]. All consensus sequences were submitted to the GenBank database and are available under accession numbers MG521356-MG521367. Moreover, DNA was extracted from all ticks used in the in vitro feeding experiments and screened for rickettsial DNA using PCR-RLB hybridisation.
In vitro feeding of ticks
Ticks were fed using silicone membranes as described previously [31], with some modifications [32, 33] and additional adjustments. Specifically, 250 ml bovine blood was collected from cattle which belonged to the Farm Animal Department, Faculty of Veterinary Medicine at Utrecht University. All animals were screened using PCR-RLB to confirm that they were free from any tick-borne pathogen. The blood was collected directly into a bottle containing heparin (final concentration 20 IU/ml). Gentamicin and glucose were immediately added to a final concentration of 5 μg/ml and 2 g/l, respectively. The blood was stored at 4 °C until further use. When the blood was used for in vitro feeding of ticks, adenosine triphosphate (ATP) was added to reach a final concentration of 0.51 μg/ml. The blood was then preheated to 37 °C and distributed into 6-well tissue culture plates at 3.1 ml blood/well.
Silicone membranes were prepared using a layer of household plastic film fixed onto a glass sheet with adhesive tape. Lens cleaning paper (Tiffen, USA) was fixed on top of the film layer. A mixture containing 15 g silicone glue (RTV-1 Elastocil E4, Wacker, Germany), 4.5 g silicone oil (DC 200, Sigma-Aldrich, Taufkirchen, Germany), 2.9 g hexane (Sigma-Aldrich) and 0.15 g of white colour paste (RAL 9010, Wacker) was prepared and a thin layer was spread evenly over the lens cleaning paper. Membranes were left to polymerise for at least 24 h with 97% humidity in a closed environment filled with sheep hair to give the membranes a typical host odour. The thickness of the prepared membranes was checked with a microcallipers, and only those between 70 μm and 100 μm thickness were used. Membranes of correct thickness were attached with silicone glue to feeding units constructed from Plexiglas tubing according to previously published specifications [31]. At the start of each experiment, the protective layer of plastic film was removed, and finely cut pieces of sheep hair were placed on top of the membrane within the feeding unit to reinforce sheep odour. During the study, it proved challenging to feed D. reticulatus using the silicone membranes. To increase the feeding success rate, D. reticulatus were pre-fed on sheep for four days. On day four, the ticks were manually removed using forceps and directly placed in feeding units so that they could re-attach and continue feeding as soon as possible. Feeding difficulties were not observed with R. sanguineus (s.l.) ticks and therefore selected ticks were used directly, without pre-feeding.
Depending on availability and the activity of ticks, we used a combination of five male and five female ticks per feeding unit when possible. We selected only ticks that showed questing behaviour or movement after stimulation with CO2. For D. reticulatus we used six feeding units. In units 1–5 we placed five female and five male ticks. Feeding unit 6 contained five female ticks that were left over. Due to a lack of active R. sanguineus (s.l.) ticks, we were only able to use four feeding units. In unit 1 we placed five female and five male ticks. In units 2 and 3 we placed solely male ticks, ten and 13, respectively. The last feeding unit, unit 4, contained five female and nine male ticks. After applying the ticks, the feeding units were carefully lowered into wells of a 6-well tissue culture plate containing 3.1 ml pre-warmed bovine blood supplemented as described above. Plates containing D. reticulatus ticks were incubated at 37 °C with 90% relative humidity and 4% CO2. Plates containing R. sanguineus (s.l.) were placed in a water bath at 37 °C with a 100% relative humidity.
To limit disturbance of D. reticulatus during feeding, and thus prevent premature tick detachment, the blood was changed at 24-h intervals instead of 12-h intervals as previously described [32]. When the blood was changed, the part of the membrane in contact with the blood was rinsed with sterile PBS before placing the unit in a well containing fresh prewarmed blood. Tick attachment rates were checked, and blood samples were taken after resuspending the sedimented blood cells and collecting 1 ml of blood per well. The D. reticulatus ticks were allowed to feed for a total of 96 h.
For R. sanguineus (s.l.), the first blood change and sampling were at 8 h after placing the ticks in the feeding units. Other blood changes and samplings were at 24-h intervals, starting from the placement of the ticks in the feeding units. Final blood samples were taken at 72 h. Whereafter, the feeding was terminated.
All blood samples were stored at -20 °C. At the end of the artificial feeding experiments, ticks were stored in 70% ethanol until further use.
Extraction of DNA from blood and in vitro fed ticks
Samples (200 μl) of collected blood were used for DNA extraction with the GeneJET Genomic DNA Purification Kit (Thermo Scientific, Karlsruhe, Germany), according to the manufacturer’s instructions. For DNA extraction from ticks, all were washed with demineralised water in an ultrasonic bath and then placed in individual tubes. Each tick was cut into smaller pieces using a sterile scalpel. Sectioned ticks were further disrupted in lysis buffer using the TissueLyser LT bead mill (Qiagen, Venlo, The Netherlands) with 7 mm metal beads at 50 oscillations for 3 min as described by the manufacturer. Subsequently, DNA was extracted using a GeneJET Genomic DNA Purification Kit. All the genomic DNA eluates were stored at -20 °C.
PCR-reverse line blot hybridisation
To screen for rickettsial DNA, a PCR fragment (≈ 360bp) of the 16S rRNA gene was amplified with primers Rick-F1 (5'-GAA CGC TAT CGG TAT GCT TAA CAC A -3') and Rick-R2 (biotin 5'-CAT CAC TCA CTC GGT ATT GCT GGA-3') published by Christova et al. [34] and modified by Nijhof et al. [35]. Cycling conditions for PCR reactions in 25 μl volumes were as described by Giangaspero et al. [36]. The obtained amplicons were used in the RLB hybridisation assay to detect specific rickettsial species as previously described [34, 37]. Briefly, 10 μl PCR product was diluted with 150 μl 2× SSPE/0.1% SDS buffer to a final volume of 160 μl. The diluted PCR samples were denatured for 10 min at 100 °C and placed directly on ice. Samples were then centrifuged at 4 °C for 30 s and 11,000× g. Subsequently, 150 μl of each denatured PCR product was loaded into the slots of an MN45 miniblotter (Immunetics, Cambridge, MA, USA) and hybridised onto a membrane prepared with covalently bound specific oligonucleotide probes, for 60 min at 42 °C. Samples were removed by aspiration, and the membrane was removed from the miniblotter. To remove any falsely annealed PCR products, the membrane was washed twice with preheated 2× SSPE/0.5% SDS at 50 °C for 10 min with shaking. The membrane was then incubated at 42 °C with 50 ml 2× SSPE/0.5% SDS containing 5 μl streptavidin horseradish peroxidase conjugate at 500 U conjugate/ml (Streptavidin-POD conjugate, Roche, Woerden, The Netherlands) for 30 min under gentle shaking. To remove unbound conjugate, the membrane was washed twice with 2× SSPE/0.5% SDS at 42 °C for 10 min, followed by two washes with 2 × SSPE at room temperature for 5 min. Finally, membranes were incubated with ECL reagents (Amersham, Buckinghamshire, UK) and the chemiluminescence reactions were detected using an ECL hyperfilm (Amersham), which was developed using an automated X-ray developer.
Results
Sequencing of rickettsial DNA
PCR-RLB analysis of DNA extracted from a random selection of ticks from the tick colonies before the in vitro feeding assays confirmed the presence of R. raoultii in D. reticulatus ticks and R. massiliae in R. sanguineus (s.l.) ticks. The obtained rickettsial sequences were analysed using BLAST (http://blast.ncbi.nlm.nih.gov/). All rickettsial sequences originating from D. reticulatus ticks were similar (99–100%) to R. raoultii strain Khabarovsk genome (CP010969.1); all sequences obtained from R. sanguineus (s.l.) ticks were identical (100%) to R. massiliae strain AZT80 genome (CP003319.1) available in GenBank.
Attachment of D. reticulatus and R. sanguineus (s.l.) during in vitro feeding
Following pre-feeding of D. reticulatus on sheep, the in vitro attachment rates were 29.1% after 24 h, 41.8% after 48 h and 43.6% after 96 h (Table 1).
Attachment rates of R. sanguineus (s.l.) were 40.4% after 8 h, 70.2% after 24 h, 85.1% after 48 h and 70.2% after 72 h (Table 2).
Detection of R. raoultii and R. massiliae DNA in blood samples
Pre-feeding of D. reticulatus on sheep prevented an accurate estimation of the earliest time frame wherein R. raoultii could be transmitted; we did, however, detect the transmission of this agent for the first time in an artificial feeding system. The blood sample taken 24 h after the ticks were applied to feeding unit #2 tested positive for R. raoultii DNA (Table 1), samples remained positive for up to 72 h post-tick application. Blood samples from unit #5 tested positive at 48 h and 96 h; samples from unit #1 were positive at 72 h, unit #4 at 96 h and unit #6 at 96 h (Table 1).
Rhipicephalus sanguineus (s.l.) ticks were more willing to feed through the silicone membranes and were less sensitive during the handling of the feeding units. This allowed us to change the blood meal and collect samples 8 h after the ticks were applied to the units. Thus, the earliest time point at which R. massiliae DNA was detected was 8 h after the ticks were placed in the feeding units (unit #3, Table 2). Samples from feeding unit #3 remained positive until 48 h. Blood samples from unit #1 tested positive at 24 h and 72 h; samples from unit #4 tested positive at 24 h, 48 h and 72 h (Table 2).
PCR-RLB analysis of tick DNA after in vitro feeding
PCR-RLB analysis of extracted tick DNA revealed that 90.9% (50/55) of the in vitro fed D. reticulatus tested positive for R. raoultii and 40.4% (19/47) of R. sanguineus (s.l.) for R. massiliae.
Discussion
Our experiments confirm the effectiveness of in vitro feeding assays using silicone membranes, although we were more successful with R. sanguineus (s.l.) than with D. reticulatus ticks. In vitro feeding systems using silicone membranes were initially developed for Ixodes ricinus, whereby attachment rates of 90% have been achieved [38,39,40]. Other tick species have also been adapted to feed in vitro, in particular, Amblyomma americanum, with attachment rates between 50% and 75% [41]; Hyalomma dromedarii and H. anatolicum with rates of 55% and 75%, respectively [42], and Ixodes scapularis ticks which reached an attachment rate of 45% in vitro [33].
The relatively low attachment rate of D. reticulatus ticks (29.1% after 24 h, up to 43.6% after 96 h) was also reported in a recent study conducted by Krull et al., wherein the average attachment rate was 31.6% [43]. In the same study, it was demonstrated that an increased CO2 level could act as a feeding stimulant and therefore be improving the attachment rate [43]. Further studies are required to enhance the in vitro feeding behaviour of D. reticulatus ticks.
The attachment rates of R. sanguineus (s.l.) were significantly higher than those of D. reticulatus ticks; namely, 70.2% after 24 h, up to 85.1% after 48 h. This was also observed in a study conducted by Fourie et al., wherein the attachment rate was as high as 72.5% after only 24 hours [32].
The observed differences in attachment and feeding behaviour of different tick species indicate that there is still much to learn about the behaviour biology of ticks, whereby feeding stimulants that work for one tick species do not necessarily work for another species. Further optimisation of feeding conditions is required as in vitro assays prove vital for studying the vectorial capacity of ticks without the influence of laboratory animals.
Although the vectorial capacity of D. reticulatus and R. sanguineus (s.l.) regarding the respective Rickettsia species has already been documented [18, 29, 30, 44,45,46], here we demonstrate for the first time that both rickettsial pathogens are transmitted by in vitro feeding vector ticks. The detection of R. raoultii and R. massiliae DNA indicated that both organisms were transmitted in vitro, although in theory, it could have been DNA only. Rickettsia massiliae was detected as early as 8 h after the ticks were placed within the feeding units, confirming that transmission of tick-borne microorganisms can occur at a relatively early stage after tick attachment. We were unable to determine whether R. raoultii could also be transmitted at an early stage after tick attachment, because of the necessity to pre-feed D. reticulatus ticks on sheep. We did, however, observe R. raoultii transmission 24 h after the ticks were placed in the feeding units, proving the efficacy of the artificial feeding system in studying the transmission dynamics of tick-borne pathogens. Throughout the artificial feeding assays, we observed ticks attaching and feeding, but rickettsial DNA was not always found in the blood of the respective wells. This might indicate that the ticks feeding at that specific time point were negative or had just started feeding, as we were unable to observe tick attachment and feeding behaviour continuously. Furthermore, ticks may have been disturbed while feeding on the silicone membrane and re-attached at a later time, or Rickettsia-positive ticks might have fed during different time periods in the same feeding unit. This could explain why feeding unit #1, in the R. sanguineus (s.l.) feeding assay, was positive for R. massiliae DNA at 24 h, negative at 48 h and became positive again 72 h after tick application (Table 2).
Finally, our results further confirm the importance of removing ticks as soon as possible to minimise the risk of infection with tick-borne bacteria. More recently, Ehrlichia canis was transmitted as soon as 6 hours after tick attachment in vivo on dogs and within 8 hours by in vitro feeding R. sanguineus (s.l.) ticks [32]. The relatively short period observed for rickettsiae to be transmitted might be because of the presence of the bacteria within the salivary glands [47] and haemolymph [30, 48] before feeding. This contrasts with other bacteria such as Borrelia burgdorferi (s.l.) which are present in the midgut before feeding. The earliest transmission period for Borreliella burgdorferi (s.l.) is witnessed as early as 17 hours after attachment of infected I. ricinus ticks [49]. This is due to that the spirochetes are attached to the midgut of ticks and require external stimuli to pass through the midgut into the haemolymph and reach the salivary glands before transmission can occur [50].
Conclusions
In our study, D. reticulatus and R. sanguineus (s.l.) ticks successfully fed in artificial feeding systems. Rickettsia massiliae DNA was detected as early as 8 h after the ticks were applied to the feeding units; Rickettsia raoultii DNA was detected 24 h after (pre-fed) ticks were placed within the feeding units. The early transmission time of tick-borne rickettsial species emphasises the importance of removing ticks as soon as possible and the use of tick repellents to minimise the risk of exposure to tick-borne microorganisms.
Abbreviations
- ATP:
-
adenosine triphosphate
- DEBONEL:
-
Dermacentor-borne necrosis erythema lymphadenopathy
- RLB:
-
reverse line blot
- SDS:
-
sodium dodecyl sulphate
- SENLAT:
-
scalp eschar neck lymphadenopathy
- SFG:
-
spotted fever group
- TIBOLA:
-
tick-borne lymphadenopathy
References
Raoult D, Roux V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin Microbiol Rev. 1997;10:694–719.
Lakos A. Tick-borne lymphadenopathy - a new rickettsial disease? Lancet. 1997;350:1006.
Mediannikov O, Matsumoto K, Samoylenko I, Drancourt M, Roux V, Rydkina E, et al. Rickettsia raoultii sp. nov., a spotted fever group rickettsia associated with Dermacentor ticks in Europe and Russia. Int J Syst Evol Microbiol. 2008;58:1635–9.
Angelakis E, Pulcini C, Waton J, Imbert P, Socolovschi C, Edouard S, et al. Scalp eschar and neck lymphadenopathy caused by Bartonella henselae after tick bite. Clin Infect Dis. 2010;50:549–51.
J a O, Portillo A. Tick-borne rickettsioses in Europe. Ticks Tick Borne Dis. 2012;3:271–8.
Rieg S, Schmoldt S, Theilacker C, de With K, Wölfel S, Kern WV, et al. Tick-borne lymphadenopathy (TIBOLA) acquired in southwestern Germany. BMC Infect Dis. 2011;11:167.
Špitalská E, Štefanidesová K, Kocianová E, Boldiš V. Rickettsia slovaca and Rickettsia raoultii in Dermacentor marginatus and Dermacentor reticulatus ticks from Slovak Republic. Exp Appl Acarol. 2012;57:189–97.
Portillo A, Santibáñez S, García-Álvarez L, Palomar AM, Rickettsioses in Europe OJA. Microbes Infect. 2015;17:834–8.
Jia N, Zheng YC, Ma L, Huo QB, Ni XB, Jiang BG, et al. Human infections with Rickettsia raoultii, China. Emerg Infect Dis. 2014;20:866–8.
Jongejan F, Ringenier M, Putting M, Berger L, Burgers S, Kortekaas R, et al. Novel foci of Dermacentor reticulatus ticks infected with Babesia canis and Babesia caballi in the Netherlands and in Belgium. Parasit Vectors. 2015;8:232.
Hofmeester TR, van der Lei P-B, Docters van Leeuwen A, Sprong H, van Wieren SE. New foci of Haemaphysalis punctata and Dermacentor reticulatus in the Netherlands. Ticks Tick Borne Dis. 2015;7:10–3.
Dautel H, Dippel C, Oehme R, Hartelt K, Schettler E. Evidence for an increased geographical distribution of Dermacentor reticulatus in Germany and detection of Rickettsia sp. RpA4. Int J Med Microbiol. 2006;296:149–56.
Široký P, Kubelová M, Bednář M, Modrý D, Hubálek Z, Tkadlec E. The distribution and spreading pattern of Dermacentor reticulatus over its threshold area in the Czech Republic - how much is range of this vector expanding? Vet Parasitol. 2011;183:130–5.
Kiewra D, Czulowska A. Evidence for an increased distribution range of Dermacentor reticulatus in south-west Poland. Exp Appl Acarol. 2013;59:501–6.
Li H, Zhang P-H, Huang Y, Du J, Cui N, Yang Z-D, et al. Isolation and identification of Rickettsia raoultii in human cases: a surveillance study in 3 medical centers in China. Clin Infect Dis. 2018;66:1109–15.
Igolkina Y, Krasnova E, Rar V, Savelieva M, Epikhina T, Tikunov A. et al. Detection of causative agents of tick-borne rickettsioses in Western Siberia, Russia: identification of Rickettsia raoultii and Rickettsia sibirica DNA in clinical samples. Clin Microbiol Infect. 2018;24:e9–199.e12
Vitale G, Mansueto S, Rolain JM, Raoult D. Rickettsia massiliae human isolation. Emerg Infect Dis. 2006;12:174–5.
Eremeeva ME, Bosserman EA, Demma LJ, Zambrano ML, Blau DM, Dasch GA. Isolation and identification of Rickettsia massiliae from Rhipicephalus sanguineus ticks collected in Arizona. Appl Environ Microbiol. 2006;72:5569–77.
Milhano N, Palma M, Marcili A, Núncio MS, de Carvalho IL, De Sousa R. Rickettsia lusitaniae sp. nov. isolated from the soft tick Ornithodoros erraticus (Acarina: Argasidae). Comp Immunol Microbiol Infect Dis. 2014;37:189–93.
Fernández-Soto P, Pérez-Sánchez R, Díaz Martín V, Encinas-Grandes A, Álamo Sanz R. Rickettsia massiliae in ticks removed from humans in Castilla y León, Spain. Eur J Clin Microbiol Infect Dis. 2006;25:811–3.
Scarpulla M, Barlozzari G, Marcario A, Salvato L, Blanda V, De Liberato C, et al. Molecular detection and characterisation of spotted fever group rickettsiae in ticks from central Italy. Ticks Tick Borne Dis. 2016;7:1052–6.
Cicuttin GL, De Salvo MN, La Rosa I, Dohmen FEG. Isolation of Rickettsia massiliae from Rhipicephalus sanguineus ticks, Buenos Aires (Argentina). J Parasitol. 2015;101:711–2.
García-García JC, Portillo A, Núñez MJ, Santibáñez S, Castro B, Oteo JA. Case report: a patient from Argentina infected with Rickettsia massiliae. Am J Trop Med Hyg. 2010;82:691–2.
Cascio A, Torina A, Valenzise M, Blanda V, Camarda N, Bombaci S, et al. Scalp eschar and neck lymphadenopathy caused by Rickettsia massiliae. Emerg Infect Dis. 2013;19:836–7.
Beeler E, Abramowicz KF, Zambrano ML, Sturgeon MM, Khalaf N, Hu R, et al. A focus of dogs and Rickettsia massiliae-infected Rhipicephalus sanguineus in California. Am J Trop Med Hyg. 2011;84:244–9.
Movilla R, Altet L, Serrano L, Tabar M-D, Roura X. Molecular detection of vector-borne pathogens in blood and splenic samples from dogs with splenic disease. Parasit Vectors. 2017;10:131.
Schötta A-M, Wijnveld M, Stockinger H, Stanek G. Approaches for reverse line blot-based detection of microbial pathogens in Ixodes ricinus ticks collected in Austria and impact of the chosen method. Appl Environ Microbiol. 2017;83:e00489–17.
Chmielewski T, Podsiadly E, Karbowiak G, Tylewska-Wierzbanowska S. Rickettsia spp. in ticks, Poland. Emerg Infect Dis. 2009;15:486–8.
Alberdi MP, Nijhof AM, Jongejan F, Bell-Sakyi L. Tick cell culture isolation and growth of Rickettsia raoultii from Dutch Dermacentor reticulatus ticks. Ticks Tick Borne Dis. 2012;3:349–54.
Wijnveld M, Schötta A-M, Pintér A, Stockinger H, Stanek G. Novel Rickettsia raoultii strain isolated and propagated from Austrian Dermacentor reticulatus ticks. Parasit Vectors. 2016;9:567.
Kröber T, Guerin PM. An in vitro feeding assay to test acaricides for control of hard ticks. Pest Manag Sci. 2007;63:17–22.
Fourie JJ, Stanneck D, Luus HG, Beugnet F, Wijnveld M, Jongejan F. Transmission of Ehrlichia canis by Rhipicephalus sanguineus ticks feeding on dogs and on artificial membranes. Vet Parasitol. 2013;197:595–603.
Andrade JJ, Xu G, Rich SM. A silicone membrane for in vitro feeding of Ixodes scapularis (Ixodida: Ixodidae). J Med Entomol. 2014;51:878–9.
Christova I, Van De Pol J, Yazar S, Velo E, Schouls L. Identification of Borrelia burgdorferi sensu lato, Anaplasma and Ehrlichia species, and spotted fever group rickettsiae in ticks from Southeastern Europe. Eur J Clin Microbiol Infect Dis. 2003;22:535–42.
Nijhof AM, Bodaan C, Postigo M, Nieuwenhuijs H, Opsteegh M, Franssen L, et al. Ticks and associated pathogens collected from domestic animals in the Netherlands. Vector Borne Zoonotic Dis. 2007;7:585–95.
Giangaspero A, Marangi M, Papini R, Paoletti B, Wijnveld M, Jongejan F. Theileria sp. OT3 and other tick-borne pathogens in sheep and ticks in Italy: molecular characterisation and phylogeny. Ticks Tick Borne Dis. 2015;6:75–83.
Schouls LM, Van De Pol I, Rijpkema SG, Schot CS. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J Clin Microbiol. 1999;37:2215–22.
Bonnet S, Jouglin M, Malandrin L, Becker CAM, Agoulon A, L’Hostis M, et al. Transstadial and transovarial persistence of Babesia divergens DNA in Ixodes ricinus ticks fed on infected blood in a new skin-feeding technique. Parasitology. 2007;134:197–207.
Campbell EM, Burdin M, Hoppler S, Bowman AS. Role of an aquaporin in the sheep tick Ixodes ricinus: assessment as a potential control target. Int J Parasitol. 2010;40:15–23.
Kröber T, Guerin PM. In vitro feeding assays for hard ticks. Trends Parasitol. 2007;23:445–9.
Bullard R, Allen P, Chao CC, Douglas J, Das P, Morgan SE, et al. Structural characterisation of tick cement cones collected from in vivo and artificial membrane blood-fed lone star ticks (Amblyomma americanum). Ticks Tick Borne Dis. 2016;7:880–92.
Tajeri S, Razmi GR. Hyalomma anatolicum anatolicum and Hyalomma dromedarii (Acari: Ixodidae) imbibe bovine blood in vitro by utilising an artificial feeding system. Vet Parasitol. 2011;180:332–5.
Krull C, Böhme B, Clausen P-H, Nijhof AM. Optimization of an artificial tick feeding assay for Dermacentor reticulatus. Parasit Vectors. 2017;10:60.
Földvári G, Široký P, Szekeres S, Majoros G, Sprong H. Dermacentor reticulatus: a vector on the rise. Parasit Vectors. 2016;9:314.
Milhano N, Popov V, Vilhena M, Bouyer DH, de Sousa R, Walker DH. Quantitative study of Rickettsia massiliae in Rhipicephalus sanguineus organs. Ticks Tick Borne Dis. 2014;5:709–14.
Földvári G, Rigó K, Lakos A. Transmission of Rickettsia slovaca and Rickettsia raoultii by male Dermacentor marginatus and Dermacentor reticulatus ticks to humans. Diagn Microbiol Infect Dis. 2013;76:387–9.
Qiu Y, Nakao R, Ohnuma A, Kawamori F, Sugimoto C. Microbial population analysis of the salivary glands of ticks; a possible strategy for the surveillance of bacterial pathogens. PLoS One. 2014;9:e103961.
Burgdorfer W. Hemolymph Test. Am J Trop Med Hyg. 1970;19:1010–4.
Kahl O, Janetzki-Mittmann C, Gray JS, Jonas R, Stein J, de Boer R. Risk of Infection with Borrelia burgdorferi sensu lato for a host in relation to the duration of nymphal Ixodes ricinus feeding and the method of tick removal. Zentralblatt für Bakteriol. 1998;287:41–52.
Tilly K, Rosa PA, Stewart PE. Biology of Infection with Borrelia burgdorferi. Infect Dis Clin North Am. 2008;22:217–34.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets used in this study and the extracted DNA from ticks are available upon request. All consensus DNA sequences are available in the GenBank database under the accession numbers MG521356-MG521367 .
Author information
Authors and Affiliations
Contributions
EO and MW are equal contributors to the study, which was conceptualised by FJ. MB carried out the Rhipicephalus experiments, and EO did the experiments with the Dermacentor ticks. LB provided PCR/RLB training. MW and EO wrote the first draft of the manuscript, which was edited by FJ. Both FV and MTM supervised the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The animal experiment committee approved blood collection from cattle and pre-feeding of ticks on sheep (CCD no: AVD 10800 2016 709).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Olivieri, E., Wijnveld, M., Bonga, M. et al. Transmission of Rickettsia raoultii and Rickettsia massiliae DNA by Dermacentor reticulatus and Rhipicephalus sanguineus (s.l.) ticks during artificial feeding. Parasites Vectors 11, 494 (2018). https://doi.org/10.1186/s13071-018-3075-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-018-3075-2