Abstract
Background
Transfluthrin vapour prevents mosquito bites by disrupting their host-seeking behaviors. We measured the additional benefits of combining transfluthrin-treated sisal decorations and long-lasting insecticidal nets (LLINs) with an aim of extending protection against early evening, indoor-biting malaria vectors when LLINs are ineffective.
Methods
We investigated the indoor protective efficacy of locally made sisal decorative baskets (0.28 m2) treated with 2.5 ml and 5.0 ml transfluthrin, in terms of mosquito density, exposure to bites and 24 h mortality. Experiments were conducted in experimental huts, located in Lupiro village, Ulanga District, south-eastern Tanzania. Human landing catches (HLC) were used to measure exposure to bites between 19:00–23:00 h. Each morning, at 06:00 h, mosquitoes were collected inside huts and in exit traps and monitored for 24 h mortality.
Results
Sisal decorative baskets (0.28 m2) treated with 2.5 ml and 5.0 ml transfluthrin deterred three-quarters of Anopheles arabiensis mosquitoes from entering huts (relative rate, RR = 0.26, 95% confidence interval, CI: 0.20–0.34, P < 0.001 and RR= 0.29, 95% CI: 0.22–0.37, P < 0.001, respectively). Both treatments induced a 10-fold increase in 24 h mortality of An. arabiensis mosquitoes (odds ratio, OR = 12.26, 95% CI: 7.70–19.51, P < 0.001 and OR = 18.42, 95% CI: 11.36–29.90, P < 0.001, respectively).
Conclusions
Sisal decorative items treated with spatial repellents provide additional household and personal protection against indoor biting malaria and nuisance mosquitoes in the early evening, when conventional indoor vector control tools, such as LLINs, are not in use. We recommend future studies to investigate the epidemiological relevance of combining LLINs and transfluthrin decorated baskets in terms of their effect on reduction in malaria prevalence.
Similar content being viewed by others
Background
Long-lasting insecticidal nets (LLINs), indoor residual spraying (IRS), improved diagnosis and treatment have brought about substantial decline in malaria transmission, particularly in sub-Saharan Africa [1,2,3]. Despite these achievements, residual malaria transmission that occurs even with high coverage of LLINs and/or IRS continues to threaten efforts towards malaria elimination. Additionally, insecticide resistance in Africa is another challenge in consolidating, and sustaining the gains accrued by vector control tools [4,5,6,7].
Effectiveness of LLINs depends on factors that influence human-vector contact, such as time and place of malaria-transmitting mosquito bites [8], user’s sleeping hours, proper use, installation and maintenance of nets, as well as user’s compliance [9]. When LLINs are not available, the risk of exposure to infectious bites increases during meal times, at social events and or when students are doing homework. In addition, in rural Africa most people live in houses that are not sufficiently proofed to prevent mosquito entry [10].
Topical repellents [11, 12] and protective clothing [13] represent some of the options used as personal protection against mosquito bites when LLINs are not in use. Although these tools confer some protection, they have some limitations: (i) they divert mosquitoes to non-users [14]; (ii) they require reapplication often hourly; and (iii) they often fail due to non-compliance by users [15]. Additionally, topical repellents are unlikely to be practical for daily use, and may not be affordable for continuous use in low and middle-income populations [16]. Due to high temperatures in some regions, and costs required for re-application, the use of protective clothing may not be feasible in most tropical countries. Development of new, efficacious, low-cost, context specific, practical and scalable vector control tools, that target indoor biting mosquitoes when LLINs are not in use, would complement the protective efficacy of LLINs and IRS.
Spatial repellents are vapour-phase insecticides that incapacitate mosquitoes and prevent them from locating hosts and obtaining blood meals [17]. Examples of spatial repellent delivery formats include pyrethroid-treated mosquito coils, vaporizer mats, aerosols, and paper strips as well as traditional practices such as burning and smoldering plants [18].
Previous studies have shown that transfluthrin prevents mosquitoes from feeding [19], and induces mosquito mortality [20]. Here, we quantified the potential benefits of combining spatial repellent with LLINs, as a complementary strategy against indoor biting mosquitoes in the early evening, when LLINs are not in use.
Methods
Study area
The study was conducted in Lupiro village (8.385°S, 36.670°E), Ulanga District, south-eastern Tanzania [21]. Annual rainfall is 1200–1600 mm with temperature ranging between 20–32.6 °C [21]. The main malaria vectors in this area are An. arabiensis and Anopheles funestus (sensu lato) [22]. The main vector control intervention in the area is LLINs, with a first universal mass LLINs distribution campaign conducted between 2010 and 2011 [23, 24]. A more recent LLINs mass campaign was conducted between 2015 and 2016 [25]. Preceding studies indicated that both An. arabiensis and An. funestus were pyrethroid (i.e. permethrin: 77% and 65%, respectively) resistant [26] and findings from a more recent study indicated that An. funestus (s.l.) was also resistant to pyrethroids (i.e. permethrin: 10.5%) [27].
Preparation of transfluthrin-treated sisal fabrics
Circular pieces of sisal 0.28 m2 were treated with either 2.5 ml or 5 ml of 97% transfluthrin (Shenzhen Sunrising Industry Company, Limited, Shenzhen, China) following the method previously described [28,29,30]. Control pieces were soaked in a mixture of water and detergent only as previously described [28,29,30]. All pieces were enclosed in colorfully beaded iron welded baskets as previously described [30].
Rationale for delivering transfluthrin using sisal decorative baskets
Sisal fabrics are versatile products from the sisal plant, available in most of the tropical countries like Tanzania. These fabrics can be made into various household products, such as mats, baskets, curtains, wall picture frame, etc. The uniqueness of the sisal fabrics are: (i) they have relatively high absorbance of liquid such as water; and (ii) they allow slow release of transfluthrin in air, this way transfluthrin-treated sisal fabrics may remain effective for a duration of more than six months or a year [28, 31]. Nevertheless, as the sisal products fits for different households decorative items, using these items indoor, when are treated with transfluthrin, may serve two purposes: decorate house and act as an indoor vector control tool.
Study design
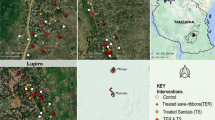
Experiments were conducted from 6th January 2015 to 7th February 2015. The effect of combining transfluthrin-treated sisal baskets and permethrin-treated LLINs on the proportion of indoor mosquito density, the proportion of early evening indoor mosquito bites and survival of mosquitoes in experimental huts (Fig. 1a) was investigated. The treatments included: (i) control arm with permethrin-treated LLIN and four untreated sisal baskets; (ii) four transfluthrin-treated (2.5 ml) sisal baskets and one permethrin-treated LLIN; and (iii) four transfluthrin-treated sisal baskets (5 ml) and one permethrin-treated LLIN. Initially, treatments were randomly allocated to 3 experimental huts, using a lottery method and later treatment and control arms were rotated between 3 huts after 9 consecutive experimental nights using a 3 × 3 Latin square design. A sisal basket (Fig. 1b) [30], was suspended in each of the four corners of the huts (Fig. 1c). They were placed 1.84 m off the ground and 0.52 m from the wall. In each hut, a male volunteer conducted human landing catches from 19:00 to 23:00 h. This coincided with the time when most people within this community are likely to be awake but not protected by LLINs. Moreover, mosquitoes were also collected from exit traps, fitted on eaves and windows of the huts as well as on the floor at 06:00 h. All mosquitoes were kept in a field insectary situated approximately 50 m from the nearest experimental hut. The temperature in the experimental huts was 26.94 °C during the day and 25.65 °C at night and relative humidity was 81.0% during the day and 86.5% at night. Mosquitoes were provided 10% glucose solution for 24 h after which mortality was recorded. After 24 h, mosquitoes were sorted and recorded as dead, live, blood-fed or unfed. Morphological identification keys [32] were used to identify mosquitoes to their genus and species. Standard polymerase chain reaction (PCR) [33, 34] was used to differentiate a subsample of sibling species of An. gambiae (s.l.) and An. funestus (s.l.) mosquitoes that were randomly selected each day. The primary outcomes measured included: (i) mosquito deterrence, which is reduction in the density of indoor mosquitoes; (ii) indoor human mosquito biting rate, which is the proportion of mosquitoes that landed and attempted to bite volunteers that were conducting HLC; and (iii) insecticide-induced 24 h mortality.
Outside view of Ifakara experimental hut design and sisal baskets decorative prototype as previously described [30]. a An outside view of the Ifakara experimental hut. b A sisal decorative basket [30]. c Inside view of Ifakara experimental hut with suspended sisal decorative baskets about 1.8 m from the floor and 0.52 m from the wall. The arrow indicates the position of the suspended sisal decorative basket
Data analysis
Deterrence was determined statistically using log-normal Poisson generalized linear mixed effects models (GLMMs) in R statistical software version 3.1.3, with lme4 package [35]. The response variable was the total number of mosquitoes collected from experimental huts including those collected indoors by those conducting HLC. Experimental huts and day of experiment were treated as random independent variables, while treatment was coded as a fixed variable. An over-dispersion random variable accounting for the random fluctuating nature of mosquito count data on different experimental days was included. An. arabiensis, An. funestus (s.l.) and Culex species mosquitoes were analyzed in separate models. The same analysis was used to measure reduction in the proportion of biting mosquitoes in the early evening. The total number of mosquitoes collected by HLC in experimental huts between 19:00 and 23:00 h was fitted as the dependent variable. The hut and the day of experiment were treated as random variables, while treatment arm was coded as a fixed variable. Insecticide induced mortality was determined by fitting a GLMMs with a binomial distribution and a logit-link function. The proportion of dead and live mosquitoes was coded as dependent binomial variable, treatment arms as fixed variable whereas day of experiment and experimental huts were treated as random variables.
Results
The total number of mosquitoes collected was 7125. These included 4157 Culex spp.; 1672 An. arabiensis; 1165 An. funestus (s.l.); 121 Mansonia spp.; 4 Coquilettidia spp.; 3 An. coustani; and 3 Aedes spp. Of 91 An. gambiae (s.l.) samples amplified by PCR, all of the 86% (n = 78) successful amplifications achieved were An. arabiensis. Sixty-eight An. funestus (s.l.) samples were analyzed by PCR, and 71% (49/68) were successful amplifications. Of the successful amplifications, 96% (47/49) were An. funestus (sensu stricto), 2% (1/49) were An. leesoni, and the remaining 2% (1/49) were An. rivulorum.
Deterrence
Relative to LLINs with untreated sisal baskets, sisal decorative baskets treated with 2.5 ml and 5.0 ml transfluthrin, in combination with permethrin LLINs, reduced almost three quarters of indoor An. arabiensis mosquitoes (2.5 ml: RR = 0.26, 95% confidence interval, CI: 0.2–0.34, P < 0.001) and (5.0 ml: RR = 0.29, 95% CI: 0.22–0.37, P < 0.001) (Table 1, Fig. 2). Adding either 2.5 ml or 5 ml transfluthrin-treated baskets to LLIN huts, did not reduce indoor densities of An. funestus (s.l.) mosquitoes (2.5 ml: RR = 0.83, 95% CI: 0.60–1.14, P < 0.230; and 5.0 ml: RR = 0.82, 95% CI: 0.6–1.13; P < 0.240). Huts with transfluthrin-treated sisal baskets and LLINs had nearly one third less Culex sp. mosquitoes compared to those with LLINs and untreated baskets (RR = 0.72, 95% CI: 0.61–0.85, P < 0.001) for 2.5 ml transfluthrin and (RR = 0.70, 95% CI: 0.6–0.83, P < 0.001) for 5 ml transfluthrin. As shown in Fig. 2 and Table 4, there were no differences in effect between the 2.5 ml and 5 ml treatments in reducing indoor mosquito entry.
Mean indoor entry rate (mosquitoes caught per hut per night) of An. arabiensis (a), An. funestus (b) and Culex spp. (c) between the huts that had transfluthrin-treated sisal baskets and LLINs to those with untreated counterparts and LLINs. The error bars represent the 95% confidence intervals, CI. Abbreviations: LLINs, long-lasting insecticidal nets; TF, transfluthrin
Indoor human mosquito biting rate
Figure 3 and Table 2 show that both 2.5 ml and 5.0 ml transfluthrin-treated baskets, combined with LLINs, reduced the proportion of An. arabiensis mosquito bites by more than three quarters (2.5 ml: RR = 0.10, 95% CI: 0.05–0.23, P < 0.001; and 5.0 ml: RR = 0.12, 95% CI: 0.06–0.26, P < 0.001) compared to LLINs with untreated baskets. In addition, the two interventions reduced An. funestus (s.l.) mosquitoes bites by nearly half (2.5 ml: RR = 0.48, 95% CI: 0.27–0.87, P < 0.016 and 5 ml: RR = 0.56, 95% CI: 0.31–0.98, P < 0.043). The addition of transfluthrin-treated baskets reduced exposure to Culex spp. mosquitoes by approximately two thirds (2.5 ml and LLINs: RR = 0.33, 95% CI: 0.25–0.42, P < 0.001; and 5 ml and LLINs: RR = 0.27, 95% CI: 0.21–0.35, P < 0.001). Furthermore, as shown in Fig. 3 and Table 5, there were no differences in effect between the 2.5 ml and 5 ml treatments in reducing indoor mosquito biting rate.
Mean biting rate (biting per person per night) against indoor bites of An. arabiensis (a), An. funestus (b) and Culex spp. (c) between the huts that had transfluthrin-treated sisal baskets and LLINs to those with untreated counterparts and LLINs. The error bars represent the 95% confidence intervals, CI. Abbreviations: LLINs, long-lasting insecticidal nets; TF, transfluthrin
Insecticide-induced 24 h mortality
Adding transfluthrin treated baskets in experimental huts with LLINs, induced a 10-fold increase in 24 h mortality of An. arabiensis (OR = 12.26, 95% CI: 7.70–19.51, P < 0.001 for 2.5 ml and OR = 18.43, 95% CI: 11.36–29.90, P < 0.001 for 5 ml). Compared to the control arm, adding transfluthrin-treated sisal baskets in experimental hut with LLINs, did not have impact on inducing mortality of An. funestus (s.l.) mosquitoes 24 h post-exposure (OR = 0.54, 95% CI: 0.53–0.54, P < 0.001 and OR = 0.69, 95% CI: 0.69–0.70, P < 0.001, respectively). Neither 2.5 ml (1.57, 95% CI: 0.95–2.57, P < 0.076 ) nor 5.0 ml (OR = 1.67, 95% CI: 0.98–2.86, P < 0.061) transfluthrin-treated baskets combined with LLINs increased mortality of Culex spp. mosquitoes (Table 3, Fig. 4). Additionally, as shown in Fig. 4 and Table 6, there were no differences in effect between the 2.5 ml and 5 ml treatments in inducing mosquito mortality rate.
Mortality rate (mortality per 24 h post-exposure) of An. arabiensis (a), An. funestus (b) and Culex spp. (c) between the huts that had transfluthrin-treated sisal baskets and LLINs to those with untreated counterparts and LLINs. The error bars represent the 95% confidence intervals, CI. Abbreviations: LLINs, long-lasting insecticidal net, TF, transfluthrin
Discussion
Here, we investigated the complementary effects of transfluthrin treated baskets combined with LLINs in terms of mosquito deterrence, biting rate and 24 h mortality. We show that transfluthrin treated baskets provided comprehensive protection against An. arabiensis than An. funestus or Culex spp.
Long-lasting insecticidal nets confer protection via a range of modes of action, including excito-repellency, induced mortality of mosquitoes as well as providing physical barrier [36]. However, the emergence of insecticide resistance is undermining the benefits of LLINs and efforts towards malaria elimination [4,5,6,7]. Changing biting behavior and residual malaria transmission [37,38,39] have also significantly reduced the outputs of LLINs and IRS, which calls for complementary strategies.
A combination of transfluthrin-treated sisal baskets and LLINs reduced the overall numbers of indoor density of An. arabiensis mosquitoes by three quarters, compared to LLINs with untreated sisal baskets (Table 1, Fig. 2). However, this reduction was not observed for An. funestus and for Culex spp. Preceding studies, for example Hill et al. [40], demonstrated that a combination of transfluthrin-treated mosquito coil and LLINs resulted in massive reduction of indoor mosquito densities. Similarly, Ogoma et al. [20] demonstrated that combination of transfluthrin-treated coils and LLINs resulted in reduction of indoor mosquito densities. These studies suggest the combination of transfluthrin-based spatial repellents and LLINs may reduce the number of mosquitoes entering dwellings, thereby reducing the risk of malaria transmission.
Our findings support a most recently developed mathematical model, which suggested that combining a highly-toxic insecticide and an efficacious repellent could combat insecticide resistance while protecting people from mosquito bites [41].
Secondly, transfluthrin-treated sisal baskets reduced exposure to early evening bites of An. arabiensis mosquitoes, where LLINs alone may not have been effective (Table 2, Fig. 3). A similar effect, albeit lower, was observed with An. funestus and Culex spp. Didzie et al. [12] demonstrated a dramatic reduction in indoor mosquitoes bites when LLINs were used in combination with topical repellent (NO MAS, a water-based lotion with its principle active ingredient para-methane-diol and lemongrass). Similarly, Syafruddin et al. [42] demonstrated that a combination of LLINs and topical repellent (picaridin, KBR3023, SC Johnson, Racine, WI, USA) reduced indoor mosquito biting rates. The risk of malaria transmission is highest before bed time, considering the fact that LLINs will not be in use at that time. Spatial repellents that provide protection to multiple people in a wide area would be a complementary strategy to LLNs [43].
Mathematical models applied in previous studies postulate that a combination of repellent and LLINs attenuate community-wise benefit by diverting the vectors away from lethal, insecticide treated surfaces [44]. Surprisingly, a 10-fold increase in mortality of An. arabiensis was observed when transluthrin was used in combination with LLINs (Table 3, Fig. 4). Previously, Ogoma et al. [20] also demonstrated an increase in mortality of An. arabiensis and An. gambiae (s.s.) in the presence of transfluthrin coils. However, we did not observe any added benefits of combining transfluthrin decorated baskets with LLINs in terms of inducing mortality of An. funestus and Culex spp. The low mortality observed for An. funestus may be partly explained by pyrethroid resistance exhibited by these mosquitoes as demonstrated previously [26], and confirmed recently [27]. The findings from this study indicate that the efficacy of both 2.5 ml and 5.0 ml 97% transfluthrin treatments was similar (Tables 4, 5 and 6, Figs. 2, 3 and 4). Therefore, a lower dose is recommended for use in future studies.
Combining transfluthrin-treated household decorations and permethrin-treated LLINs was beneficial, and potentially enhanced protection by LLINs, against indoor biting malaria vectors by reducing indoor mosquito density and biting rate and increasing 24 h mortality. Transfluthrin is a pyrethroid, and its efficacy was less pronounced on suspected pyrethroid resistant An. funestus (s.l.). This calls for frequent insecticide susceptibility tests to monitor emergence of resistance.
Conclusions
Here, we have demonstrated that transfluthrin-treated emanators combined with LLINs reduce indoor mosquito entry and protect people against indoor mosquito bites when LLINs are not in effect. The emanators increase mortality of major malaria vectors in the area. Future studies should focus on measuring epidemiological endpoints of these combined interventions.
Abbreviations
- GLMMs:
-
Generalized linear mixed effects models
- HLC:
-
Human landing catch
- IRS:
-
Indoor residual spraying
- IHI:
-
Ifakara Health Institute
- IRB:
-
Institutional review board
- LLINs:
-
Long-lasting insecticidal nets
- mRDT:
-
Malaria rapid diagnostic test
- NIMR:
-
National Institute for Medical Research
- OR:
-
Odds ratio
- PCR:
-
Polymerize chain reaction
- RR:
-
Relative rate
- TF:
-
Transfluthrin
References
Noor AM, Kinyoki DK, Mundia CW, Kabaria CW, Mutua JW, Alegana VA, et al. The changing risk of Plasmodium falciparum malaria infection in Africa: 2000-10: a spatial and temporal analysis of transmission intensity. Lancet. 2014;383:1739–47.
Steketee RW, Campbell CC. Impact of national malaria control scale-up programmes in Africa: magnitude and attribution of effects. Malar J. 2010;9:299.
O'Meara WP, Mangeni JN, Steketee R, Greenwood B. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect Dis. 2010;10:545–55.
Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27:91–8.
Gatton ML, Chitnis N, Churcher T, Donnelly MJ, Ghani AC, Godfray HC, et al. The importance of mosquito behavioural adaptations to malaria control in Africa. Evolution. 2013;67:1218–30.
Ranson H, Lissenden N. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria ontrol. Trends Parasitol. 2016;32:187–96.
Hemingway J, Ranson H, Magill A, Kolaczinski J, Fornadel C, Gimnig J, et al. Averting a malaria disaster: will insecticide resistance derail malaria control? Lancet. 2016;387:1785–8.
Pates H, Curtis C. Mosquito behavior and vector control. Annu Rev Entomol. 2005;50:53–70.
Alaii JA, van den Borne HW, Kachur SP, Shelley K, Mwenesi H, Vulule JM, et al. Community reactions to the introduction of permethrin-treated bed nets for malaria control during a randomized controlled trial in western Kenya. Am J Trop Med Hyg. 2003;68:128–36.
Kirby MJ, Green C, Milligan PM, Sismanidis C, Jasseh M, Conway DJ, et al. Risk factors for house-entry by malaria vectors in a rural town and satellite villages in The Gambia. Malar J. 2008;7:2.
Deressa W, Yihdego YY, Kebede Z, Batisso E, Tekalegne A, Dagne GA. Effect of combining mosquito repellent and insecticide treated net on malaria prevalence in southern Ethiopia: a cluster-randomised trial. Parasit Vectors. 2014;7:132.
Dadzie S, Boakye D, Asoala V, Koram K, Kiszewski A, Appawu M. A community-wide study of malaria reduction: Evaluating efficacy and user-acceptance of a low-cost repellent in northern Ghana. Am J Trop Med Hyg. 2013;88:309–14.
Kimani E, Vulule J, Kuria I, Mugisha F. Use of insecticide-treated clothes for personal protection against malaria: a community trial. Malar J. 2006;5:63.
Moore SJ, Davies CR, Hill N, Cameron MM. Are mosquitoes diverted from repellent-using individuals to non-users? Results of a field study in Bolivia. Tropical Med Int Health. 2007;12:532–9.
Wilson AL, Chen-Hussey V, Logan JG, Lindsay SW. Are topical insect repellents effective against malaria in endemic populations? A systematic review and meta-analysis. Malar J. 2014;13:446.
Sangoro O, Kelly AH, Mtali S, Moore SJ. Feasibility of repellent use in a context of increasing outdoor transmission: a qualitative study in rural Tanzania. Malar J. 2014;13:347.
Schreck CE, Gilbert IH, Weidhaas DE, Posey KH. Spatial action of mosquito repellents. J Econ Entomol. 1970;63:1576–8.
World Health Organization. Guidelines for efficacy testing of household insecticide products mosquito coils, vaporizer mats, liquid vaporizers, ambient emanators and aerosols. Geneva: WHO; 2009.
Ogoma SB, Ngonyani H, Simfukwe ET, Mseka A, Moore J, Maia MF, et al. The mode of action of spatial repellents and their impact on vectorial capacity of Anopheles gambiae sensu stricto. PLoS One. 2014;9:e110433.
Ogoma SB, Lorenz LM, Ngonyani H, Sangusangu R, Kitumbukile M, Kilalangongono M, et al. An experimental hut study to quantify the effect of DDT and airborne pyrethroids on entomological parameters of malaria transmission. Malar J. 2014;13:131.
Okumu FO, Madumla EP, John AN, Lwetoijera DW, Sumaye RD. Attracting, trapping and killing disease-transmitting mosquitoes using odor-baited stations - the Ifakara odor-baited stations. Parasit Vectors. 2010;3:12.
Okumu FO, Killeen GF, Ogoma S, Biswaro L, Smallegange RC, Mbeyela E, et al. Development and field evaluation of a synthetic mosquito lure that is more attractive than humans. PLoS One. 2010;5:e8951.
Renggli S, Mandike R, Kramer K, Patrick F, Brown NJ, McElroy PD, et al. Design, implementation and evaluation of a national campaign to deliver 18 million free long-lasting insecticidal nets to uncovered sleeping spaces in Tanzania. Malar J. 2013;12:85.
THMIS. Results from the 2011-12 Tanzania HIV/AIDS and Malaria Indicator Survey and Malaria Indicator Survey. 2012.
President's Malaria Iniative. Tanzania Malaria Operational Plan For Year 2016. 2016.
Lwetoijera DW, Harris C, Kiware SS, Dongus S, Devine GJ, McCall PJ, et al. Increasing role of Anopheles funestus and Anopheles arabiensis in malaria transmission in the Kilombero Valley, Tanzania. Malar J. 2014;13:331.
Kaindoa EW, Matowo NS, Ngowo HS, Mkandawile G, Mmbando A, Finda M, et al. Interventions that effectively target Anopheles funestus mosquitoes could significantly improve control of persistent malaria transmission in south-eastern Tanzania. PLoS One. 2017;12:e0177807.
Ogoma SB, Ngonyani H, Simfukwe ET, Mseka A, Moore J, Killeen GF. Spatial repellency of transfluthrin-treated hessian strips against laboratory-reared Anopheles arabiensis mosquitoes in a semi-field tunnel cage. Parasit Vectors. 2012;5:54.
Govella NJ, Ogoma SB, Paliga J, Chaki PP, Killeen G. Impregnating hessian strips with the volatile pyrethroid transfluthrin prevents outdoor exposure to vectors of malaria and lymphatic filariasis in urban Dar es Salaam, Tanzania. Parasit Vectors. 2015;8:322.
Masalu JP, Finda M, Okumu FO, Minja EG, Mmbando AS, Sikulu-Lord MT, et al. Efficacy and user acceptability of transfluthrin-treated sisal and hessian decorations for protecting against mosquito bites in outdoor bars. Parasit Vectors. 2017;10:197.
Ogoma SB, Mmando AS, Swai JK, Horstmann S, Malone D, Killeen GF. A low technology emanator treated with the volatile pyrethroid transfluthrin confers long term protection against outdoor biting vectors of lymphatic filariasis, arboviruses and malaria. PLoS Negl Trop Dis. 2017;11:e0005455.
Gillies MT, Coetzee M. A Supplement to Anophelinae of African of South of the Sahara (Afrotropical region). S African Inst Med Research. 1987;50:1–143.
Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–9.
Koekemoer LL, Kamau L, Hunt RH, Coetzee M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg. 2002;66:804–11.
Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Soft. 2015;67:1–48.
Killeen GF, Smith TA, Ferguson HM, Abdulla S, Mshinda H, Lengeler C, Kachur SP. Preventing childhood malaria in Africa by protecting adults from mosquitoes with insecticide treated-nets. PLoS Med. 2007;4:e229.
Govella NJ, Ferguson H. Why use of interventions targeting outdoor biting mosquitoes will be necessary to achieve malaria elimination. Front Physiol. 2012;3:199.
Russell T, Govella N, Azizi S, Drakeley C, Kachur SP, Killeen G. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80.
Killeen GF. Characterizing, controlling and eliminating residual malaria transmission. Malar J. 2014;13:330.
Hill N, Zhou HN, Wang P, Guo X, Carneiro I, Moore SJ. A household randomized, controlled trial of the efficacy of 0.03% transfluthrin coils alone and in combination with long-lasting insecticidal nets on the incidence of Plasmodium falciparum and Plasmodium vivax malaria in Western Yunnan Province, China. Malar J. 2014;13:208.
Lynch PA, Boots M. Using evolution to generate sustainable malaria control with spatial repellents. eLife. 2016;5:e15416.
Syafruddin D, Bangs MJ, Sidik D, Elyazar I, Asih PB, Chan K, et al. Impact of a spatial repellent on malaria incidence in two villages in Sumba, Indonesia. Am J Trop Med Hyg. 2014;91:1079–87.
Achee NL, Bangs MJ, Farlow R, Killeen GF, Lindsay S, Logan JG, et al. Spatial repellents: from discovery and development to evidence-based validation. Malar J. 2012;11:164.
Killeen GF, Chitnis N, Moore SJ, Okumu FO. Target product profile choices for intra-domiciliary malaria vector control pesticide products: repel or kill? Malar J. 2011;10:207.
Acknowledgements
We sincerely thank volunteers who collected mosquitoes in experimental huts, as well field technicians Mr Severine Ignas Chanja and Mr Shabani Amidu Dulango who conducted the morphological identification of mosquitoes. Laboratory staff at Ifakara Health Institute, Mr Francis Tumbo, Faraji Abilah and Mr Said Abbasi who conducted PCR analysis of mosquito samples.
Funding
The study was funded by Grand Challenges Canada (Grant number: S5 0440-01). FOO was also supported by Wellcome Trust Intermediate Fellowship in Public Health and Tropical Medicine (Grant number: WT102350/Z/13/Z). ASM is also supported by Wellcome Trust Masters Fellowship (Grant number: WT106356/Z/14/Z).
Availability of data and materials
Data supporting the conclusions of this article are included within the article.
Author information
Authors and Affiliations
Contributions
JPM designed and conducted experiments, analyzed the data and drafted the manuscript. FOO contributed to the study design, data analysis and revised the manuscript. ASM revised the manuscript. MTSL conceived the idea, helped to acquire funds and edited the manuscript. SBO conceived the study, obtained funding, guided data analysis, edited and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institute Review Board of Ifakara Health Institute IHI/IRB/EXT/07 and Medical Research Coordinated Committee of the National Institute for Medical Research of the United Republic of Tanzania (NIMR/HQ/R.8a/Vol.1X/2199). Study participants were recruited after signing informed consent forms. Participants were trained on conducting HLC and how to avoid excessive exposures to mosquitoes bites. To stop malaria transmission during HLC, volunteers were given malaria prophylaxis drugs: Mefloquine (Mepha LLC, Aesch-Basel Switzerland). They were also screened for malaria parasites once every week for the entire experimental period using rapid diagnostic test kits mRDT- (MAL- Pf®, ICT Diagnostics, Cape Town, South Africa). There were no reported cases of malaria throughout the experiment. This manuscript has been approved by Dr Susan F. Rumisha, on behalf of the Director General of The National Institute for Medical Research, United Republic of Tanzania, with reference number: NIMR/HQ/P.12 VOL. XXIII/20.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Masalu, J.P., Okumu, F.O., Mmbando, A.S. et al. Potential benefits of combining transfluthrin-treated sisal products and long-lasting insecticidal nets for controlling indoor-biting malaria vectors. Parasites Vectors 11, 231 (2018). https://doi.org/10.1186/s13071-018-2811-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-018-2811-y