Abstract
Background
Previous studies have shown that insulin receptors in schistosomes, triggered by host insulin, play an important role in parasite growth, development and fecundity by regulating glucose metabolism. However, limited information is available on the recently identified endogenous insulin-like peptide (ILP) in blood flukes.
Results
We isolated ILPs from Schistosoma japonicum (SjILP) and S. recognised (SmILP) and present results of their molecular and structural analysis. SjILP shares 63% amino acid identity with SmILP, but only 18% identity with human insulin. There is high cross immunological reactivity between the S. japonicum and S. mansoni ILPs as observed in western blots using an anti-SjILP polyclonal antibody. ADP binding/hydrolysis ability was observed in both SjILP and SmILP, but not in human insulin, suggesting a parasite-specific role for ILP compared with host insulin. Protein binding assays using the Octet-RED system showed SjILP binds S. japonicum IRs (SjIR1 and SjIR2) strongly. An anti-phospho antibody against extracellular signal-regulated kinase (Erk) recognised a 44-kDa target band in an extract of adult worms after stimulation by rSjILP in vitro, suggesting an important role for SjILP in activating SjIRs and in regulating downstream signal transduction. Immunolocalisation showed SjILP is located on the tegument and the underlying musculature, similar to that observed for SjIR1, but it is also present throughout the parenchyma of males and in the vitelline cells of females, the same locations as SjIR2 described in an earlier published study of ours. The same localisation of SjILP and the SjIRs is suggestive of an interaction between the insulin-like peptide and the IRs. In addition to binding host insulin, schistosomes also can express their own endogenous ILPs, which can activate the parasite insulin signal pathway, thereby playing a critical role in worm growth, development and fertility.
Conclusions
These findings shed new light on ILPs in schistosomes, providing further insight into the distinct and specialised functions of SjIR1 and 2 in S. japonicum and their interaction with host insulin.
Similar content being viewed by others
Background
The life-cycle of schistosomes involves snail intermediate hosts and definitive mammalian hosts including humans, in whom schistosomes can survive for decades [1]. Remarkably, the highly sophisticated relationship between schistosomes and their mammalian hosts seems to involve exploitation by these parasites of host endocrine and immune signals [2, 3]. By binding many ligands including hormones, growth factors, cytokines, receptor tyrosine kinases (RTKs), which are high-affinity cell surface receptors, can be activated to trigger different signalling cellular cascades for the regulation of cell proliferation, differentiation and survival [4]. Numerous data have demonstrated that schistosome RTKs can bind host growth factors, activating conserved kinase signalling pathways which are involved in worm growth and development [5]. RTK signalling is active in the reproductive organs of schistosomes where it likely regulates gametogenesis, sexual maturation and egg production [6]. Given the fact that schistosome eggs play key roles in both parasite transmission and pathogenesis in mammalian hosts, RTKs and their signalling partners represent potentially important targets for chemotherapeutic attack. Genome-wide searches have recently reported the presence of an insulin signalling pathway (a typical RTK signal pathway) in S. japonicum [7], S. mansoni [8] and S. haematobium [9, 10]. Further, classic downstream components of the insulin pathway have been identified in schistosomes, including Src homology-containing (SHC), Src homology 2-B proteins, phosphoinositide-3-kinase (PI3K), extracellular signal-regulated kinase (ERK), glycogen synthase (GYS) and glucose transport protein 4 (GTP4) [7–11]. Previous studies have shown that host insulin can stimulate the metabolism, growth and development of adult [12] and larval schistosomes [13] by increasing glucose uptake and enhancing the survival of schistosome larvae [14]. Moreover, the presence of human insulin increases the glucose content of adult male and female S. japonicum 1.7- and 2.9-fold, respectively, in vitro [15], suggesting insulin plays a key role in regulating glucose uptake in this parasite. Our previous microarray analysis also demonstrated that host insulin plays an important role in insulin signalling in schistosomes by stimulating glucose metabolism through up-regulation of the phosphoinositide-3-kinase (PI3K) sub-pathway [15]. In addition, host insulin is also necessary for worm fecundity due to its activation of the mitogen-activated protein kinases (MAPK)/ERK sub-pathway [15], thereby regulating cell proliferation, differentiation and survival. Two types of insulin receptors have been isolated from S. mansoni (SmIR-1 and SmIR-2) [16] and S. japonicum (SjIR1 and SjIR2) [17], which were shown to bind human insulin in two-hybrid analysis and the Octet-RED system [18]. In summary, these studies imply that insulin signalling, activated by host insulin, plays an important role in the growth, development and fecundity of schistosomes.
There have been 37 insulin-like ligands (ILPs) identified in free-living Caenorhabditis. elegans [19] but limited information is available on the presence and biological importance of insulin-like peptides (ILPs) in schistosomes and other platyhelminths. Genome-wide searches for ILPs have recently reported the presence of two ILPs in Taenia and Echinococcus spp. [20]. Moreover, in Taenia solium, one ligand, ILP-1, was shown to be predominantly expressed in ovarian tissues, suggesting an important role in worm fecundity [20]. However, to date, only one ILP has been identified in S. japonicum and S. mansoni [20], and there is no information to indicate how schistosomes utilise the ILP, and what its function might be in these parasites. In this study, we employed molecular structural analysis, protein interaction and phosphorylation assays, and used immunolocalisation procedures to explore the functional roles of ILPs in schistosomes.
Methods
Parasites
Schistosoma japonicum adult worms were collected by perfusion of female Animal Resource Centre (ARC) Swiss mice infected percutaneously with 60 cercariae (Anhui population, mainland China) shed from Oncomelania hupensis hupensis snails as described [21] at the QIMRB animal facility. S. mansoni adult worms were collected by perfusion of ARC Swiss mice infected percutaneously with 160 cercariae shed from Biomphalaria glabrata snails [22]. Infected B. glabrata snails were kindly provided by the NIAID Schistosomiasis Resource Centre, Biomedical Research Institute, Rockville, Maryland, USA.
Cloning insulin-like peptides (ILP) from S. japonicum, S. mansoni
A Qiagen RNeasy kit (Qiagen, Hilden, Germany) was used to purify total RNA from adult S. japonicum and S. mansoni. A one-step RT-PCR (Qiagen) kit was employed to amplify specific cDNA. Based on a sequence in GenBank for an ILP in S. japonicum (AY814982), primers for SjILP were designed (Additional file 1: Table S1) to obtain the full-length cDNA. Using BLAST on the SjILP sequence in SchistoDB (http://schistodb.net/schisto), we obtained predicted genomic sequences for ILP in S. mansoni (SmILP), S. haematobium (ShILP) and designed appropriate primers for amplifying full-length cDNA sequences for SmILP (Additional file 1: Table S1).
Sequence and structural analysis
Searches for homologous insulin-like peptide sequences were performed using BLAST on the NCBI website (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and the WormBase ParaSite website (http://parasite.wormbase.org/Multi/Tools/Blast). Molecular weight and isoelectric point determinations were performed using the ExPASy-Compute pI/Mw tool (http://web.expasy.org/compute_pi/). Signal sequences were identified using the SignalP 4.1 server (http://www.cbs.dtu.dk/services/SignalP/) [23]. The PHYRE2 protein fold recognition server (http://www.sbg.bio.ic.ac.uk/phyre2/) was used to generate three-dimensional (3D) models [24] of the schistosome ILP, and binding site predictions were carried out using the 3DLigandSite (http://www.sbg.bio.ic.ac.uk/3dligandsite/) [25].
Protein expression, purification and antibody generation
C-terminal fragments of SjILP (from T31 to S130) and SmILP (T32-N133) were amplified and cloned into the pET28b vector (Novagen, Madison, USA) using a forward primer with a BamH I restriction site (underlined) and a reverse primer with a Sal I restriction site (underlined). Forward (5'-AAG GAT CCG ACA CAT AGT TTA CCA GAA TTA CAA A-3') and reverse (5'-AAG TCG ACG CTA GGA TTG CAA AAT TGT TCT-3') primers for SjILP and forward (5'- AAG GAT CCG ACA CAA ACT TTA ACA GAA TTG AAT AC-3') and reverse (5'- AAG TCG ACA TTT GGA TTA CAA AAT TGT TCT-3') primers for SmILP were used for amplification and cloning into the pET28b vector.
The reconstructed vectors were then transformed into Escherichia coli (BL21 strain) for expression induced with 1 mM IPTG (isopropyl thio-b-D-galactoside) at 37 °C for 3 h. Recombinant SjILP (rSjILP) and SmILP (rSmILP) proteins were purified from inclusion bodies by chromatography using a Ni-NTA His-tag affinity kit (Novagen) under denaturing conditions using 6 M guanidine according to the manufacturer’s instructions. Purified proteins were then refolded in buffer (55 mM Tris-HCl, 21 mM NaCl, 0.88 mM KCl, 2 mM reduced glutathione, 0.4 mM oxidized glutathione, 1 mM EDTA, 10% w/v sucrose, pH 8.2).
Polyclonal antibodies were raised against the SjILP fusion protein in 8 weeks old female Balb/c mice. Briefly, five mice were immunised three times each with 25 μg recombinant protein adjuvanted with Quil A (Superfos, Denmark) at 3-week intervals [26]. The immunisations were delivered by intraperitoneal injection. Blood was collected 2 weeks after the third injection. The titre of the antibody was determined using an enzyme-linked immunosorbent assay (ELISA). Briefly, Maxisorb immunoplates (Nalge Nune International, USA) were coated overnight at 4 °C with rSjILP protein (100 μl of 0.5 μg/ml) in coating buffer (100 μl/well). After three washes with 0.05% (v/v) Tween in PBS (PBST), wells were blocked with 200 μl of 5% (v/v) skim milk in PBS (SMP) and incubated for 1 h at 37 °C. The mouse anti-SjILP serum was serially diluted (from 1:200 to 1:102,400) in SMP, and 100 μl in duplicate of each dilution were added to individual wells. After incubation at 37 °C for 1 h, the wells were washed with PBST for three times and 100 μl (1:2,000 dilution) of horseradish peroxidise (HRP)-conjugated goat anti-mouse IgG (Invitrogen) was added. After incubation at 37 °C for 1 h, the wells were washed with PBST for five times, 100 μl of substrate solution [2,2-azino-di-(ethyl-benzithiozolin sulfonate)] (Sigma-Aldrich, Castle Hill, Australia) was added, the wells were then incubated at room temperature and read on a plate reader using Microplate manager software (Bio-Rad, Mississauga, Canada).
Western blot analysis
The mouse anti-SjILP serum was used in Western blotting to probe electrophoresed proteins; these proteins included: 1) Purified recombinant SjILP and SmILP; 2) Tegument protein and residual carcass protein [27] extracted from adult S. japonicum freshly perfused from mice percutaneously infected with S. japonicum as described earlier; 3) Native S. mansoni protein extracts prepared [17] from adult worms freshly perfused from mice percutaneously infected with S. mansoni. An adult worm antigen preparation (SWAP) was made from schistosomes as described [17], after adult worms were washed in perfusion buffer (8.5 g NaCl and 15 g NaCitrate in 1 l of water) three times, to minimise contamination of the schistosome protein extract with host components. To determine whether there was any immunological cross-reactivity between human insulin (Sigma-Aldrich) and SjILP, the mouse anti-SjILP antibody, a mouse anti-human insulin monoclonal antibody (Abcam, Cambridge, UK) and naïve mouse sera as control were used in Western blotting to probe equal quantities of electrophoresed human insulin and rSjILP. The concentrations of all proteins used were determined using the Bio-Rad protein assay dye reagent (Bio-Rad).
Protein samples were separated on 15% (w/v) SDS-PAGE gels and transferred to an Immun-Blot low fluorescence-PVDF membrane. Overnight blocking was performed with Odyssey buffer containing 4% (v/v) goat serum at 4 °C. Then, the membrane was subjected to incubation with the mouse anti-SjILP or the anti-human insulin monoclonal antibody (diluted in Odyssey buffer and 0.1% (v/v) Tween-20) for 1 h followed by incubation with IRDye-labeled 680LT goat anti-mouse IgG antibody (Li-COR Biosciences) (1:15,000 diluted in Odyssey buffer with 0.1% Tween-20 and 0.01% SDS) for 1 h on a shaker in a dark chamber. After a final wash with distilled water, the membrane was allowed to dry in the dark and visualised using the Odyssey CLx Infrared Imaging System [22].
Detection of mitogen-activated protein kinase (MAPK) using an anti-phospho-P44/42 MAPK (Erk1/2) antibody
It had been shown previously that an anti-phospho-P44/42 MAPK (Erk1/2) antibody could recognize exclusively activated (phosphorylated) forms of this kinase in S. mansoni [28] because of the high sequence homology in key phosphorylation sites between kinases from schistosomes and humans, and because phosphorylation at these sites is vital for enzyme activation [28]. Based on the fact that the p44/42 MAPK (Erk1/2) signalling pathway can be activated in response to extracellular stimuli including growth factors [29], an anti-phospho-P44/42 MAPK (Erk1/2) antibody (Cell Signaling Technology, New England Biolabs, Ipswisch, USA) was used to detect the presence of an Erk band in an extract of adult S. japonicum following the incubation of adult worms in the presence of human insulin (Sigma-Aldrich) or rSjILP. Briefly, freshly perfused adult S. japonicum worms were cultured over night at 37 °C in DMEM (Gibco, Waltham, USA); then the worms were incubated for 30 min in fresh DMEM containing: 1). rSjILP (1 μM); 2). rSjILP (1 μM) + human insulin (1 μM); 3). human insulin (1 μM).; and 4). neither rSjILP nor human insulin. Worms from the different groups were then collected for SWAP extraction which was undertaken in the presence of HALT protease/phosphatase inhibitor cocktail (ThermoFisher, Waltham, USA). SDS-PAGE/Western blotting was carried out as described above using the Odyssey system. Tyrosine kinase activity was detected using a mouse anti-phospho-P44/42 MAPK (Erk1/2) antibody (1:1,000) as primary antibody (1:1,000 dilution in Odyssey buffer and 0.1% Tween-20) for 1 h followed by incubation with IRDye-labeled 680LT goat anti-mouse IgG antibody (Li-COR Biosciences, Lincoln, USA) (1:15,000 diluted in Odyssey buffer with 0.1% Tween-20 and 0.01% SDS). An anti-actin antibody (Sigma-Aldrich) (1:150) [30] was used to assess protein-loading differences. OdysseyClassic 3.0 software was used for Western blot quantification.
Immunolocalisation
Horseradish peroxidise (HRP) labelling was used for the immunolocalisation of SjILP in adult S. japonicum as described [27]. Freshly perfused male and female worms were fixed in 100% methanol, embedded in Tissue-Tek Optimal Cutting Temperature (OCT) compound (ProSciTech, Queensland, Australia), and 7.0 μm cryostat sections were produced. HRP labelling was performed according to standard procedures [17]. The primary antibody solution was a 1:100 dilution of the mouse anti-SjILP serum and normal mouse serum (1:100) was used as a control. Non-specific antibody binding was inhibited by incubating the sections in 10% (v/v) normal goat serum in PBS. ImmPRESS HRP Anti-mouse IgG (Peroxidase) Polymer (Vector Labs, California, USA) was used as a secondary antibody to detect immunolocalisation. Slides were scanned and digitised using a ScanScope XT (Aperio, California, USA).
HRP labelling was also used with a mouse anti-human insulin monoclonal antibody (Abcam, Cambridge, UK) to determine whether human insulin could bind to freshly perfused adult S. japonicum worms washed 3 times with perfusion buffer. The HRP labelling was performed according to standard procedures as described above. The primary antibody solution was a 1:100 dilution of the mouse anti-human insulin monoclonal antibody and normal mouse serum (1:100) was used as a control. Non-specific antibody binding was inhibited by incubating the section in 10% (v/v) normal goat serum in PBS.
Adenosine diphosphate (ADP) assays
To measure the level of hydrolysis of ADP by the schistosome ILP, ADP assay kits (Sigma-Aldrich) were used to measure ADP levels of recombinant schistosome ILPs after their interaction with different concentrations of ADP. ADP levels in various proteins (0.13 mg/ml) were determined following their incubation for 20 min with different concentrations of ADP (0, 5, 15, 30 μM). These proteins included: (i) rSjILP; (ii) rSmILP; (iii) S. japonicum tegumental protein as a positive control [31, 32], extracted from adult S. japonicum using the freeze/thaw/vortex method [33]; (iv) human insulin; and (v) S. japonicum Kunitz type protease inhibitor (rSjKI-1) [34]. Human insulin and rSjKI-1 were employed as negative controls, both of which were shown to have no ADP binding sites after analysis using the 3DLigandSite software.
Binding between SjILP/insulin and S. japonicum insulin receptors
Binding assays were performed using the Octet Red system (FortéBio, Menlo Park, CA, USA) in 96-well microplates at 25 °C as described [18]. Briefly, assays were carried out by placing the Streptavidin Biosensors (FortéBio, Fremont, USA) in the microplate wells and measuring changes in layer thickness (in nanometers, nm) over time (in seconds). Protein/peptides were biotinylated using a NHS-PEO4-biotin kit (Thermo Scientific, Rockford, IL, USA) and then they were immobilised to the Streptavidin Biosensors. First, a duplicate set of sensors was rinsed in kinetic buffer (1 mM phosphate, 15 mM NaCl, 0.1 mg/ml BSA, 0.002% Tween-20) for 300 s which served as the baseline. Secondly, sensors were immobilised for 600 s with 200 μl culture medium containing biotinylated protein/peptide. Thirdly, sensors were washed in kinetic buffer for another 600 s. Finally, sensors were exposed to a series of diluted samples run in 200 μl volumes in the same assay. BSA was used as a negative control. The association of the two proteins was monitored for 1000 s followed by dissociation in the kinetic buffer for 1000 s. Data analysis from the FortéBio Octet RED instrument included a double reference subtraction. Sample subtraction was performed using the BSA as a reference control, and sensor subtraction was performed on all samples automatically using Octet User Software 7 [35].
Two binding assays were undertaken as follows:
-
(i)
To test the binding ability between rSjILP and recombinant proteins coding the ligand domain of SjIR1 (rSjLD1)/ligand domain of SjIR2 (rSjLD2) [18]. Briefly, biotinylated rSjILP (150 ng/μl) was immobilised to the Streptavidin Biosensors as described above and then exposed to a series of dilutions of the rSjLD1 or rSjLD2 proteins (7, 14, 28, 56, 112 ng/μl), after washing in the kinetic buffer. The association of the two proteins was monitored followed by dissociation in the kinetic buffer as described above.
Furthermore, to compare the binding affinity of SjLDs to rSjILP/human insulin, biotinylated rSjILD1 (150 ng/μl)/SjLD2 (100 ng/μl) was immobilized to the Streptavidin Biosensors, and then exposed to rSjILP and human insulin at 65 and 130 ng/μl, respectively, followed by dissociation by kinetic buffer. The association between the rSjLDs and rSjILP was real-time monitored followed by dissociation in the kinetic buffer.
-
(ii)
To compare the binding affinity of rSjILP to SjIR peptides (including analogues 1, 3, 13, 15, the first two derived from SjLD1 and the latter two from SjLD2) [36] which have been demonstrated as parasite-specific insulin binding epitopes with the strong binding ability to human insulin. The positions of the four analogues used in this study are provided in the Additional file 2: Figure S1. Peptides were synthesised and purified as previously described [36]. SjILP immobilized sensors were exposed to analogue 1 (1.85–11.7 μM), analogue 3 (0.5–1.85 μM), analogue 13 (4.1–15.3 μM) and analogue 15 (0.21–1.07 μM) followed by dissociation by kinetic buffer. The association between rSjILP and the different peptides was real-time monitored followed by dissociation in the kinetic buffer.
Results
Sequences of schistosome insulin-like peptides
Complete cDNA sequences obtained for schistosome ILPs comprised an open reading frame (ORF) of 390 bp encoding 130 amino acids in S. japonicum (SjILP), an ORF of 399 bp encoding 133 amino acids in S. mansoni (SmILP). The cDNA sequence for ShILP was predicted based on genomic data published recently for S. haematobium [9, 10]; it showed an ORF of 396 bp encoding 132 amino acids in S. haematobium (ShILP). SjILP shares 63% and 65% amino acid sequence identity with SmILP and ShILP, respectively. There is 79% amino acid sequence identity between SmILP and ShILP. SjILP shares only 18% amino acid identity with human insulin. Using the SignalP 4.1 server, we found SjILP contains a signal peptide (M1-E24), a B chain (I25-F75) and an A chain (F94-S129); SmILP has a B chain (M26-K78) and an A chain (F97-N133) but no predicted signal peptide; ShILP contains a signal peptide (M1-E24), a B chain (I25-L76) and an A chain (F116-N152).
Schistosome ILPs have a basic insulin structure (Additional file 3: Figure S2) containing an A peptide and B peptide linked by disulfide bridges. The structures show conserved cysteines in the A chain with the signature amino acid sequence CCCX(2)CX(8)C present.
SjILP, SmILP and ShILP have the same exon-intron arrangement whereby two exons are separated by a large intron of 8207 bp, 11,235 bp and 11,868 bp in S. japonicum, S. mansoni and S. haematobium, respectively.
The tertiary protein structures of SjILP, SmILP and ShILP were predicted using PHYRE2, and they share very similar structure as shown in Additional file 3: Figure S2, which is the predicted SjILP 3D structure. Schistosome ILPs contain 85–87% of Alpha helix and 12% transmembrane helix (Y15-T30 in SjILP, L15-D30 in SmILP, H15-T30 in ShILP). Of note, we found three predicted ADP binding sites located at HIS59, ARG62, and ARG120 residues in SjILP (Fig. 2b); SmILP has two ADP binding site located at ASN60 and ARG123 (Additional file 3: Figure S2), whereas no ADP binding sites were predicted in ShILP indicating S. haematobium may bind ADP in a different manner compared with the other two species.
ADP assays
Adenosine diphosphate (ADP) is produced from adenosine triphosphate via the action of ATPases and plays a critical role in energy transfer reactions and is more stable than ATP.
To determine the hydrolysis of ADP of schistosome ILPs, we used an ADP assay to measure the ADP levels maintained in proteins following their incubation for 20 min with different concentrations of ADP. Following incubation with ADP, the negative controls, human insulin and rSjKI-1, produced, as expected, similar curves as the assay kit ADP standard curve (Fig. 1). The adult S. japonicum tegument protein extract hydrolysed 75–81% of ADP after incubation with ADP (5–30 μM), confirming the schistosome tegument possesses a protein/proteins which can hydrolyze ADP [32]. SjILP and SmILP consumed 29–39% and 21–34% of ADP, respectively. There was no significant difference in the levels of consumed ADP between SjILP and SmILP following their incubation with different concentrations of ADP, suggesting that both have similar ADP hydrolysis ability.
Adenosine diphosphate (ADP) assays with recombinant proteins (including SjILP and SmILP) and S. japonicum tegument protein. By using an ADP assay kit, ADP concentration (μM) was measured following incubation for 20 min with different proteins (0.13 mg/ml), including SjILP, SmILP, the positive control (adult S. japonicum worm tegument protein extract; SjT) and negative controls (human insulin and rSjKI-1). The assay kit ADP standard curve was obtained using different concentrations of ADP alone. Error bars represent the standard error of the mean (SEM). This experiment was performed three times
Western blot analysis
SDS-PAGE showed purified, and refolded rSjILP and rSmILP each migrated as a single band with the predicted sizes of approximately 15.6 kDa and 16 kDa, respectively (Fig. 2a). ELISA determinations indicated the titer of the mouse anti-SjILP serum used in the Western blot analysis was 1:25,600. Both rSjILP, rSmILP were recognised by the mouse anti-SjILP antibody (1:500) in a western-blot (Fig. 2a). Neither rSjILP nor rSmILP was recognised by naïve mouse sera (data not shown). Further, a band of approximately 16 kDa in extracts of both carcass protein (60 μg/well) and adult S. japonicum tegument protein (60 μg/well) was recognised by the anti-SjILP antibody (1:100) (Fig. 2b) with the signal being more pronounced in the latter. Cross immunological reactivity among the schistosome ILPs was observed in western blots using the anti-SjILP antibody (1:50), as it bound a band of approximately 16 kDa in SWAP from adult S. mansoni (45 μg/well) (Fig. 2b), thereby correlating well with the calculated molecular size of both ILPs; no bands were detectable when SWAP from either schistosome species was probed with naïve mouse serum (data not shown). Furthermore, no cross immunological reactivity was evident between SjILP, and recombinant human insulin was observed in western blots. Anti-SjILP antibody (1:500) only recognised rSjILP (20 μg/well), but did not bind to recombinant human insulin (20 μg/well), while anti-human insulin monoclonal antibody (1:50) only recognised human insulin (0.5 μg/well) at the expected molecular size of approximately 5.8 kDa, but did not react with rSjILP (0.5 μg/well).
Western blot analysis using anti-SjILP serum to probe recombinant proteins (SjILP, SmILP and human insulin) and total extracts from adult schistosomes. a The SDS-PAGE gel of purified and refolded recombinant proteins SjILP (Lane 1) and SmILP (Lane 2). Mouse anti-SjILP antibody was used to probe rSjILP (Lane 3), rSmILP (Lane 4); b Mouse anti-SjILP antibody was used to probe carcass protein (Lane 1) and tegument protein (Lane 2) extracted from adult S. japonicum, and SWAP extracted from adult S. mansoni (Lane 3); c Mouse anti-human insulin monoclonal antibody was used to probe recombinant human insulin (Lane 1) and rSjILP (Lane 2). Mouse anti-SjILP antibody was used to probe recombinant human insulin (Lane 3) and rSjILP (Lane 4). Lanes M1 and M2, PageRuler pre-stained protein ladder in a different size. This experiment was performed three times
To determine whether there was immunological cross-reactivity between rSjILP and human insulin, we used a mouse anti-human insulin monoclonal antibody and the mouse anti-SjILP antibody in Western blots to probe electrophoresed human insulin and rSjILP (Fig. 2). Whereas the anti-SjILP antibody bound rSjILP, it did not bind to human insulin, while the anti-human insulin antibody bound human insulin but not rSjILP.
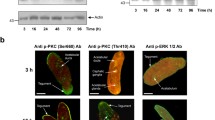
An anti-phospho p44/42 MAPK antibody was used to detect the phosphorylation of Erk in adult S. japonicum stimulated by host insulin or rSjILP. An Erk band, approximately the same size (44 kDa) as observed previously in S. mansoni [37], was recognised by the anti-phospho antibody in protein extracts of adult S. japonicum worms incubated with or without rSjILP and insulin (Fig. 3). A 44 kDa Erk band was recognised in extracts prepared from worms stimulated with rSjILP and from worms co-stimulated with rSjILP and insulin, with no significant difference evident in band intensity between the two extracts (Fig. 3). The Erk band was also detectable in an extract from worms incubated with human insulin only but, compared with that of the worms co-stimulated with rSjILP and insulin, its intensity was reduced by 58% [t (6) = 31.39, P = 0.001] (Fig. 3). The Erk band was detectable in an extract of worms incubated without human insulin and rSjILP, but its intensity was reduced by 30% [t (6) = 4.353, P = 0.049] compared with that in the worm extract incubated with insulin only (Fig. 3) (*P ≤ 0.05, **P ≤ 0.001, ***P ≤ 0.0001).
Effect of stimulation with rSjILP or human insulin on extracellular signal-regulated kinase (Erk) in adult S. japonicum worms. a Anti-actin antibody (upper) and anti-phospho p44/42 MAPK (Erk) antibody (lower) were used to probe in Western blot analysis a protein extract of adult S. japonicum incubated for 30 min with: rSjILP (Lane 1); both rSjILP and human insulin (Lane 2); human insulin (Lane 3); neither rSjILP nor insulin (Lane 4). b Signal intensities of bands recognised by anti-phospho p44/42 MAPK (Erk) antibody in panel A (lower), were measured using the Odyssey Classic Infrared Imager with a scan intensity setting of 5 and sensitivity of 5. Error bars represent the standard error of the mean (SEM). This experiment was performed twice
Distribution of SjILP and human insulin in adult S. japonicum
Indirect immunohistochemistry, incorporating HRP labelling (Fig. 4), indicated SjILP immunoreactivity occurred in the tegument, and the underlying musculature, similar to that observed previously for SjIR1 [17] but was also present throughout the parenchyma of males and vitelline cells of females, the same locations as SjIR2 [17]. No labelling was evident with control sera from pre-immunized mice.
Immunolocalisation of SjILP and human insulin in adult S. japonicum. a Adult male and b female worm sections were labelled with mouse anti-SjILP antibody coupled with anti-mouse HRP and scanned using an Aperio scanner. c Negative control sections of the female worm were incubated with mouse pre-immune serum. d Adult male and e female worm sections were labelled with mouse anti-human insulin monoclonal antibody coupled with anti-mouse HRP and scanned using an Aperio scanner. f Negative control sections of the male worm were incubated with mouse pre-immune serum. Scale-bars: a-f, 10 μm
To investigate further the interaction of human insulin with adult S. japonicum, we used HRP labelling to determine the distribution of human insulin in male and female worms (Fig. 4). Human insulin was detected only on the surface of adult male and female S. japonicum, co-located with SjIR1 [17], probed using the anti-human insulin monoclonal antibody. No labelling was evident with control mouse serum.
Binding assays
Octet RED technology was used to measure the binding affinity between SjILP and SjLDs and between SjILP and peptides, including analogues 1, 3 derived from SjLD1, and analogues 13, 15 derived from SjLD2 [36]. Our previous study showed that analogues 1, 3, 13 and 15 are mainly responsible for host insulin binding affinity in S. japonicum [36]. Interactions between rSjILP and SjLDs are presented in Fig. 5; there was strong interaction in vitro between rSjILP and rSjLD1 [KD = 2.255e-10, coefficient of determination (r 2) = 0.99] and rSjILP and rSjLD2 (KD = 1.6e-10, r 2 = 0.96) detected in the protein concentration range 7–112 μg/ml. Increasing the concentration of SjLD1/2 led to an increased binding response with the dissociation phase slowly decreasing. To compare the binding affinity of the SjLDs to SjILP and human insulin, SjLD1/2 immobilised sensors were exposed to SjILP or insulin at 65 and 130 ng/μl, respectively. At the same protein concentration, SjILP exhibited 4.6–5.5 times stronger binding to SjLD1 (Fig. 5c) and 2.8–3.5 times stronger binding to SjLD2 (Fig. 5d) than human insulin.
Binding affinity assays between SjILP/human insulin and SjLD1/2 using the Octet RED system. The real-time binding response between rSjILP and (a) rSjLD1, (b) rSjLD2 at different protein concentrations (ng/μl) was measured in seconds (s). The parameters of the binding response (nm) and the KD value (M) of the binding between SjILP and SjLD1/2 (7–112 ng/μl) are shown. c A comparison of the SjLD1 binding ability to SjILP and human insulin at concentrations of 65 and 130 ng/μl. d A comparison of the SjLD2 binding ability to SjILP and human insulin at concentrations 65 and 130 ng/μl. The coefficient of determination (R 2) for all the interactions was close to 1.0, indicating a good curve fit
rSjILP strongly bound analogues 1 and 3, exhibiting KD values of 1.99e-09 (r 2 = 0.92, in the peptide concentration range 5.6–36.5 ng/μl) and 6.35e-10 (r 2 = 0.99, in the peptide concentration range 2.8–7.9 ng/μl), respectively (Fig. 6a, b). SjILP was also able to bind analogues 13 and 15 with KD values of 1.68e-09 (r 2 = 0.93, in the peptide concentration range 12.8–57.5 ng/μl) (Fig. 6c) and 2.22e-11 (r 2 = 0.98, in the peptide concentration range 0.9–5.8 ng/μl) (Fig. 6d), respectively.
Binding affinity assays between SjILP and analogues 1, 3, 13, 15 derived from SjLDs, respectively, using the Octet RED system. The binding response between rSjILP and analogues (a) 1, (b) 3, (c) 13, (d)15 at different protein concentrations (μg/ml) was measured in seconds (sec). The parameters of the binding response (nm) and the KD value (M) of the binding affinity between SjILP and peptides at different concentrations are shown. The coefficient of determination (R 2) for all the interactions was close to 1.0, indicating a good curve fit
Discussion
It has been demonstrated that two types of insulin receptors (IR-1 and IR-2) from S. japonicum [17], S. mansoni [16], and E. multilocularis [38] can bind to human insulin, thereby activating the insulin signalling pathway which plays an important role in the regulation of worm glucose uptake, adult worm fecundity and the differentiation of germline stem cell populations [16, 17, 38].
Genome-wide searches have recently identified insulin-like peptides (ILPs) in parasitic flatworms [20], including schistosomes, but no further studies on blood fluke ILPs have been carried out. The ILPs, which initiate insulin signalling by binding to their insulin receptors, are a large multigene family that have been characterised extensively from invertebrates [39, 40]. To better understand the characterisation of ILPs in schistosomes, we isolated the ILP genes from S. japonicum (SjILP) and S. mansoni (SmILP) and showed they exhibit 63% protein sequence similarity and a high level of cross immunological reactivity observed in western blots using an anti-SjILP antibody. Schistosome ILPs may possess conserved features shared by the members of the insulin/insulin-like family, including tertiary structures comprising 85–87% alpha and 12% transmembrane helices; a gene structure featuring two small exons separated by a much larger sized intron; conserved splicing sites, and six strictly conserved cysteines forming three disulfide bonds. The disulfide bonds are predicted to form two inter chain bridges across the two chains and one intra-chain bond on the A chain, which is deemed to be pivotal for the acquisition of the functional structure of the mature dimeric peptide [41].
We were able to confirm that both SjILP and SmILP can bind ADP, as predicted using PHYRE2, whereas, no ADP binding ability was observed with human insulin, suggesting a specific role for ILPs in schistosomes. It has been demonstrated previously that schistosome tegumental extracts possess ATP and ADP hydrolysing activity [31, 32] and ATP hydrolysis commonly leads to the generation of ADP, which is a major agonist of the recruitment and aggregation of platelets [42]. Further studies discovered that the binding or hydrolysis of exogenous ADP led to the inhibition of platelet aggregation and thrombus formation around the worms [43]. It is known that the peptide growth factor insulin-like growth factor (IGF)-1, which is structurally related to insulin, can affect ADP-ribosylation processes and interactions with glucocorticoids which are important in the regulation of glucose metabolism during the maturation and differentiation of astroglial cells [44, 45]. Based on our ADP assays showing SjILP hydrolysed 29–39% of ADP compared with human insulin, we hypothesis that SjILP, with common conserved signatures shared with members of the insulin/insulin-like family, may also function in a similar role as IGF-1 after binding to exogenous ADP, but this requires further in vivo experimental investigation.
By sharing the same binding epitopes (analogues 1, 3 and 13, 15) [18], SjILP has a stronger bind affinity with the SjIRs than human insulin. Our previous research and that of others have shown that human insulin can stimulate the schistosome insulin pathway by binding parasite IRs and activating the downstream Erk/MAPK and PI3K/AKt sub-pathways [15], thereby playing an essential role in worm growth and development [46]. To further determine if the binding between rSjILP/human insulin and SjIRs could activate downstream Erk/MAPK signal transduction, we used an anti-phospho antibody against Erk to detect the phosphorylation of Erk in adult S. japonicum following incubation with host insulin/rSjILP. We found that following stimulation with either rSjILP or human insulin, adult S. japonicum was able to induce the phosphorylation of Erk. However, a weak phosphor-p44 band was also observed in the SWAP of S. japonicum cultured in DMEM without insulin/rSjILP, indicating ILP expressed by the worm itself can activate Erk. In addition, more phosphorylated Erk was observed in worms stimulated with human insulin compared with worms cultured without insulin, further supporting our previous report that by binding to SjIR, host insulin can activate the Erk/MAPK sub-pathways in schistosomes. [15]. We found Erk was strongly phosphorylated in worms stimulated with exogenous rSjILP or co-stimulated with SjILP and insulin, compared with those incubated with human insulin alone. There was no significant difference between worms treated with rSjILP and co-treated with rSjILP and human insulin, suggesting that SjILP binds SjIRs and activates downstream signalling more strongly than human insulin; this may due to the stronger binding of SjLD-SjILP compared to that of SjLD-human insulin (Fig. 5).
The immunolocalisation analysis showing that SjILP is distributed on the worm tegument and in the parenchyma/vitelline tissue of adult S. japonicum matches the western blot results indicating that native SjILP can be recognised by the anti-SjILP antibody both in the S. japonicum tegument and in carcass protein (Fig. 2b). Furthermore, the co-distribution of SjILP and SjIR1 on the worm tegument and same location of SjILP and SjIR2 in the parenchyma/vitelline tissue in adult parasite provides are suggestive of an interaction between the schistosome ILP and SjIR1 and 2, respectively, in different locations in S. japonicum. The immunolocalization study showing human insulin was detected on the tegument of adult worms with less HRP labelling compared to that observed in SjILP, supports the results of our binding assays showing the SjILP has a stronger bind affinity with SjLD than with human insulin. In addition, the distribution of human insulin only on the surface of worms, indicates that it may be able to bind SjIR1 on the surface with no opportunity to cross though the tegument and bind to SjIR2 inside the parasite, although human insulin has been shown to bind strongly with both SjIR1 and SjIR2 which share similar binding epitopes [18]. Compared with SjIR2, SjIR1 may be more involved in utilising host insulin, having a specialised function in the parasite to exploit the mammalian host hormone, with the IR-1 homologue having been lost by other taxa during evolution [17]. Given the important role of insulin in glucose uptake in S. japonicum and the same location of SjILP/human insulin and SjIR1 on the surface of adult worms leads to a logical hypothesis that the transport of glucose from host blood into schistosomes may be regulated by the binding between SjILP/human insulin and SjIR1 occurring at the parasite surface although this requires further verification. Once glucose is taken up into the schistosome worm, it would be transferred into different tissues and cells with this process being regulated by the binding between SjILP and SjIR2. Both molecules are co-located in the parenchyma of males and the vitelline cells of females and play important roles in the regulation of growth, adult fertility and the differentiation of germline stem cell populations [15, 18, 20].
Conclusions
The findings presented here shed new light on the function and utilisation of ILPs in schistosomes and provide further insight on the distinct and specialised functions of SjIR1 and 2 in S. japonicum and their interaction with host insulin.
Abbreviations
- ADP:
-
Adenosine diphosphate
- ELISA:
-
Enzyme-linked immunosorbent assay
- Erk:
-
Extracellular signal–regulated kinase
- GTP4:
-
Glucose transport protein 4
- GYS:
-
Glycogen synthase
- HRP:
-
Horseradish peroxidise
- ILP:
-
Insulin-like peptide
- IR:
-
Insulin receptor
- LD:
-
Ligand domain of insulin receptor
- MAPK:
-
Mitogen-activated protein kinase
- OCT:
-
Optimal cutting temperature
- PI3K:
-
Phosphatidylinositide 3-kinase
- RTKs:
-
Receptor tyrosine kinases
- SHC:
-
Src homology-containing
- SWAP:
-
Soluble adult worm antigen preparation
References
Braschi S, Wilson RA. Proteins exposed at the adult schistosome surface revealed by biotinylation. Mol Cell Proteomics. 2006;5:347–56.
Amiri P, Locksley RM, Parslow TG, Sadick M, Rector E, Ritter D, et al. Tumour necrosis factor alpha restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature. 1992;356:604–7.
Davies SJ, Grogan JL, Blank RB, Lim KC, Locksley RM, McKerrow JH. Modulation of blood fluke development in the liver by hepatic CD4+ lymphocytes. Science. 2001;294:1358–61.
Alberts GF, Hsu DK, Peifley KA, Winkles JA. Differential regulation of acidic and basic fibroblast growth factor gene expression in fibroblast growth factor-treated rat aortic smooth muscle cells. Circ Res. 1994;75:261–7.
Dissous C, Khayath N, Vicogne J, Capron M. Growth factor receptors in helminth parasites: signalling and host-parasite relationships. FEBS Lett. 2006;580:2968–75.
Morel M, Vanderstraete M, Hahnel S, Grevelding CG, Dissous C. Receptor tyrosine kinases and schistosome reproduction: new targets for chemotherapy. Front Genet. 2014;5:238.
Schistosoma japonicum Genome S, Functional Analysis C. The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature. 2009;460:345–51.
Berriman M, Haas BJ, LoVerde PT, Wilson RA, Dillon GP, Cerqueira GC, et al. The genome of the blood fluke Schistosoma mansoni. Nature. 2009;460:352–8.
Stroehlein AJ, Young ND, Jex AR, Sternberg PW, Tan P, Boag PR, et al. Defining the Schistosoma haematobium kinome enables the prediction of essential kinases as anti-schistosome drug targets. Sci Rep. 2015;5:17759.
Young ND, Jex AR, Li B, Liu S, Yang L, Xiong Z, et al. Whole-genome sequence of Schistosoma haematobium. Nat Genet. 2012;44:221–5.
Wang L, Yang Z, Li Y, Yu F, Brindley PJ, McManus DP, et al. Reconstruction and in silico analysis of the MAPK signaling pathways in the human blood fluke, Schistosoma japonicum. FEBS Lett. 2006;580:3677–86.
Saule P, Vicogne J, Delacre M, Macia L, Tailleux A, Dissous C, et al. Host glucose metabolism mediates T4 and IL-7 action on Schistosoma mansoni development. J Parasitol. 2005;91:737–44.
Vicogne J, Cailliau K, Tulasne D, Browaeys E, Yan YT, Fafeur V, et al. Conservation of epidermal growth factor receptor function in the human parasitic helminth Schistosoma mansoni. J Biol Chem. 2004;279:37407–14.
Levi-Schaffer F, Smolarsky M. Schistosoma mansoni: effect of insulin and a low-molecular-weight fraction of serum on schistosomula in chemically defined media. Exp Parasitol. 1981;52:378–85.
You H, Zhang W, Moertel L, McManus DP, Gobert GN. Transcriptional profiles of adult male and female Schistosoma japonicum in response to insulin reveal increased expression of genes involved in growth and development. Int J Parasitol. 2009;39:1551–9.
Khayath N, Vicogne J, Ahier A, BenYounes A, Konrad C, Trolet J, et al. Diversification of the insulin receptor family in the helminth parasite Schistosoma mansoni. FEBS J. 2007;274:659–76.
You H, Zhang W, Jones MK, Gobert GN, Mulvenna J, Rees G, et al. Cloning and characterisation of Schistosoma japonicum insulin receptors. PLoS One. 2010;5:e9868.
You H, Gobert GN, Cai P, Mou R, Nawaratna S, Fang G, et al. Suppression of the insulin receptors in adult Schistosoma japonicum impacts on parasite growth and development: further evidence of vaccine potential. PLoS Negl Trop Dis. 2015;9:e0003730.
Leevers SJ. Growth control: invertebrate insulin surprises! Curr Biol. 2001;11:R209–12.
Wang S, Luo X, Zhang S, Yin C, Dou Y, Cai X. Identification of putative insulin-like peptides and components of insulin signaling pathways in parasitic platyhelminths by the use of genome-wide screening. FEBS J. 2014;281:877–93.
Jones MK, McManus DP, Sivadorai P, Glanfield A, Moertel L, Belli SI, et al. Tracking the fate of iron in early development of human blood flukes. Int J Biochem Cell Biol. 2007;39:1646–58.
Ranasinghe SL, Fischer K, Gobert GN, McManus DP. Functional expression of a novel Kunitz type protease inhibitor from the human blood fluke Schistosoma mansoni. Parasit Vectors. 2015;8:408.
Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8:785–6.
Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc. 2009;4:363–71.
Wass MN, Kelley LA, Sternberg MJ. 3DLigandSite: predicting ligand-binding sites using similar structures. Nucleic Acids Res. 2010;38:W469–73.
You H, Gobert GN, Duke MG, Zhang W, Li Y, Jones MK, et al. The insulin receptor is a transmission blocking veterinary vaccine target for zoonotic Schistosoma japonicum. Int J Parasitol. 2012;42:801–7.
You H, Gobert GN, Du X, Pali G, Cai P, Jones MK, et al. Functional characterisation of Schistosoma japonicum acetylcholinesterase. Parasit Vectors. 2016;9:328.
Walker AJ, Ressurreicao M, Rothermel R. Exploring the function of protein kinases in schistosomes: perspectives from the laboratory and from comparative genomics. Front Genet. 2014;5:229.
Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–39.
Ressurreicao M, Kirk RS, Rollinson D, Emery AM, Page NM, Walker AJ. Sensory protein kinase signaling in Schistosoma mansoni Cercariae: host location and invasion. J Infect Dis. 2015;212:1787–97.
Vasconcelos EG, Nascimento PS, Meirelles MN, Verjovski-Almeida S, Ferreira ST. Characterization and localization of an ATP-diphosphohydrolase on the external surface of the tegument of Schistosoma mansoni. Mol Biochem Parasitol. 1993;58:205–14.
Vasconcelos EG, Ferreira ST, Carvalho TM, Souza W, Kettlun AM, Mancilla M, et al. Partial purification and immunohistochemical localization of ATP diphosphohydrolase from Schistosoma mansoni. Immunological cross-reactivities with potato apyrase and Toxoplasma gondii nucleoside triphosphate hydrolase. J Biol Chem. 1996;271:22139–45.
Jia X, Schulte L, Loukas A, Pickering D, Pearson M, Mobli M, et al. Solution structure, membrane interactions, and protein binding partners of the tetraspanin Sm-TSP-2, a vaccine antigen from the human blood fluke Schistosoma mansoni. J Biol Chem. 2014;289:7151–63.
Ranasinghe SL, Fischer K, Gobert GN, McManus DP. A novel coagulation inhibitor from Schistosoma japonicum. Parasitology. 2015;142:1663–72.
Golubovskaya VM, Ho B, Zheng M, Magis A, Ostrov D, Morrison C, et al. Disruption of focal adhesion kinase and p53 interaction with small molecule compound R2 reactivated p53 and blocked tumor growth. BMC Cancer. 2013;13:342.
Stephenson RJ, Toth I, Liang J, Mangat A, McManus DP, You H. Identification of host insulin binding sites on Schistosoma japonicum insulin receptors. PLoS One. 2016;11:e0159704.
Ressurreicao M, De Saram P, Kirk RS, Rollinson D, Emery AM, Page NM, et al. Protein kinase C and extracellular signal-regulated kinase regulate movement, attachment, pairing and egg release in Schistosoma mansoni. PLoS Negl Trop Dis. 2014;8:e2924.
Hemer S, Konrad C, Spiliotis M, Koziol U, Schaack D, Forster S, et al. Host insulin stimulates Echinococcus multilocularis insulin signalling pathways and larval development. BMC Biol. 2014;12:5.
Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–6.
Nasonkin IO, Alikasifoglu A, Barrette T, Cheng MM, Thomas PM, Nikitin AG. Cloning, characterization, and embryonic expression analysis of the Drosophila melanogaster gene encoding insulin/relaxin-like peptide. Biochem Biophys Res Commun. 2002;295:312–8.
Favero-Retto MP, Palmieri LC, Souza TA, Almeida FC, Lima LM. Structural meta-analysis of regular human insulin in pharmaceutical formulations. Eur J Pharm Biopharm. 2013;85:1112–21.
Gachet C. Regulation of platelet functions by P2 receptors. Annu Rev Pharmacol Toxicol. 2006;46:277–300.
Da’dara AA, Bhardwaj R, Skelly PJ. Schistosome apyrase SmATPDase1, but not SmATPDase2, hydrolyses exogenous ATP and ADP. Purinergic Signal. 2014;10:573–80.
Avola R, Condorelli DF, Surrentino S, Turpeenoja L, Costa A, Giuffrida Stella AM. Effect of epidermal growth factor and insulin on DNA, RNA, and cytoskeletal protein labeling in primary rat astroglial cell cultures. J Neurosci Res. 1988;19:230–8.
Marchetti B. The LHRH-astroglial network of signals as a model to study neuroimmune interactions: assessment of messenger systems and transduction mechanisms at cellular and molecular levels. Neuroimmunomodulation. 1996;3:1–27.
Vanderstraete M, Gouignard N, Cailliau K, Morel M, Lancelot J, Bodart JF, et al. Dual targeting of insulin and venus kinase Receptors of Schistosoma mansoni for novel anti-schistosome therapy. PLoS Negl Trop Dis. 2013;7:e2226.
Acknowledgements
We thank Mary Duke (QIMR Berghofer Medical Research Institute) for the maintenance of the parasite lifecycle. We are grateful to Tony Walker (Kingston University, London, UK) for suggestions about recognising phosphorylated forms of kinase in schistosomes. We gratefully acknowledge the NIAID Schistosomiasis Resource Centre of the Biomedical Research Institute (Rockville, MD) for providing schistosome-infected Biomphalaria glabrata and Bulinus truncatus.
Funding
We are grateful for funding provided by an Australian Infectious Disease Research Centre Seed Grant and a Program from the National Health and Medical Research Council (NHMRC) of Australia (APP 1037304).
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its Additional files 1, 2 and 3. The datasets generated and analysed during the current study are available in GenBank (accession numbers AY814982 for SjILP).
Authors’ contributions
Conceived and designed the experiments: HY, DPM and WH. Performed the experiments: XD and HY. Analysed the data: XD, HY, DPM and PC. Contributed reagents/materials/analysis tools: XD, HY, DPM, WH and PC. Wrote the paper: HY, XD and DPM. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval
The conduct and procedures involving animal experimentation were approved by the Animal Ethics Committee of the QIMR Berghofer Medical Research Institute (project number A0108-054). This study was performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Additional files
Additional file 1: Table S1.
Primers used in PCR to obtain full-length cDNA sequences encoding schistosome insulin-like peptides in S. japonicum (SjILP) S. mansoni (SmILP). (DOC 28 kb)
Additional file 2: Figure S1.
Alignment of amino acid sequences of extracellular regions of different insulin receptors using CLUSTAL W. The extracellular regions of SjIR1 and 2 were aligned with those from insulin receptors in Homo sapiens (HIR) and Drosophila melanogaster (DmIR). Black boxes indicate identical amino acids and grey boxes denote sequence similarity. Peptides P13, P1, are boxed in red and P15, P3 are shown in green. These peptides all bound SjILP or human insulin using the Octet RED system. SjLD1 contains amino acid sequence from D59-E411 in SjIR1 and SjLD2 contains sequence from R37-C525 in SjIR2. (DOC 56 kb)
Additional file 3: Figure S2.
(A) Schematic representation of the predicted structures of insulin-like peptides in Schistosoma japonicum (SjILP), S. mansoni (SmILP) and S. haematobium (ShILP). Schistosome ILPs contain an A peptide (thick underline) and a B peptide (double underline) linked by disulfide bridges; a signal peptide was predicted only in SjILP and ShILP (boxed in green). The conserved cysteines in the A chain with the amino acid sequence CCCX(2)CX(8)C are indicated in dark red. The putative disulfide bonds between conserved cysteines are indicated by the S-S bridge in red. (B) Predicted tertiary protein structures of schistosome ILPs and the location of adenosine diphosphate (ADP) binding sites. (a) Three-dimensional model of SjILP determined using Phyre2. Image coloured by rainbow from N to C terminus, Model dimensions (Å): X:28.233, Y:29.795, Z:26.620) are the same as those of SmILP and ShILP. (b) Three predicted ADP binding sites of SjILP located at HIS59, ARG62, and ARG120 residues. (c) Two ADP binding sites of SmILP presented at ASN60 and ARG123. No ADP binding sites were predicted in ShILP. (TIF 1820 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Du, X., McManus, D.P., Cai, P. et al. Identification and functional characterisation of a Schistosoma japonicum insulin-like peptide. Parasites Vectors 10, 181 (2017). https://doi.org/10.1186/s13071-017-2095-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-017-2095-7