Abstract
Background
Hwa-byung (HB) is an anger syndrome caused by an inadequate release of accumulated anger that leads to somatic and psychiatric symptoms. As HB results from long-term inadequately treated negative emotions, its symptoms are complex, intractable and concomitant with other psychiatric disorders. Therefore, studies aiming to develop effective and safe treatment options for HB are needed. We plan to conduct a pilot study for a future, full-scale, randomized controlled trial (RCT) of an optimal acupuncture procedure using semi-individualized acupuncture points that consider participants’ personal disposition and type of emotional stress.
Method/design
This randomized, sham-controlled, participant- and assessor-blinded pilot trial aims to determine the study feasibility of acupuncture for HB and to explore its clinical effects and safety. This clinical trial will be conducted with two groups: one treated with real acupuncture and the other with sham acupuncture for 10 sessions over 4 weeks. The experimental group (EG) will receive semi-individualized acupuncture, whereas the control group (CG) will receive sham acupuncture, namely minimal acupuncture on non-acupuncture points. The recruitment, compliance, and completion rate and clinical evaluations, including a Visual Analogue Scale (VAS), the Korean version of the Beck Depression Inventory (BDI), the short form of the Stress Response Inventory (SRI-short form) and the Instrument of the Oriental Medical Evaluation for HB (IOME-HB), will be assessed to evaluate feasibility and possible effects and safety. Four weeks after completing treatment, follow-up assessments will be performed.
Discussion
As this is a pilot study mainly aiming to investigate trial feasibility, the results of this study will be analyzed descriptively and interpreted for the study purposes. Cohen’s d will be reported to determine the effect of acupuncture for HB and to enable comparisons with other treatment methods. This protocol is significant in that it provides optimal semi-individualized acupuncture treatment. We expect this study to offer information about the feasibility of this treatment and data about the possible effects and safety.
Trial registration
Clinical Research Information Service (CRIS), Republic of Korea: KCT0001732. Registered on 14 December 2015.
Similar content being viewed by others
Background
Hwa-byung (HB) is an anger syndrome that is considered a cultural syndrome of Korea. In the Diagnostic and Statistical Manual of Mental Disorders, version 4 (DSM-IV), HB is listed as a Korean culture-bound syndrome in the glossary of culture-bound syndromes [1], and the disease has been given an independent disease code in the Korean Standard Classification of Diseases 7 (KCD-7) [2]. Research is currently underway to diagnose HB separately from other mental disorders, as diagnostic criteria change with the culture of the time. Accordingly, the HB Diagnostic Interview Schedule (HBDIS) and the Instrument of Oriental Medical Evaluation for HB (IOME-HB) were developed [3,4,5]. HB has distinct characteristics that differ from those of other known psychiatric disorders, and is typically accompanied by major depressive disorder (MDD) and generalized anxiety disorder (GAD) [6,7,8].
The symptoms of HB can be physical and include chest palpitations, flushing, surges of feelings, and lumps in the throat or solar plexus; however, they can also be emotional and include resentment, bitterness, and hard feelings [3]. HB results from anger that has accumulated over a long period of time without being released and is caused both by an individual’s disposition and by repeated stress from a society that imposes unfair and unreasonable restrictions on its members [3, 6, 7]. The prevalence of HB ranges from 4.2% to 13.3% in Korea depending on the age group and region [3, 9] and is reported to be higher in women than in men [10]. Self-perceived HB is relatively prevalent, with a community-based study reporting a rate of 12% [11].
One review study indicated that a multi-disciplinary approach, including religious help and traditional methods, is necessary for the treatment of HB [12]. Another study investigating paroxetine revealed improvements in the symptoms of HB and Hamilton Depression Rating Scale (HAM-D) and State-Trait Anger Expression Inventory (STAXI) scores, but the study was limited to a single-group, open trial [13]. In terms of non-pharmacological treatments, one qualitative study investigating loving-kindness meditation reported therapeutic effects in four of nine participants, partial effects in four participants, and no effect in one participant [14]. Another qualitative study investigating a mindfulness-based stress reduction program indicated improvement in the symptoms of HB and STAXI, Hospital Anxiety and Depression Scale-Anxiety (HAD-A), and Hospital Anxiety and Depression scale-Depression (HAD-D) score [15]; however, these two studies included small sample sizes (9 and 10 participants, respectively) and were single-arm, qualitative studies whose findings are difficult to generalize.
Acupuncture has been used for various psychiatric disorders [16], and its clinical effect against depression [17] and anxiety [18, 19] has been reported in previous studies. For studies on HB, one study reported improvement in the symptoms of HB and Beck Depression Inventory (BDI) score but not in STAXI and State-Trait Anxiety Inventory (STAI) scores [20]. Several studies reported contrasting results, and did not find significant improvement in symptoms of HB or in BDI, STAXI, or STAI scores [21, 22]. These three studies included a control group that received shallow, penetrating, sham acupuncture at non-acupuncture points, which may have contributed to a failure to detect differences between the groups. In addition, the acupuncture used in these studies was focused on traditional eastern philosophy using the Five Elements Theory; thus, the method exhibited limitations in the possible widespread use by global researchers and practitioners. Two studies focused on concurrent symptoms, such as insomnia and anxiety, but remained limited in that one used a waiting list and the other included a single-group, open-trial design [23]. In 2013, clinical guidelines for HB were developed encompassing various studies. The guidelines indicated the limitations as a standardized treatment regimen could not be determined. The clinical guidelines proposed the use of acupuncture to treat HB based on previous evidence and noted that individualized treatment that considers the specific disposition and level and type of stress must be simultaneously provided [24]. Given the need for further research regarding individualized treatment, the present clinical study protocol was developed to use both fixed acupuncture points related to the major symptoms of HB, and variable acupuncture points based on traditional Korean medicine (TKM) principles.
Accordingly, we designed a randomized, sham-controlled, participant- and assessor-blinded, parallel-design pilot study using sham-treatment control and a 1:1 allocation. We will assess the clinical trial feasibility, and explore the effect and safety of acupuncture as a treatment for HB by comparing an experimental group (EG) receiving semi-individualized acupuncture with a control group (CG) receiving sham acupuncture.
Method/design
Study design
This study is a randomized, sham-controlled, participant- and assessor-blinded, parallel-design clinical trial. Participants will be assigned to the EG or the CG. The EG will be treated with real acupuncture, whereas the CG will be treated with sham acupuncture.
Setting of the study
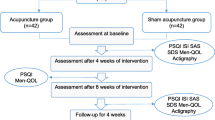
This clinical trial will be conducted at the outpatient department of a university hospital. Public recruitment advertising will be used to recruit participants to voluntarily apply to the trial, and a Korean medical doctor (KMD) will explain the details of the trial and will supply documented explanations. When the participants decide to enroll in the trial, they will be asked to submit written consent and to undergo evaluation to ensure compliance with inclusion/exclusion criteria. Participants who are eligible for the study will be randomly assigned to one of two groups and will receive the planned intervention and evaluation according to their corresponding group. Both groups will receive the intervention for a total of 10 sessions for 4 weeks (two to three sessions per week). A post-treatment assessment will be performed at 4 weeks after visit 1, and the study will be completed 8 weeks after visit 1 (Fig. 1).
Recruitment
A participant recruitment document, including the study title, target participants, period, location and contents of the intervention, will be distributed as printed material, newspaper advertisements, and posters displayed at the clinical trial sites.
Randomization and allocation concealment
An independent statistician will generate a table of random sampling numbers using Strategic Applications Software (SAS) ® Version 9.4 (SAS institute, Inc., Cary, NC, USA). The statistician will place the groups to be assigned into each double-layered, opaque envelope in sequence, seal the envelopes and write the numbers in order. The randomization code will be secured in a double-locked cabinet. As practitioners cannot be blinded by the nature of the intervention, the practitioners will assign the participants to one of the groups by opening the sealed envelopes in order in front of the participants. The opened envelope will be separately stored in a safe. The practitioner will confirm the eligibility before opening the envelope. If the participants do not meet the inclusion criteria, they will be removed from the study, and the randomization will not be performed (the envelope will not be opened). The reason for the lack of randomization will be recorded in a screening log and a Case Report Form (CRF).
Sample size calculation
This study aims to evaluate clinical trial feasibility and to investigate basic information about effects and safety of acupuncture for the treatment of HB, rather than to satisfy hypothesis testing. Therefore, the sample size was not decided based on power calculations but based on the precision of mean and standard deviation (SD), the rationale of feasibility, and ethical issues that prohibit over-recruitment of participants. We ensured that the sample size exceeded the minimal number needed to assure the validity of the mean, SD and effect size and rationale of feasibility [25] and considered the study period and research budget [26, 27]. Accordingly, a sample size of 26 participants was estimated.
Blinding of participants and assessors
As this study will use minimal acupuncture for the CG, the practitioners cannot be blinded; however, the participants and assessors will be blinded. Participants will be informed that they will be treated with either classical acupuncture or non-classical acupuncture [28]. Independent assessors who are not in charge of the intervention will ask only the questions necessary to evaluate and record in the CRF and will not know the assigned group throughout the study period. Un-blinding will be considered only under circumstances in which it is necessary for the rights and safety of the participants, including the occurrence of serious adverse effects. In such cases, the researchers will notify the principal investigator (PI) and document the subsequent processes.
Characteristics of participants
Inclusion criteria
-
1.
Men and women who are aged 20 to 65 years
-
2.
Meet the diagnostic criteria of the HBDIS: participants should have at least three of the four major symptoms (including chest discomfort, burning sensation of the face or chest, a surge of feeling, and sensation of a mass in the throat or in the epigastrium), one of two major psychological symptoms (including feelings of unfairness and mortification, and emotional resentment or ill will), two of four related somatic symptoms, and two of three related psychological symptoms. The symptoms should be related to stress, should cause psychosocial hypo-function, and should not be caused by a medical disorder
-
3.
Provide written informed consent after being informed of the objectives and details of the clinical trial and agreeing to participate in the trial
Exclusion criteria
-
1.
History of serious psychiatric or neurologic disorder: hallucination, delusion, abuse of or dependence on alcohol or drugs, cerebrovascular disease, or degenerative disease of the central nervous system
-
2.
Use of medications related to HB, such as anti-depressants or anti-anxiety, during the preceding 1 month
-
3.
A seriously unstable medical condition: needing active medical treatment promptly, abnormal vital signs, or intolerable pain or poor food intake causing difficulty in activities in daily living (ADL)
-
4.
Participation in other clinical trials
-
5.
Female participants who are pregnant, breast-feeding, or planning to become pregnant
-
6.
Residents of collective dwelling facilities, such as social welfare institutions
-
7.
Failure to provide written informed consent
-
8.
Lack of eligibility for the trial for other reasons: illiteracy, questionnaires are not understood or answered, or if the patient is not expected to be compliant with the treatment and evaluation visit schedule
Intervention
Intervention of experimental group
Ten acupuncture sessions will be conducted at fixed and individualized acupuncture points for 4 weeks by inserting an acupuncture needle perpendicular to the skin at a depth of 5.0–25.0 mm with manipulation for the de-qi and maintaining the position for 20 min each session. Disposable, sterile, 0.25 × 30-mm stainless steel acupuncture needles (Dongbang, South Korea) will be used. The fixed acupuncture points will be GV20, CV17, HT7, and ST36, and the individualized points will be decided according to the participants’ specific symptoms [24, 29, 30]. The locations of the acupuncture points are presented in the Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA) (Additional file 1). Qualified TKM doctors (KMD) with over 4 years of clinical experience will be in charge of the intervention.
Treatment of control group
The CG will receive minimal acupuncture on non-acupuncture points 10 times over 4 weeks. The same type of acupuncture needle will be inserted perpendicular to the skin at a depth of 1.0–3.0 mm without manipulation for the de-qi and maintained for 20 min. The number of acupuncture points, duration, and frequency of the sessions will be the same as those for the EG, and the same practitioner will conduct the intervention.
-
Location of non-acupuncture points:
-
1.
Upper extremities (UE) 1 (bilateral): 1 cm lateral from 5 cm below the midpoint of the transverse cubital crease
-
2.
Abdomen 1: 3 cm lateral from a point 13.5 cm above the umbilicus
-
3.
Lower extremities (LE) 1 (bilateral): 1.5 cm above EX-LE2 (the center of the upper border of the patella)
-
4.
LE 2 (bilateral): point at the upper one third of the medial part of the tibia
-
5.
LE 3 (bilateral): 1.5 cm below LE 2
-
1.
Allowed or prohibited concomitant treatment
All treatments for HB and symptom management will be prohibited. Other subjects’ treatments for underlying chronic diseases will be allowed if they do not change during the study period. If a violation occurs during the study period, the patient will be removed from the study.
Outcome measure
Feasibility outcomes
We will evaluate the feasibility of the study by assessing recruitment, compliance, and completion rates. Acceptability of acupuncture will be also evaluated by investigating effect and safety. The acupuncture treatment will be regarded as an acceptable intervention for a full-scale study if the effect size is greater than 0.2 in at least three of six major symptoms. With respect to safety, a full-scale study will be planned if there is no serious adverse event (SAE) associated with the intervention and if the incidence of adverse events (AEs) is not significantly different from the CG.
The integrity of the study will be examined as a qualitative indicator with regard to whether the study proceeds without any problems and whether a full-scale study is possible without modification of the pilot study design. This will be measured using the Acceptance Checklist for Clinical Effectiveness Pilot Trials (ACCEPT) [31]. We will check the blinding index to determine whether the blinding procedure requires modification. The blinding index ranges from − 1 to 1, with 0 being the most favorable blindness level [32].
Clinical outcomes
Clinical outcomes include information regarding the possible effects and safety of acupuncture. The outcome measures regarding clinical effect will be assessed at baseline, week 3, week 5, and week 9. A Visual Analogue Scale (VAS) score for the four major somatic symptoms and two major psychological symptoms of HB will be the primary outcome measure. The major symptoms are chest discomfort, burning sensation of the face or chest, a surge of feeling, sensation of a mass in the throat or in the epigastrium, frequent feelings of unfairness and mortification, and emotional resentment or ill will.
The VAS is widely used for the assessment of variations in the severity of subjective symptoms. Participants mark their symptom severity on a 100-mm straight line whose extreme left end indicates “absence of symptoms” and whose extreme right end indicates “unbearably severe symptoms” [33, 34].
The secondary clinical outcome measures will be the VAS score for subjective anger, the BDI and the short-form of the Stress Response Inventory (SRI-short form). The IOME-HB and serum serotonin levels will be evaluated at baseline and week 5. The BDI is a self-reported inventory to evaluate the severity of depression with 21 questions, including somatic-affective and cognitive-affective factors. Each question is assigned one of 0 to 3 points, and the total score ranges from 0 to 63 [35, 36]. The SRI-short form is used to assess responses to stress. It contains 22 questions, including somatization factors (nine items), depression factors (eight items), and anger factors (five items) [37]. The IOME-HB is used to evaluate the severity of psychiatric and somatic symptoms of each of five types of HB, according to TKM principles, by participants scoring their symptoms using a 5-point Likert scale [4, 5]. Serum serotonin levels will be measured by analyzing the blood drawn from the veins of the arm. We will quantitatively analyze the numbers themselves to see whether there are differences before and after treatment and between groups.
The follow-up evaluation will be performed 4 weeks after the completion of the treatment and will include the VAS for core symptoms, VAS for subjective anger, and the BDI and SRI-short form. To remind participants and encourage their participation in the follow-up assessment, we will inform participants about their visits by phone 1 to 7 days prior to the follow-up visit. Adverse events (AEs) will be recorded at every visit to assess the safety of the trial. The Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) schedule of enrollment, interventions, and assessments is shown in Fig. 2. The SPIRIT Checklist can be found in Additional file 2.
Criteria for dropout
-
1.
The subject or the legal representative of the subject withdraws their participation
-
2.
A violation of the inclusion/exclusion criteria is found during the course of the clinical trial
-
3.
The development of an SAE or an AE causes difficulty in continuing
-
4.
Less than 80% compliance with the treatment
-
5.
The subject violates the plan of the clinical trial
-
6.
Failure to track the subject
-
7.
The subject takes medications that affect the results during the study period
Data collection, management, and personal information protection
Data will be collected by the KMD who will be in charge of the questionnaire and evaluation. The KMD will have six or more years of specialized education and four or more years of clinical experience and will complete a workshop on evaluation standardization. Soft copies will be saved on a storage device not connected to the Internet. All data will be kept in locked storage, and only those with permission from the PI will be able to access the data. All blood samples will be disposed of immediately after they are analyzed. All retained data will contain codes to identify patients instead of personal information.
Statistical analysis
The recruitment rate, compliance rate, and completion rate will be presented as a number and percentage and will also be presented for each group. For clinical outcomes, including VAS scores for the core symptoms of HB and subjective anger, the BDI, the SRI-short form and serum serotonin levels, the mean SD at each assessment point and Cohen’s d, which is an estimated effect size that indicates the standardized difference between two mean values, will be presented [38]. Effect size will be calculated based on the value immediately after the end of the 4-week treatment (visit 10).
Analyses will mainly be performed using the full analysis set (FAS) population, while the per-protocol set (PPS) population will be analyzed as a supplement. Although the intention-to-treat (ITT) ideal includes all randomized subjects, the FAS approach acknowledges the practical infeasibility of completely following all participants until study completion and includes as complete and as similar a population to ITT as possible [39]. The FAS population does not include the following: (1) individuals who do not meet the inclusion criteria, (2) individuals who have never undergone acupuncture treatment, and (3) individuals who have never been assessed since randomization and, therefore, have no data available for collection. The PPS population will include the following: (1) individuals for whom acupuncture accounts for more than 80% of the total number of treatments received, (2) individuals who have had their evaluation parameters measured, and (3) individuals who do not violate the clinical trial protocol [40]. Missing data will be replaced by multiple imputation. Statistical analyses will be conducted by an independent statistician using SAS® version 9.4 (SAS Institute Inc., Cary, NC, USA).
Monitoring
The clinical study will be regularly monitored by independent researchers who are specifically responsible for monitoring. They will ensure that the rights and welfare of the study participants are protected, confirm the accuracy of the data, and maintain the safety and verifiability of the study. They will also verify that the clinical study complies with the study protocol and related regulations. At each monitoring visit, the researchers will confirm and store data, such as original patient records, treatment records, and storage status. Any problems observed during the course of the study will be recorded and reported. Corrective measures will be taken accordingly. If the problems are serious and compromise the safety and validity of the study, they will be reported to the Institutional Review Board (IRB), which will determine whether the clinical study can continue.
Safety issues
AEs refer to all harmful and unintended reactions that occur during the acupuncture treatment. The possible adverse effects of acupuncture include the following: local bleeding or haematoma, more than slight sharp pain, aggravation of symptoms, drowsiness, syncope, nausea, headache, seizure, peripheral nerve injury, and infection. The participants will be informed of possible AEs in advance.
Participants will be questioned about recent hospital visits, new medications taken or new symptoms arising, at every visit. The symptoms, date of occurrence, disappearance, causality, and severity of the AEs will be recorded, and the severity will be recorded using the three-level classification by Spilker et al. [41].
During the study period, all researchers will attempt to ensure patient safety. When SAEs occur, the researchers will immediately halt the study and promptly provide appropriate management. The PI will also report adverse symptoms to the IRB and research clients and halt the study partially or completely until further notice.
Discussion
HB, which literally means anger syndrome, leads to both somatic and psychological symptoms. The psychological symptoms mainly include an inadequate expression of anger and trait anger, and the somatic symptoms mainly include palpitations, chest discomfort and gastrointestinal disorders [7, 42, 43]. Regarding the causes of HB, a person’s specific disposition, stress from a difficult marital relationship or a serious life crisis can both act as risk factors of HB; enduring negative emotions for a long period of time is known to be a root cause of HB [44].
Patients with HB tend to avoid psychiatric treatment and to rely on treatments that focus only on their physical symptoms [43, 45]. These patients also receive insufficient social support [42]. Despite relatively high levels of self-awareness of HB among these patients [3, 10, 11], they have been unable to receive effective treatment; therefore, further research is needed to develop more effective and acceptable treatments for HB.
This is a pilot study that aims to evaluate the feasibility of acupuncture treatment for HB and to confirm basic information about its effects and safety [46]. In pilot studies, determining feasibility should be the first priority, and data regarding treatment effects should be descriptively analyzed to avoid exaggerated interpretations [47,48,49,50,51]. Therefore, this trial primarily aims to obtain information about the recruitment, consent, and integrity of the study protocol based on recruitment rate, compliance rate, and completion rate. Clinical effects will be assessed to explore the effects and safety of the intervention and to determine the effect size [47, 52, 53]. We will present Cohen’s d, which provides an estimated effect size, because the effect size provides numerical information about the actual difference between two outcomes whereas p values are affected by sample size and only provide a dichotomous assessment of statistical significance [54,55,56,57]. These data will provide the information needed to calculate the sample size as well as information on the intervention effects that can be easily compared to interventions applied in other studies [38, 47].
With respect to the inclusion/exclusion criteria, an age range of 20 to 65 years was selected to cover as wide a range as possible while at the same time protecting those who are underage or elderly. In addition, the exclusion of participants taking medication related to HB is essential to obtain accurate results of clinical studies, and is determined by considering the possibility of recruitment and ethical factors. As many HB patients tend not to visit hospitals and take medications for somatic symptoms only [43, 45], we judged that recruiting participants would not have serious ethical problems because it is presumed not to interrupt their proper treatments. Moreover, HB is not a life-threatening disease but reflects a chronic process. There are no specific drugs for HB, and we thus concluded that the prohibition of concurrent treatment during the total 8-week study period would not cause serious problems. Those who reside in collective dwelling facilities are excluded because they are prohibited from being subjects in clinical trials by Korean law in order to protect their human rights.
For the acupuncture points, GV20, CV17, and ST36 were selected from the points that are traditionally widely used and known to be effective based on previous research [29] textbook information [30], and guidelines [24]. This selection excluded the points that could cause severe pain or discomfort during the procedure. HT7 was selected based on a previous study that revealed positive effects on HB [23] and serotonin regulation [58].
The use of a CG is known to be reasonable and necessary to provide information that is as accurate as possible for future research [46, 59]. Therefore, we designed this pilot study with a CG to obtain realistic information under a setting similar to those used in RCTs. The CG will receive shallow acupuncture at non-acupuncture points in order to mimic true acupuncture treatment and minimize the therapeutic effects [20,21,22]. The non-acupuncture points are located far from the true acupuncture points to compensate for the limitations of the previous studies [20,21,22] and to minimize the non-specific effects of sham acupuncture [60, 61]. To assess the appropriateness of this research design, we will analyze the results of the blinding index. Accordingly, we will obtain information that will help determine the appropriate type of CG to be used in future research.
To confirm the selection of the most appropriate primary outcome measures and possible objective outcome measures, the BDI, the SRI-short form, and serum serotonin levels will be measured. HB is commonly accompanied by one or more psychiatric disorders and exhibits unique symptoms. Therefore, consistent research on the correlation between HB and other DSM diagnoses is necessary. In this study, we will explore the correlation between the symptoms of HB and other psychiatric disorders by evaluating the BDI and SRI-short form [7, 62]. Serum serotonin levels will be experimentally assessed to evaluate possible correlations between the mechanism of acupuncture’s effects on HB and serotonin and to assess the appropriateness of the evaluation in future RCTs [13, 58, 63,64,65,66,67]. Previous studies on HB and serotonin have demonstrated that the central serotonin system is related to anger, depression, and anxiety and that serotonin is involved in the behavioral patterns related to the feeling of injustice, a key appraiser in HB [68, 69]. In an animal study, acupuncture on HT7 has been reported to modulate the serotonin system in depression-related behaviors [58].
HB is listed as a culture-bound syndrome in Korea, and there are no reports of global prevalence. However, approximately 7000 to 23,000 people have immigrated abroad over the past decade [70], and approximately 1.7 million immigrants to the United States account for a high percentage of Asian Americans [71]. Moreover, a study conducted on Korean Americans revealed that 12% of them have HB [11]. In addition, considering the cumulative stress due to unfair treatment and injustice with respect to the cause of HB, there are likely to be similar subjects in countries with similar social conditions. It is possible that these patients are being treated only for individual physical symptoms not identified as HB [43, 45].
This study has some limitations. First, this clinical trial will include a small population of participants and will not primarily aim to perform hypothesis testing. Therefore, the results of this study cannot be generalized as basic data for evaluating the effect and safety of acupuncture for HB. Moreover, because the CG will receive minimal acupuncture, we cannot neglect the possibility of physiological activation caused by needle penetration. The effect of acupuncture will need to be carefully interpreted because the acupuncture points, needling depth, and de-qi will be the only differences between groups.
However, as this trial assesses the use of optimized, semi-individualized acupuncture for HB to enable the design of larger RCTs, we expect that it can provide feasibility data and basic information about the effect and safety of acupuncture for HB.
Trial status
This clinical trial is currently in the recruiting phase.
Abbreviations
- BDI:
-
Korean version of the Beck Depression Inventory
- CG:
-
Control group
- CRF:
-
Case Report Form
- DSM:
-
Diagnostic and Statistical Manual of Mental Disorders
- EG:
-
Experimental group
- FAS:
-
Full analysis set
- GAD:
-
Generalized anxiety disorder
- HB:
-
Hwa-byung
- HBDIS:
-
Hwa-byung Diagnostic Interview Schedule
- IOME-HB:
-
Instrument of Oriental Medical Evaluation for HB
- IRB:
-
Institutional Review Board
- ITT:
-
Intention-to-treat
- KMD:
-
Traditional Korean medical doctor
- LE:
-
Lower extremities
- MDD:
-
Major depressive disorder
- PI:
-
Principal investigator
- PPS:
-
Per-protocol set
- SRI-short form:
-
Short form of the Stress Response Inventory
- TKM:
-
Traditional Korean medicine
- UE:
-
Upper extremities
- VAS:
-
Visual Analogue Scale.
References
Suh S. Stories to be told: Korean doctors between hwa-byung (fire-illness) and depression, 1970–2011. Cult Med Psychiatry. 2013;37(1):81–104.
Statistics Korea. Korean Standard Classification of Diseases 7. http://kssc.kostat.go.kr/ksscNew_web/kssc/common/ClassificationContent.do?gubun=1&strCategoryNameCode=004&categoryMenu=007&addGubun=no. Accessed 20 Nov 2017.
Kim J, Kwon J, Lee M, Park D. Development of hwa-byung diagnostic interview schedule (HBDIS) and its validity test. Korean J Health Psychol. 2004;9(2):321–31.
Cheong M-H, Lee S-R, Kang W-C, Jung I-C. Preliminary study to develop the instrument of oriental medical evaluation for Hwa-Byung. J Oriental Neuropsychiatry. 2010;21(2):141–55.
Lee H-S, Choi W-C, Yu Y-S, Kang W-C, Jung I-C. Reliability and validity analysis of the Instrument of Oriental Medical Evaluation for Hwa-Byung. J Oriental Neuropsychiatry. 2014;25(4):351–8.
Park Y-J, Kim HS, Schwartz-Barcott D, Kim J-W. The conceptual structure of hwa-byung in middle-aged Korean women. Health Care Women Int. 2002;23(4):389–97.
Min SK, Suh SY. The anger syndrome hwa-byung and its comorbidity. J Affect Disord. 2010;124(1–2):211–4.
H-k K, Park J-Y. Prevalence and related factors of Hwabyung for the aged woman in rural community. J Korean Public Health Nurs. 2004;18(2):234–42.
Min SK, Namkoong K, Lee HY. An epidemiological study of hwabyung. J Korean Neuropsychiatr Assoc. 1990;29(4):867–74.
Lee J-G, Lee J-H. Study on the prevalence of Hwa-Byung diagnosed by HBDIS in general population in Kang-won province. J Oriental Neuropsychiatry. 2008;19(2):133–9.
Lin K-M, Lau JK, Yamamoto J, Zheng Y-P, Kim H-S, Cho K-H, Nakasaki G. Hwa-byung: a community study of Korean Americans. J Nerv Ment Dis. 1992;180(6):386–91.
Min SK. Treatment and prognosis of hwabyung. Psychiatry Invest. 2004;1:29–36.
Min SK, Suh SY, Jon DI, Hong HJ, Park SJ, Song KJ. Effects of paroxetine on symptoms of hwa-byung. Korean J Psychopharmacology. 2009;20(2):90–7.
Cho H, Kim J, Song S. A qualitative study about the therapeutic process of loving-kindness meditation for patients with Hwa-Byung. J Korean Psychol Couns Psychother. 2013;25(3):425–48.
Song S-Y, Cho H-J, Kim S-Y, Kim J-W. Qualitative analysis of the experiences in mindfulness-based stress reduction (MBSR) on Hwa-Byung patients. J Oriental Neuropsychiatry. 2012;23(4):153–8.
Kawakita K, Shinbara H, Imai K, Fukuda F, Yano T, Kuriyama K. How do acupuncture and moxibustion act?—Focusing on the progress in Japanese acupuncture research. J Pharmacol Sci. 2006;100(5):443–59.
Wu J, Yeung AS, Schnyer R, Wang Y, Mischoulon D. Acupuncture for depression: a review of clinical applications. Can J Psychiatry. 2012;57(7):397–405.
Pilkington K, Kirkwood G, Rampes H, Cummings M, Richardson J. Acupuncture for anxiety and anxiety disorders—a systematic literature review. Acupunct Med. 2007;25(1–2):1–10.
Eich H, Agelink M, Lehmann E, Lemmer W, Klieser E. Acupuncture in patients with minor depressive episodes and generalized anxiety. Results of an experimental study. Fortschr Neurol Psychiatr. 2000;68(3):137–44.
Choi W-J, Lee S-G, Son I-B, Sun S-H. The effects of Sa-am acupuncture Simpojeongkyeok treatment on Hwa-byung: randomized, patient-assessor blind, placebo-controlled acupuncture, pilot clinical trial. J Oriental Neuropsychiatry. 2011;22(2):1–13.
Lee S-R, Park Y-C, Hong K-E, Koo Y-S, Jo J-H, An J-J, Kang W-C, Kim J-W, Choi S-M, Jung I-C. The effect of Sa-am acupuncture treatment for major symptom of Hwa-byung: a preliminary study. J Oriental Neuropsychiatry. 2007;18(1):79–94.
Jeong I-C, Lee S-R, Park Y-C, Hong K-E, Lee Y-K, Kang W-C, Choi S-M, Choi K-W, Oh D-S, Park J-E. The effect of sa-am acupuncture simjeongkyeok treatment for major symptom of hwa-byung. J Oriental Neuropsychiatry. 2008;19(1):1–18.
Jung D-J, Lee J-H. The clinical trial for the significant effects of acupuncture on decreasing anxiety symptom of Hwa-Byung in a single institute-single-arm with Hwa-Byung, open label. J Oriental Neuropsychiatry. 2012;23(1):49–58.
The Korean Society of Oriental Neuropsychiatry. Clinical guidelines for Hwabyung. Seoul: Jipmoon; 2013. p. 65-6.
Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat. 2005;4(4):287–91.
Aisen P, Schmeidler J, Pasinetti G. Randomized pilot study of nimesulide treatment in Alzheimer’s disease. Neurology. 2002;58(7):1050–4.
Shanyinde M, Pickering RM, Weatherall M. Questions asked and answered in pilot and feasibility randomized controlled trials. BMC Med Res Methodol. 2011;11(1):1.
Linde K, Streng A, Jurgens S, Hoppe A, Brinkhaus B, Witt C, Wagenpfeil S, Pfaffenrath V, Hammes MG, Weidenhammer W, et al. Acupuncture for patients with migraine: a randomized controlled trial. JAMA. 2005;293(17):2118–25.
Lee S-G, Choi W-J, Kang H-W, Koo B-S, Kim G-W, Lee J-H. Questionnaire survey of nonherbal therapy of Hwabyung in professionals. J Oriental Neuropsychiatry. 2009;20(2):133–41.
Committee for the Textbook of the Acupuncture and Moxibustion. The acupuncture and moxibustion. Paju: Jipmoon; 2008. p. 391-417.
Charlesworth G, Burnell K, Hoe J, Orrell M, Russell I. Acceptance checklist for clinical effectiveness pilot trials: a systematic approach. BMC Med Res Methodol. 2013;13(1):78.
Bang H, Ni L, Davis CE. Assessment of blinding in clinical trials. Control Clin Trials. 2004;25(2):143–56.
Miller MD, Ferris DG. Measurement of subjective phenomena in primary care research: the Visual Analogue Scale. Fam Pract Res J. 1993;13(1):15—24.
Huskisson E. Visual Analogue Scales. Pain Meas Assessment 1983:33–37.
Sung H, Kim J, Park Y, Bai D, Lee S, Ahn H. A study on the reliability and the validity of Korean version of the Beck Depression Inventory-II (BDI-II). J Korean Soc Biol Ther Psychiatry. 2008;14(2):201–12.
Lee M, Lee Y, Jung H, Choi J, Kim S, Kim Y. A standardization study of Beck Depression Inventory 2-Korean Version (K-BDI): validity-II. Korean J Psychopathol. 1995;4:96–104.
Choi S, Kang T, Woo J. Development and validation of a modified form of the Stress Response Inventory for Workers. J Korean Neuropsychiatric Assoc. 2006;4:541–53.
Cohen J. Statistical power for the behavioral sciences. 2nd ed. Hillsdale: Lawrence Erlbaum; 1988.
ICH harmonised Tripartite Guideline. Statistical principles for clinical trials. International Conference on Harmonisation E9 Expert Working Group. Stat Med. 1999;18(15):1905-42.
Lewis JA. Statistical principles for clinical trials(ICH E 9): an introductory note on an international guideline. Stat Med. 1999;18(15):1903–42.
Spiker B, Revicki D: Taxonomy of quality of life. Quality of life and pharmacoeconomics in clinical trials 1996:25–32.
Park YJ, Lee SJ, Shin NM, Shin H, Kang HC, Jin YT, Jeon SI, Cho I. Anger, anger expression, cardiovascular risk factors, and gastrointestinal symptoms by hwa-byung symptoms in Korean adult women. Appl Nurs Res. 2015;28(4):398–403.
Rhi B-Y. Hwabyung—An overview. Psychiatry Invest. 2004;1:21–4.
Park YJ, Kim JW, Cho SH, Moon SH. Development and effectiveness of a program for relieving “Hwa-Byung” symptoms. Taehan Kanho Hakhoe chi. 2004;34(6):1035–46.
Park YJ, Kim HS, Kang HC, Kim JW. A survey of Hwa-Byung in middle-age Korean women. J Transcult Nurs. 2001;12(2):115–22.
Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. 2011;45(5):626–9.
Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004;10(2):307–12.
Bauhofer A, Stinner B, Plaul U, Torossian A, Middeke M, Celik I, Koller M, Lorenz W. Quality of life and the McPeek Recovery Index as a new outcome concept for sepsis trials tested in a pilot study of granulocyte colony-stimulating factor in patients with colorectal cancer. Br J Surg. 2001;88(8):1138–50.
Bunn V, Watling R, Ashby D, Smyth R. Feasibility study for a randomized controlled trial of oral calorie supplements in children with cystic fibrosis. In: Proceedings of 22nd European Cystic Fibrosis Conference Berlin. 1998. p. 13–9.
Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, Robson R, Thabane M, Giangregorio L, Goldsmith CH. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10(1):1.
Arnold DM, Burns KE, Adhikari NK, Kho ME, Meade MO, Cook DJ. The design and interpretation of pilot trials in clinical research in critical care. Crit Care Med. 2009;37(1):S69–74.
Ross‐McGill H, Hewison J, Hirst J, Dowswell T, Holt A, Brunskill P, Thornton J. Antenatal home blood pressure monitoring: a pilot randomised controlled trial. BJOG Int J Obstet Gynaecol. 2000;107(2):217–21.
Stevinson C, Ernst E. A pilot study of Hypericum perforatum for the treatment of premenstrual syndrome. BJOG Int J Obstet Gynaecol. 2000;107(7):870–6.
Nahm FS. Understanding effect sizes. Hanyang Med Rev. 2015;35(1):40–3.
Wilkinson L. Statistical methods in psychology journals: guidelines and explanations. Am Psychol. 1999;54(8):594.
Olejnik S, Algina J. Measures of effect size for comparative studies: applications, interpretations, and limitations. Contemp Educ Psychol. 2000;25(3):241–86.
Anderson DR, Burnham KP, Thompson WL. Null hypothesis testing: problems, prevalence, and an alternative. J Wildl Manag. 2000;64(4):912—23.
Park H, Yoo D, Kwon S, Yoo TW, Park HJ, Hahm DH, Lee H, Kim ST. Acupuncture stimulation at HT7 alleviates depression-induced behavioral changes via regulation of the serotonin system in the prefrontal cortex of maternally-separated rat pups. J Physiol Sci. 2012;62(4):351–7.
Klerman GL. Scientific and ethical considerations in the use of placebo controls in clinical trials in psychopharmacology. Psychopharmacol Bull. 1986;22(1):25-9.
Sanchez-Araujo M. Does the choice of placebo determine the results of clinical studies on acupuncture? Forschende Komplementärmedizin/Research in Complementary Medicine. 1998;5 Suppl 1:8–11.
Vincent C, Lewith G. Placebo controls for acupuncture studies. J R Soc Med. 1995;88(4):199–202.
Min SK. Clinical correlates of hwa-byung and a proposal for a new anger disorder. Psychiatry Invest. 2008;5(3):125–41.
Fava M, Rosenbaum JF, Pava JA, McCarthy MK, Steingard RJ, Bouffides E. Anger attacks in unipolar depression, Part 1: Clinical correlates and response to fluoxetine treatment. Am J Psychiatry 1993, 150:1158–1158.
Coccaro EF, Kavoussi RJ. Fluoxetine and impulsive aggressive behavior in personality-disordered subjects. Arch Gen Psychiatry. 1997;54(12):1081–8.
Cabyoglu MT, Ergene N, Tan U. The mechanism of acupuncture and clinical applications. Int J Neurosci. 2006;116(2):115–25.
Samuels N, Gropp C, Singer SR, Oberbaum M. Acupuncture for psychiatric illness: a literature review. Behav Med. 2008;34(2):55–62.
Takagi J, Yonehara N. Serotonin receptor subtypes involved in modulation of electrical acupuncture. Jpn J Pharmacol. 1998;78(4):511–4.
Hv P. Anxiety and increased aggression as pacemakers of depression. Acta Psychiatr Scand. 1998;98(s393):81–8.
Crockett MJ, Clark L, Tabibnia G, Lieberman MD, Robbins TW. Serotonin modulates behavioral reactions to unfairness. Science 2008, 320(5884):1739–1739.
STATISTICS KOREA. International migration status. 2016. http://www.index.go.kr/potal/main/EachDtlPageDetail.do?idx_cd=1684. Accessed 20 Nov 2017.
United States Census Bureau. The Asian Population: 2010. https://www.census.gov/prod/cen2010/briefs/c2010br-11.pdf. 2010 Census Briefs 2012. Accessed 20 Nov 2017.
Acknowledgements
Not applicable
Funding
This study was supported by the Korean Institute of Oriental Medicine (K16122 and K17122).
Availability of data and materials
Data generated or analyzed during this study will be included in the paper reporting our results. The results of this clinical trial will be shared through scientific articles and international conferences.
Author information
Authors and Affiliations
Contributions
HYL drafted the manuscript with interpretation and consideration of the protocol. JEK conceived and designed the study protocol and modified the manuscript draft with HYL. MKK contributed to critical revisions of the manuscript. OJK planned the statistical design of the study and wrote part of the statistical methods. ARK and HJP provided technical advice. JHC and SYC were consulted on the protocol as clinical experts. JHK directed the overall design of the study and participated in critical revisions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study has been approved by the IRB of the Daejeon Oriental Hospital of Daejeon University (approval No. djomc-133). Any modification is prohibited without the IRB’s approval, and any deviation or violation should be reported to the IRB. Written informed consent will be obtained from participants by a qualified traditional Korean medical doctor.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Checklist of Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA) (DOCX 19 kb)
Additional file 2:
SPIRIT 2013 Checklist: recommended items to address in a clinical trial protocol and related documents. (DOC 121 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lee, HY., Kim, JE., Kim, M. et al. Effect and safety of acupuncture for Hwa-byung, an anger syndrome: a study protocol of a randomized controlled pilot trial. Trials 19, 98 (2018). https://doi.org/10.1186/s13063-017-2399-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-017-2399-0