Abstract
Background
In this prospective phase 2 trial, we assessed the efficacy of trastuzumab-emtansine (T-DM1) in HER2-negative metastatic breast cancer (MBC) patients with HER2-positive CTC.
Methods
Main inclusion criteria for screening were as follows: women with HER2-negative MBC treated with ≥ 2 prior lines of chemotherapy and measurable disease. CTC with a HER2/CEP17 ratio of ≥ 2.2 by fluorescent in situ hybridization (CellSearch) were considered to be HER2-amplified (HER2amp). Patients with ≥ 1 HER2amp CTC were eligible for the treatment phase (T-DM1 monotherapy). The primary endpoint was the overall response rate.
Results
In 154 screened patients, ≥ 1 and ≥ 5 CTC/7.5 ml of blood were detected in N = 118 (78.7%) and N = 86 (57.3%) patients, respectively. ≥1 HER2amp CTC was found in 14 patients (9.1% of patients with ≥ 1 CTC/7.5 ml). Among 11 patients treated with T-DM1, one achieved a confirmed partial response. Four patients had a stable disease as best response. Median PFS was 4.8 months while median OS was 9.5 months.
Conclusions
CTC with HER2 amplification can be detected in a limited subset of HER2-negative MBC patients. Treatment with T-DM1 achieved a partial response in only one patient.
Trial registration
NCT01975142, Registered 03 November 2013
Similar content being viewed by others
Introduction
In view of the significant efficacy of anti-HER2 targeted therapies on HER2-positive breast cancers, assessment of HER2 status has become a cornerstone of the current breast cancer management. HER2 testing on tumor tissue has been standardized by successive guidelines and relies first on immunohistostaining [1,2,3]. In ambiguous cases, in situ hybridization (ISH) assays remain the gold standard as it directly assesses HER2 gene copy number and any chromosome 17 polysomy. As HER2 amplification is an early oncogenic event, HER2 status has been found to be different between primary tumors and matched metastatic tissue in fewer than 10% of patients [4,5]. When clinically feasible, HER2 status should therefore be reassessed on metastatic tissue sample in metastatic breast cancer (MBC) patients [6].
While invasive biopsy of a metastatic lesion may not always be feasible or contributive, circulating tumor biomarkers promise to become a noninvasive surrogate for tissue-based biomarkers, including HER2 status [7,8]. Many detection platforms have demonstrated that HER2 immunocytostaining and ISH techniques can be applied to circulating tumor cells (CTC) [9,10,11,12]. Some reports have also suggested a significant heterogeneity between the HER2 status of primary breast tumors and that of matched CTC sampled during the course of metastatic disease [9,13,14,15,16,17,18,19,20,21].
Within the current armamentarium of HER2-targeting drugs, trastuzumab-emtansine (T-DM1) has demonstrated its efficacy in the metastatic setting, starting from the second line of therapy [22,23]. This antibody-drug conjugate is given as a single agent and therefore represents an exquisite targeting of HER2-positive tumor cells, with no direct action on HER2-negative tumor cells.
The purpose of the phase 2 “CirCe T-DM1” trial was to investigate the clinical actionability of CTC-based HER2 status assessment. In a screening step, HER2-negative MBC patients were screened for HER2-positive CTC with the most reliable technical approach, ISH. During the treatment step, HER2-negative MBC patients with HER2-amplified (HER2amp) CTC were treated with T-DM1 given as a single agent. Final results of the screening and the treatment steps are reported here.
Materials and methods
The CirCe T-DM1 trial was approved by the regional ethics committee (CPP Ile de France I) and has been registered (EudraCT 2012-005155-16; NCT01975142). All patients provided written informed consent at inclusion both in the screening step and in the treatment step.
Screening
Main inclusion criteria for the screening step were as follows: women with HER2-negative adenocarcinoma of the breast, as assessed by immunohistostaining or/and ISH on the primary breast tumor (HER2 status reassessment on metastatic lesions was not mandatory but, when performed, had to be HER2-negative); metastatic and/or inoperable locoregional relapse progressing on at least two prior lines of systemic chemotherapy; measurable disease (RECIST v1.1); WHO performance status of 0–2; adequate laboratory parameters; and cardiac function.
Three 7.5-ml blood samples were drawn in CellSave® tubes from patients included in the screening step. Tubes were shipped at room temperature to a central laboratory (Janssen Diagnostics), located in Beerse, Belgium. The fluorescent-ISH (FISH) analysis was a multistep process, performed by Janssen Diagnostics under blinded conditions. CTC were first detected and located by immunocytofluorescence (standard CellSearch® technique); the slide was then submitted to FISH and screened a second time for fluorescent signal. Results were available within 7 days and included the following: number of CTC detected, absolute numbers of HER2 copies and chromosome 17 centromeres (CEP17) for each CTC with interpretable FISH assay, at the single cell level, and the results of internal negative controls (i.e. HER2 and CEP17 signals observed in leukocytes from the same sample). CTC displaying a HER2/CEP17 ratio of ≥ 2.2 (as per the 2007 ASCO/CAP guidelines [1]) and/or > 6 HER2 copies were considered as HER2amp. CTC with high numbers of HER2 copies without CEP17 signal available or with a HER2/CEP17 ratio < 2.2 were not considered to be HER2amp. Patients with no CTC or with non-HER2amp CTC were then considered to be off-study.
Treatment and assessment
Patients with ≥ 1 HER2amp CTC detected at the screening step were eligible for the treatment step, which consisted of T-DM1 monotherapy at the standard dose of 3.6 mg/kg IV every 3 weeks (dose reductions were allowed in the case of toxicity). Clinical and laboratory examinations were performed at each cycle, and radiological evaluation was performed every 6 weeks, according to RECIST 1.1. A second CTC count with HER2 FISH was performed after 1 cycle of therapy, but clinicians were blinded to the results.
Statistics
We hypothesized that the efficacy of T-DM1 may differ according to the absolute number of HER2amp CTC detected at the screening step; also, a very low HER2amp CTC count might be distributed by Poisson’s law of rare event and therefore turn out to be less reproducible. We therefore distinguished two populations of treated patients: HER2amp CTClow and HER2amp CTChigh populations, corresponding to patients with 1–2 HER2amp or ≥ 3 HER2amp CTC detected, respectively.
The primary objective of this study was to report the efficacy of T-DM1 in the two populations. The primary endpoint was the objective response rate among treated patients. Secondary objectives included progression-free survival (PFS; defined as the time between inclusion in the treatment step and tumor progression or death, whichever came first), overall survival (OS), duration of response, and biomarker responses.
The design of this multicenter phase 2 trial was derived from a multiple-stage Fleming design [24,25], the two populations (HER2amp CTClow and HER2amp CTChigh) being assessed as separate cohorts. In a first stage, seven patients had to be included in each of the two cohorts (N = 7 HER2amp CTClow and N = 7 HER2amp CTChigh). T-DM1 was estimated to be effective when it yielded a response rate of 25% (H1) and ineffective when it yielded a response rate < 5% (H0). After inclusion of seven patients, the study could be stopped in the corresponding population for inefficacy (no response observed) or efficacy (three or more response observed). When one to two responses were observed, another 7 patients had to be included in each cohort before drawing conclusions. In total, with an anticipated 10% detection rate of HER2amp CTC, about 280 patients could have been included in the screening step. With the abovementioned H0 and H1 hypotheses, this trial had an overall alpha risk of 0.06 and a power of 0.94. Data are available upon request.
Results
Detection of HER2amp CTC
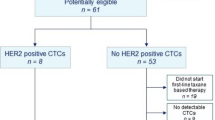
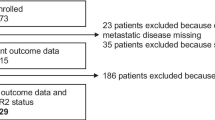
This study was open to accrual from 11/2013 to 09/2016 in 10 centers. One hundred fifty-five patients were included in the screening phase, and one patient was subsequently excluded due to HER2 positivity on metastasis. Patient characteristics are shown in Table 1. Among 154 included patients, 80 (51.9%) patients underwent a biopsy of their local/distant relapse which confirmed the HER2-negative tumor status; 87 (57.2%) patients received 3 or more lines of chemotherapy for their metastatic disease. The study flow chart is displayed in Fig. 1.
Among the 154 patients screened, ≥ 1 and ≥ 5 CTC/7.5 ml of blood were detected in N = 118 (78.7%) and N = 86 (57.3%) patients, respectively. Sixty-seven of the 79 patients (84.8%) of patients with a performance status of 1 or 2 had ≥ 1 CTC/7.5 ml detected, versus 40/63 (63.4%) patients with a performance status of 0 (p = 0.006).
FISH analysis was performed in samples with ≥ 1 CTC. For any given CTC, FISH results were considered to be interpretable when both HER2 and CEP17 signals could be assessed on at least 1 CTC and when leukocytes from the same sample showed normal HER2 and CEP17 signals. Overall, among the 7124 CTC detected by the CellSearch system in 118 patients with ≥ 1 CTC/7.5 ml at the screening stage, FISH was deemed interpretable for 1652 CTC in 79 patients (66.9% of patients with ≥ 1 CTC /7.5 ml). As expected, the probability of having ≥ 1 CTC on a blood sample with an interpretable FISH result was correlated with the total number of CTC detected (Table 1; Fig. 2a, ρ = 0.81, p = < 0.001).
Correlations between absolute CTC count and downstream CTC characterization. a Correlation between the number of CTC detected and the number of CTC with interpretable FISH results. b Correlation, in the 14 patients with ≥ 1 HER2amp CTC at the screening step, between the number of (i) CTC, (ii) CTC with interpretable FISH result, and (iii) HER2amp CTC. b Correlation between the number of CTC detected and the number of JER2amp CTC for the 14 patients with ≥ 1 HERamp CTC detected. For each patient, the number of CTC with interpretable FISH results is shown in brackets.
Overall, ≥ 1 HER2amp CTC was found in 14 patients (9.1%). The detection of HER2amp CTC was not correlated to the total number of detected CTC (Fig. 2b) but was associated with performance status (p = 0.02). Among patients with ≥ 1 HER2amp CTC, absolute CTC count, CTC with interpretable FISH, and CTC with or without HER2amp are displayed in Table 2. Seven patients (50%) had 1 HER2amp CTC. Among patients with ≥ 2 CTC with interpretable FISH results, the median ratio of HER2amp CTC among all CTC with interpretable FISH results was 8.2% (range 0.6–60%).
In September 2016, the supplier of the FISH CTC assay, blinded to the ongoing study results, discontinued development of this test. Patient screening and enrollment was therefore stopped prior to completion of the targeted study enrollment.
Patient outcomes
In the overall screening population, CTC count confirmed its prognostic value on PFS and OS in univariate and multivariate analyses (Additional file 1: Figure S1; Additional file 2: Table S1).
In patients with no CTC detected at screening, observed median PFS and OS were 11.6 months (95%CI = [7.3;17.5]) and 31 months (95%CI = [25;not reached]), respectively. In patients with ≥ 1 CTC but no HER2amp CTC detected at screening, observed median PFS and OS were 5.6 months (95%CI = [4.1;7.4]) and 10.7 months (95%CI = [8.1;14.8]), respectively.
Eleven patients with ≥ 1 HER2amp CTC detected at screening have been included in the treatment step: 9 patients in the HER2amp CTClow cohort (1 or 2 HER2amp CTC detected) and 2 patients in the HER2amp CTChigh cohort (≥3 CTC HER2amp CTC detected); all patients included in the treatment step received at least one dose of T-DM1.
Overall, one of the first seven patients (patient #03-020-1 in Table 2) included in the HER2amp CTClow cohort achieved a confirmed partial response, which allowed to enroll two more patients in that cohort prior to the study discontinuation. The overall objective response rate was therefore 11.1% (95%CI = [0.3; 48.3]) in the CTClow cohort (N = 9 patients) and 9.1% (95%CI = [0.23;41.3]) in all treated patients (N = 11 patients). The duration of this partial response was 7.1 months. At time of progression, this patient underwent a biopsy of a metastasis that responded to T-DM1, which confirmed the lack of HER2 amplification. Four patients had a stable disease as the best response. Another patient in the same cohort displayed a drop of serum tumor markers (CA15-3 and CEA) after 1 cycle of T-DM1 but was withdrawn from the study thereafter due to a grade III pneumonitis. No other toxicities potentially related to T-DM1 have been observed. Overall, in the 11 patients with HER2amp CTC, observed median PFS and OS were 4.8 months 95%CI = [2;not reached] and 9.5 months 95%CI = [4.0;not reached], respectively (Fig. 3).
Table 2 shows the total CTC count and HER2amp CTC count during therapy for the 11 treated patients, whenever available.
Discussion
This study exemplifies the promises and the pitfalls of using a “liquid biopsy” to guide patient towards a personalized therapy.
Firstly, each biomarker (such as HER2 amplification in our study) of interest on CTC requires the detection of at least one CTC. In a prior study, we reported an overall good concordance between the HER2 status of the primary breast cancer and that of CTC (assessed by immunocytofluorescence), only if a sufficient number of CTC was assessed [26]. Our results show that the feasibility of HER2 FISH on CTC is directly correlated with the number of CTC detected, which is per se an independent prognostic factor, as demonstrated elsewhere [27] and confirmed in our study. The limited number of CTC with interpretable FISH results was maybe related to the two-step process, some cells detaching from the slide after the CTC count, during the FISH procedure.
Secondly, we found out—using ISH, the most reliable assay for HER2 status—that nearly 10% of MBC patients with HER2-negative tumor exhibit HER2amp CTC. The presence of such HER2-positive CTC (detected by ISH or immunocytology) has been reported by several observational studies [9,13,14,15,16,17,18,19]. In our study, both the absolute number of HER2amp CTC and the HER2amp/HER2-negative CTC ratio were low, suggesting that these HER2amp CTC account for a minority of the tumor burden and that HER2amp subclones do not expand significantly during therapy in MBC patients.
From a statistical perspective, the main limitation of our study is the low number of patients that were treated with T-DM1, so the efficacy of T-DM1 in patients with HER2-negative tumors and HER2amp CTC, although unlikely, cannot formally be ruled out. In addition to the low objective response rate, the median PFS achieved by T-DM1 in patients with HER2amp CTC compares unfavorably to that observed in patients with ≥ 1 CTC but no HER2amp CTC.
In our study, the use of T-DM1 allowed assessing the efficacy of “pure” anti-HER2 therapy in the setting of a single-arm phase 2 study, without any other anti-tumor therapy. A different approach, investigated in the DETECT III study (NCT01619111), is to measure whether adding an anti-HER2 agent to a chemotherapy backbone would benefit to HER2-negative MBC patients with HER2-positive CTC, assessed by immunocytofluorescence (design reviewed in [28]). Results of this randomized phase III study are awaited and will complete our current understanding of the clinical actionability of HER2amp CTC in HER2-negative MBC patients.
The lack of synchronous metastatic tissue biopsy at time of treatment initiation also prevents us from comparing the HER2 amplification status between CTC and metastatic tissue. Recent preclinical experiments suggested that HER2-positive CTC retrieved from HER2-negative MBC patients are more proliferative but not addicted to HER2 signaling [14]. However, trastuzumab-deruxtecan, a newer trastuzumab-drug conjugate, demonstrated an efficacy in some patients with HER2-negative metastatic cancers [29]; this efficacy signal is now investigated in a phase 3 trial (NCT03734029).
Conclusions
CTC with HER2 amplification can be detected by ISH in about one tenth of HER2-negative MBC patients with detectable CTC. The very limited efficacy of single agent T-DM1 in that setting may be related to the fact that HER2-amplified CTC represented only a fraction of the total CTC detected in treated patients.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to privacy of clinical data but are available from the corresponding author on reasonable request.
Abbreviations
- CEP17:
-
Chromosome 17 centrosome
- CTC:
-
Circulating tumor cell
- FISH:
-
Fluorescent in situ hybridization
- HER2:
-
Human EGFR-related receptor 2
- HER2amp :
-
Human EGFR-related receptor 2-amplified
- ISH:
-
In situ hybridization
- MBC:
-
Metastatic breast cancer
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- T-DM1:
-
Trastuzumab emtansine
References
Wolff AC, Hammond MEH, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45.
Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JMS, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013.
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36:2105–22.
Aurilio G, Disalvatore D, Pruneri G, Bagnardi V, Viale G, Curigliano G, Adamoli L, Munzone E, Sciandivasci A, De Vita F, Goldhirsch A, Nolè F. A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur J Cancer. 2014;50:277–89.
Comte A, Sigal-Zafrani B, Belin L, Bièche I, Callens C, Diéras V, Bidard F-C, Mariani O, Servois V, Szwarc D, Vincent-Salomon A, Brain ECG, et al. Abstract P2-05-06: Clinical utility of systematic biopsy of first metastatic event in breast cancer: Results from a prospective multicenter trial. Cancer Res. 2016;76:P2–05–6.
Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, Harbeck N, Aguilar Lopez B, Barrios CH, Bergh J, Biganzoli L, Boers-Doets CB, et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4)†. Ann Oncol. 2018;29:1634–57.
Bidard F-C, Weigelt B, Reis-Filho JS. Going with the flow: from circulating tumor cells to DNA. Sci Transl Med. 2013;5:207ps14.
Cabel L, Proudhon C, Gortais H, Loirat D, Coussy F, Pierga J-Y, Bidard F-C. Circulating tumor cells: clinical validity and utility. Int J Clin Oncol. 2017;22:421–30.
Fehm T, Becker S, Duerr-Stoerzer S, Sotlar K, Mueller V, Wallwiener D, Lane N, Solomayer E, Uhr J. Determination of HER2 status using both serum HER2 levels and circulating tumor cells in patients with recurrent breast cancer whose primary tumor was HER2 negative or of unknown HER2 status. Breast Cancer Res. 2007;9:R74.
Fehm T, Müller V, Aktas B, Janni W, Schneeweiss A, Stickeler E, Lattrich C, Löhberg CR, Solomayer E, Rack B, Riethdorf S, Klein C, et al. HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res Treat. 2010;124:403–12.
Mayer JA, Pham T, Wong KL, Scoggin J, Sales EV, Clarin T, Pircher TJ, Mikolajczyk SD, Cotter PD, Bischoff FZ. FISH-based determination of HER2 status in circulating tumor cells isolated with the microfluidic CEE™ platform. Cancer Genet. 2011;204:589–95.
Perez-Toralla K, Mottet G, Guneri ET, Champ J, Bidard F-C, Pierga J-Y, Klijanienko J, Draskovic I, Malaquin L, Viovy J-L, Descroix S. FISH in chips: turning microfluidic fluorescence in situ hybridization into a quantitative and clinically reliable molecular diagnosis tool. Lab Chip. 2015;15:811–22.
Meng S, Tripathy D, Shete S, Ashfaq R, Saboorian H, Haley B, Frenkel E, Euhus D, Leitch M, Osborne C, Clifford E, Perkins S, et al. uPAR and HER-2 gene status in individual breast cancer cells from blood and tissues. Proc Natl Acad Sci U S A. 2006;103:17361–5.
Jordan NV, Bardia A, Wittner BS, Benes C, Ligorio M, Zheng Y, Yu M, Sundaresan TK, Licausi JA, Desai R, O’Keefe RM, Ebright RY, et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature. 2016;537:102–6.
Wallwiener M, Hartkopf AD, Riethdorf S, Nees J, Sprick MR, Schönfisch B, Taran F-A, Heil J, Sohn C, Pantel K, Trumpp A, Schneeweiss A. The impact of HER2 phenotype of circulating tumor cells in metastatic breast cancer: a retrospective study in 107 patients. BMC Cancer. 2015;15:403.
Agelaki S, Dragolia M, Markonanolaki H, Alkahtani S, Stournaras C, Georgoulias V, Kallergi G. Phenotypic characterization of circulating tumor cells in triple negative breast cancer patients. Oncotarget. 2017;8:5309–22.
Beije N, Onstenk W, Kraan J, Sieuwerts AM, Hamberg P, Dirix LY, Brouwer A, de Jongh FE, Jager A, Seynaeve CM, Van NM, Foekens JA, et al. Prognostic impact of HER2 and ER status of circulating tumor cells in metastatic breast cancer patients with a HER2-negative primary tumor. Neoplasia. 2016;18:647–53.
B a. S J, Neugebauer J, Andergassen U, Melcher C, Schochter F, Mouarrawy D, Ziemendorff G, Clemens M, V Abel E, Heinrich G, Schueller K, Schneeweiss A, et al. The HER2 phenotype of circulating tumor cells in HER2-positive early breast cancer: A translational research project of a prospective randomized phase III trial. PLoS One. 2017;12:e0173593.
Chen W, Zhang J, Huang L, Chen L, Zhou Y, Tang D, Xie Y, Wang H, Huang C. Detection of HER2-positive circulating tumor cells using the LiquidBiopsy system in breast cancer. Clin Breast Cancer. 2019;19(1):e239-46.
Pestrin M, Bessi S, Galardi F, Truglia M, Biggeri A, Biagioni C, Cappadona S, Biganzoli L, Giannini A, Di Leo A. Correlation of HER2 status between primary tumors and corresponding circulating tumor cells in advanced breast cancer patients. Breast Cancer Res Treat. 2009;118:523–30.
Munzone E, Nolé F, Goldhirsch A, Botteri E, Esposito A, Zorzino L, Curigliano G, Minchella I, Adamoli L, Cassatella MC, Casadio C, Sandri MT. Changes of HER2 status in circulating tumor cells compared with the primary tumor during treatment for advanced breast cancer. Clin Breast Cancer. 2010;10:392–7.
Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh D-Y, Diéras V, Guardino E, Fang L, Lu MW, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91.
Krop IE, Kim S-B, González-Martín A, LoRusso PM, Ferrero J-M, Smitt M, Yu R, Leung ACF, Wildiers H. TH3RESA study collaborators. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:689–99.
Tournoux-Facon C, De Rycke Y, Tubert-Bitter P. Targeting population entering phase III trials: a new stratified adaptive phase II design. Stat Med. 2011;30:801–11.
Tournoux-Facon C, De Rycke Y, Tubert-Bitter P. How a new stratified adaptive phase II design could improve targeting population. Stat Med. 2011;30:1555–62.
Ligthart ST, Bidard F-C, Decraene C, Bachelot T, Delaloge S, Brain E, Campone M, Viens P, Pierga J-Y, Terstappen LWMM. Unbiased quantitative assessment of Her-2 expression of circulating tumor cells in patients with metastatic and non-metastatic breast cancer. Ann Oncol. 2013;24:1231–8.
Bidard F-C, Peeters DJ, Fehm T, Nolé F, Gisbert-Criado R, Mavroudis D, Grisanti S, Generali D, Garcia-Saenz JA, Stebbing J, Caldas C, Gazzaniga P, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15:406–14.
Bidard F-C, Fehm T, Ignatiadis M, Smerage JB, Alix-Panabières C, Janni W, Messina C, Paoletti C, Müller V, Hayes DF, Piccart M, Pierga J-Y. Clinical application of circulating tumor cells in breast cancer: overview of the current interventional trials. Cancer Metastasis Rev. 2013;32:179–88.
Doi T, Shitara K, Naito Y, Shimomura A, Fujiwara Y, Yonemori K, Shimizu C, Shimoi T, Kuboki Y, Matsubara N, Kitano A, Jikoh T, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol. 2017;18:1512–22.
Acknowledgements
The study was designed at the 2011 ESMO-ECCO-AACR-EORTC “Methods in Clinical Cancer Research” workshop (aka “Flims workshop”), with major contributions from Profs S. Hilsenbeck (Houston, TX) and J. de Bono (London, UK).
Funding
This work was supported by Roche France and Institut Curie SIRIC2 (grant INCa-DGOS-INSERM_12554).
Author information
Authors and Affiliations
Contributions
FCB, JYP, and FB conceived the study; all authors participated to the acquisition and interpretation of data; all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The CirCe T-DM1 trial was approved by the regional ethics committee (CPP Ile de France I).
Consent for publication
Not applicable.
Competing interests
WJ: honoraria and travel grants from Roche. PC: research support from Roche. FCB and JYP: research support from Janssen Diagnostics, research support, honoraria, and travel grants from Roche. The other authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Figure S1. Survival by CTC count at the screening step. a: Progression-Free Survival. b: Overall Survival.
Additional file 2.
Table S1. Univariate and multivariate analyses for PFS and OS (screened population). For multivariate analyses, only significant results are shown. MBC: metastatic breast cancer.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Jacot, W., Cottu, P., Berger, F. et al. Actionability of HER2-amplified circulating tumor cells in HER2-negative metastatic breast cancer: the CirCe T-DM1 trial. Breast Cancer Res 21, 121 (2019). https://doi.org/10.1186/s13058-019-1215-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13058-019-1215-z