Abstract
Background
BRCA phenocopies are individuals with the same phenotype (i.e. cancer consistent with Hereditary Breast and Ovarian Cancer syndrome = HBOC) as their affected relatives, but not the same genotype as assessed by blood germline testing (i.e. they do not carry a germline BRCA1 or BRCA2 mutation). There is some evidence of increased risk for HBOC-related cancers in relatives of germline variant carriers even though they themselves test negative for the familial variant (BRCA non-carriers). At this time, BRCA phenocopies are recommended to undergo the same cancer surveillance as individuals in the general population. This raises the question of whether the increased cancer risk in BRCA non-carriers is due to alterations (germline, somatic or epigenetic) in other cancer-associated genes which were not analyzed during BRCA analysis.
Methods
To assess the nature and potential clinical significance of somatic variants in BRCA phenocopy tumors, DNA from BRCA non-carrier tumor tissue was analyzed using next generation sequencing of 572 cancer genes. Tumor diagnoses of the 11 subjects included breast, ovarian, endometrial and primary peritoneal carcinoma. Variants were called using FreeBayes genetic variant detector. Variants were annotated for effect on protein sequence, predicted function, and frequency in different populations from the 1000 genomes project, and presence in variant databases COSMIC and ClinVar using Annovar.
Results
None of the familial BRCA1/2 mutations were found in the tumor samples tested. The most frequently occurring somatic gene variants were ROS1(6/11 cases) and NUP98 (5/11 cases). BRCA2 somatic variants were found in 2/6 BRCA1 phenocopies, but 0/5 BRCA2 phenocopies. Variants of uncertain significance were found in other DNA repair genes (ERCC1, ERCC3, ERCC4, FANCD2, PALB2), one mismatch repair gene (PMS2), a DNA demethylation enzyme (TET2), and two histone modifiers (EZH2, SUZ12).
Conclusions
Although limited by a small sample size, these results support a role of selected somatic variants and epigenetic mechanisms in the development of tumors in BRCA phenocopies.
Similar content being viewed by others
Introduction
Cancer predisposition in hereditary breast and ovarian cancer (HBOC) syndrome is caused by pathogenic germline variants in the BRCA1/2 genes (germline BRCA variants) and is inherited in an autosomal dominant pattern. The lifetime risk of breast cancer in female germline BRCA variant carriers is up to 85%; the lifetime risk of ovarian cancer is up to 50% [2, 6, 8, 11, 33, 34, 36, 38] Genetic alterations in the BRCA1/2 genes cause over 90% of cases of HBOC [6, 33, 34, 36]. The genetic test recommended for patients suspected of carrying a germline BRCA variant involves sequencing of their BRCA1 and BRCA2 genes with deletion/duplication analyses, most commonly in blood and less frequently in saliva. A relative of a germline BRCA variant carrier who has tested negative for the known familial alteration is deemed to have normal (wild-type) germline BRCA1 and BRCA2 genes and is sometimes called a BRCA non-carrier.
There are conflicting reports on the relative risk ratio (RR) of breast and ovarian cancers in BRCA non-carriers when there is a known familial BRCA genetic alteration. Some authors argue that their cancer risk is the same as in the general population [24, 42], some conclude that their risk is the same as high risk families without identified germline BRCA pathogenic variants [20], but, overall, most authors agree that the risk is increased with a breast cancer RR of up to 5.1 [14, 27, 32, 35]. The studies are difficult to compare due to differences in methodology and patient populations [17] as well as different prognoses of breast cancers with BRCA1 vs. BRCA2 alterations [12, 25]. Germline BRCA non-carriers that develop breast or ovarian cancers are referred to as “BRCA phenocopies,” meaning that they have the same phenotype (affected by cancer) as germline BRCA carriers, but do not have the same genotype (the known BRCA alteration as shown by germline genetic testing).
Explanations offered for HBOC malignancies in BRCA phenocopies include sporadic cancer related to familial lifestyle and/or environmental factors or pathogenic variants in other, possibly not yet identified, genes that cause HBOC. All of these explanations assume that cancers in BRCA non-carriers are not related to the familial BRCA variant. The risk of BRCA non-carriers developing an HBOC cancer is clinically important because it determines their cancer surveillance and prevention recommendations [16].
Undetectable germline variants in blood might also be due to revertant mosaicism where a rare spontaneous correction of a pathogenic variant might occur in BRCA pedigrees and result in false-negative testing for the familial variant. However, a study by Azzollini et al. [4] did not find the familial variants in tumor samples, blood leukocytes, buccal mucosa nor urine of BRCA phenocopies. We previously explored natural chimerism as an alternative explanation for BRCA phenocopies [28]. We hypothesized that breast and ovarian cancer can still be caused by familial BRCA variants, but transmitted in an alternative, non-mendelian fashion (e.g., through maternal-fetal or tetragametic chimerism) so that the altered genes are present in chimeric tissues rather than in blood. Since BRCA mutant cells are much more likely to give rise to cancer than non-mutants cells, in a chimeric organism, the tumor would be BRCA-mutant. We analyzed tumor tissue in BRCA phenocopies for the known familial variant using targeted PCR and qPCR methods [28]. In our cohort of 11 cases, we did not find the familial alteration in the tumor tissues. In the current study, we analyzed the tumor samples from the same cohort of patients. We used next generation sequencing (NGS) to investigate the possibility of other (somatic and/or germline) gene variants driving the cancer phenotype and the possibility of BRCA1/2 epigenetic silencing in the context of familial cancer predisposition.
Methods
Patients, clinical assessment, and germline genetic testing
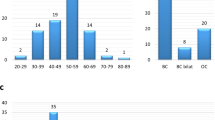
Patients for this study were selected based on an HBOC cancer phenotype in the absence of a known familial BRCA mutation found in a first-degree relative. With approval by the Rush University Medical Center Institutional Review Board, each subject signed an informed consent form and tumor specimens were obtained from the Department of Pathology, Rush University Medical Center (Chicago, IL) and Pathology Departments of other institutions where participants had their cancer surgery. Cancer diagnoses were obtained from pathology reports and histologic evaluation. Clinical data were established from chart review and self-reported history forms. Patients were eligible if they were affected by cancer but had previously tested negative for a known familial pathogenic variant. Breast cancer patients under 45 (invasive or non-invasive), women with ovarian, fallopian tube, or primary peritoneal cancer at any age, endometrial cancer, patients with male breast cancer or pancreatic cancer were considered eligible for this study. Eleven cases that met these criteria were found. Familial mutations included BRCA1 c.186_187delAG (p.L22_E23LVfs; 2 patients), c.1793delA (p.G559Vfs), c.17 + 3A > G, c.2841A > T (p.K947 N), c.3109_3110insAA (p.K1037 fs), c.5215G > A (p.D1739N), c.8107G > A, and BRCA2 c.6794_6975 insA, c.5645C > A (p.S882*) and c.6174delT (p.F2058Lfs).
Four patients underwent expanded commercial germline genetic testing in addition to the BRCA1/2 testing. Patients 1 and 9 underwent gene panel testing that included 23 genes: ATM, BARD1, BRCA1, BRCA2, BRIP1, CDH, CHEK2, EPCAM, MLH1, MRE11A, MSH2, MSH6, MUTYH, NFN, NF1, PALB2, PMS2, PTEN, RAD50, RAD51C, RAD51D, STK11, and TP53. Patient 5 underwent a more comprehensive gene panel test due to her significant personal and family history of cancer that included 49 genes: APC, ATM, BAP1, BARD1, BRCA1, BRCA2, BRIP1, BMPR1A, CDH1, CDK4, CDKN2A, CHEK2, EPCAM FH, FLCN, GREM1, MAX, MEN1, MET, MITF, MLH1, MRE11A, MSH2, MSH6, MUTYH, NBN, NF1, PALB2, PMS2, POLD1, POLE, PTEN, RAD50, RAD51C, RAD51D, RET, SDHA, SDHAF2, SDHB, SDHC, SDHD, SMAD4, SMARCA4, STK11, TMEM127, TP53, TSC1, TSC2, and VHL. Patient 3 underwent a gene panel specific to breast cancer risk that included 14 genes: ATM, BARD1, BRIP1, CDH1, CHEK2, MRE11A, MUTYH, NBN, PALB2, PTEN, RAD50, RAD51C, STK11, and TP53. Patient 10 underwent Lynch syndrome testing (MLH1, MSH2, and MSH6) in addition to BRCA1/2 due to a personal history of early onset endometrial cancer. No pathogenic variants were identified in the other genes tested.
DNA isolation
Hematoxylin and eosin-stained tissue 4 μm sections cut adjacent to unstained sections were examined by a pathologist. Using the stained slide as a guide, approximately 2mm2 of tumor tissue was manually scraped from the unstained slides. The tissue was digested in a solution of 1.0 mg/mL proteinase K (Sigma) in10mM Tris, pH 8.3, and 50 mM KCl. Digestions proceeded overnight at 56 °C. After fluorometric confirmation of adequate DNA concentration, the lysate was used directly for sequencing analysis.
Library preparation
The Nimblegen Cancer Gene panel was used in this study. Sequences were selected using biotinylated capture probes (Nimblegen, Hoffmann-La Roche, Ltd., Basel, Switzerland). The captured DNA was fragmented to an average DNA fragment size is 180–220 bp and end-repaired for ligation of adaptor sequences carrying primer binding sites. The DNA was then amplified with primers tailed with sequencing primer binding sites and patient-specific indexes.
Tumor DNA sequencing
That captured library (576 target genes) was sequenced on the MiSeq™ system (Illumina). The products of the indexing amplification were denatured and introduced to the flow cell for in situ amplification by bridge PCR on the MiSeq. The resulting clusters of immobilized templates were subjected to reversible dye terminator sequencing.
Variant calling
Raw reads were aligned to human reference genome hg19 using BWA MEM [23]. Apparent PCR duplicates were removed using Picard Mark Duplicates [40]. Variants were called using FreeBayes variant detection [21] annotated for effect on protein sequence, predicted function, and frequency in different populations from the 1000 genomes project, and presence in variant databases COSMIC and ClinVar using Annovar [39].
Results
Variants
The eleven patients studied carried diagnoses of infiltrating ductal carcinoma, ductal carcinoma in situ, invasive lobular carcinoma, ovarian adenocarcinoma and primary peritoneal carcinoma. One patient with endometrial cancer (non-HBOC) was included. Patient ages at diagnosis ranged from 26 to 66 years. None of the 11 patient tumors tested displayed the familial BRCA variant (Table 1). All tumors underwent expanded gene panel testing and were confirmed negative for the familial variants.
Between 2000 and 12,000 variants were detected per sample using the Nimblegen cancer gene panel. This panel is directed at high coverage of 572 genes with a reported role in carcinogenesis. Table 1 shows the analysis data for variants with potential clinical significance.
Variant data was annotated and screened for coverage (read depth or number of times sequenced). Samples 3, 6, 8, 10 and 11 had limiting DNA, resulting in lower coverage. The effect of the variant change on the protein product was calculated using PolyPhen for variants located in coding regions. The impact of the amino acid variants on protein structure (and function) was predicted from analysis of multiple sequence alignments and protein 3D-structures, predicting the effect of the DNA change on the cell (tumor) phenotype. All samples had at least one variant previously reported in the COSMIC or ClinVar databases. There are, however, caveats to using databases where submitted variants are at most classified into levels of clinical relevance as a source of information. Defined evidence categories provided by contributors may not sufficiently describe the medical significance of a variant [3].
The most frequently observed mutated gene was ROS1 (6/11 cases), displaying variants p.S1109 L and p.I537M, neither of which predict a deleterious effect on protein function, and p.S1109 L, which may affect protein function (Polyphen score 0.908). ROS1 encodes a receptor tyrosine kinase related to anaplastic lymphoma kinase (ALK), along with members of the insulin-receptor family. ROS1 gene rearrangements lead to fusion of the entire tyrosine kinase domain of ROS1 with 1 of 12 different partners, including fusions with: TPM3, SDC4, SLC34A2, CD74 and EZR. ROS1gene rearrangements are present in about 1% of lung cancers where they are therapeutic targets for an FDA-approved agent Crizotinib. The same deleterious RET mutation p.S649 L (Polyphen score 1) was present in cases 8 and 4. RET is another receptor tyrosine kinase [10].
The next most frequent variant found was NUP98, found in 5/11 cases. This gene encodes a 186 kDa precursor protein that undergoes auto-proteolytic cleavage to generate a 98 kDa nucleoporin and 96 kDa nucleoporin, the latter portion is a scaffold component of the nuclear core complex that regulates transport of macromolecules between the nucleus and cytoplasm. Translocations between this gene and many other partner genes have been observed in myeloid leukemia and myelodysplastic syndrome [41].
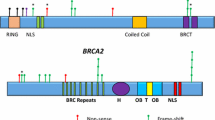
Somatic BRCA2 variants were observed in three tumors, two of which were from BRCA1 phenocopies, and each had two BRCA2 variants: N289H and N991D, only one of which is deleterious (Polyphen scores 0.991 and 0, respectively). A third tumor from a BRCA1 phenocopy had a BRCA1 Q309R deleterious variant (Polyphen score .999). All BRCA variants have been reported in COSMIC or ClinVar. None of familial BRCA pathogenic variants were found among the variants.
A PALB2 p.E672Q variant (Polyphen score 0.275) was present in one tumor and a deleterious PALB2 p.G998E variant found in another (Polyphen score 1). Both tumors were from BRCA2 phenocopies. PALB2 serves as the molecular scaffold in the formation of the homologous recombination BRCA1-PALB2-BRCA2 complex [37].
Fanconi anemia complementation group D2, FANCD2 variants p.N545S and p.R174Q were present in separate samples, the latter variant having predicted effects on protein function (Polyphen score .96), but also having low sequence depth. PALB2 and BRCA2 are members of this complementation group.
Other DNA repair complexes are represented amongst the variants including PMS2 with p.V738F and p.R20Q, ERCC3 p.S704 L and ERCC4 p.R415Q. These variants, however, had low sequence coverage (< 50).
A somatic p.S1079 L variant in WRN was found in one case. Germline WRN variants are associated with premature aging. The WRN gene also functions in DNA repair and may have implications in tumorigenesis [9, 31].
Variants in genes that regulate histone and DNA methylation were present in three cases. TET1 p.A256V has unlikely protein effect, while TET2 p.P29R has a high Polyphen score .993. The ten-eleven translocation (TET) genes encode oxidases that demethylate methylated cytosine in DNA. A histone methylase gene variant, EZH2 p.D146H (Polyphen score .898) was found in a different specimen. EZH2 encodes a member of the Polycomb-group (PcG) protein family. PcG family members form multimeric protein complexes which maintain the transcriptional repressive state of genes.
Variants in genes that regulate histone and DNA methylation were present in three cases. TET1 p.A256V is unlikely to affect the protein function, while TET2 p.P29R has a high Polyphen score 0.993. The ten-eleven translocation (TET) genes encode oxidases that demethylate methylated cytosine in DNA. A histone methylase gene variant, EZH2 p.D146H (Polyphen score .898) was found in a different specimen. EZH2 encodes a member of the Polycomb-group (PcG) protein family. PcG family members form multimeric protein complexes which maintain the transcriptional repressive state of genes.
Discussion
The current study addresses the origin of a disease phenocopy, an HBOC syndrome, in the absence of a familial (germline) gene pathogenic variant. We previously tested DNA from 11 tumors from women who come from families carrying BRCA1 or BRCA2 pathogenic variants, but who do not carry the variant themselves as defined by blood testing [28]. Although genetic alterations in any of the 11 tumor samples have not been demonstrated, several potential driver mutations were observed through testing with the Agilent 572 oncogene panel. There were no HBOC-related gene variants shared in all cases, although other somatic variants were present including BRCA variants in three cases and a PALB2 variant in one case. The familial pathogenic variants in 6/11 cases are frameshift insertions and deletions and one intronic variant, while most of the detected pathogenic variants were exonic single nucleotide variant (SNV). This may be due to the gene panel used which is designed to detect SNV in exonic regions.
In the absence of available normal tissue from most of these cases, the identification of germline variants was limited. Based on allele frequencies highly divergent from 50% and low frequency in population studies of germline variation, some of the reported variants might be somatic. Some apparently benign variants such as BRCA2 p.N991D and PPARG p.P12A are reported in ClinVar. ClinVar hosts germline and somatic variants. It contains all categories of germline variants including pathogenic, likely pathogenic, variants of uncertain significance (VUS), likely benign, and benign. ClinVar has 18 reports of BRCA2 p.N991D, including familial breast and breast-ovarian cancer, Fanconi anemia, and HBOC syndrome. A germline mutation PPARG p.P12A was reported as a risk factor for obesity, noninsulin-dependent diabetes mellitus, and familial partial lipodystrophy was reported in a single study. Both of these variants were included in Table 1 because of their presence in the annotated database.
There was limited DNA for four of the 11 specimens, which is reflected in the coverage levels in Table 1. As a result of low coverage, the variants may not be included in a clinical report. For variants with strong clinical significance based on clinical data (called Tier 1 variants) [22], repeat sequencing or confirmation by other methods would be recommended. The rarity of BRCA phenocopies also limits this study. Additional samples would further confirm the lack of familial pathogenic variants as well as any commonalities in family variants or in the somatic variants of the phenocopies. Interestingly, the one patient with non-HBOC phenotype (endometrial carcinoma) had a complex family history of maternal HBOC history (breast and ovarian cancers) with pathogenic 6794 insA variant in BRCA2 and paternal Lynch syndrome history (colon, lung and bone cancers) without any familial variant reported [28]. The possibility exists, therefore, that this patient is a phenocopy of a paternally inherited variant.
In addition to genetic drivers, epigenetic changes may also contribute to the disruption of cell phenotype and tumorigenesis. DNA hypermethylation in gene promoters is a mechanism for loss of function of genes. Epigenetic mechanisms independent of DNA sequence can affect phenotype in a heritable manner. DNA methylation patterns are reprogrammed during gamete production by erasure of epigenetic tags (DNA methylation and histone alterations). This should reset the DNA imprinting but rare survival of imprinted genes may persist [18, 29]. Furthermore, loss of function epigenetic regulators such as TET and EZH2 through DNA mutation, may result in aberrant methylation in offspring.
The presence of variants that can alter methylation state led to the investigation of BRCA 1/2 gene promoter methylation in the phenocopy DNA as measured by cytosine methylation. Aberrant DNA methylation patterns in gene promoters are strong regulators of gene expression and phenotype. In a preliminary analysis, BRCA promoter methylation status in tumor tissue DNA from phenocopies was compared to the DNA methylation in tumor tissue of BRCA carriers. Results so far in a small number of samples show a threefold increase in BRCA2 promoter methylation in phenocopy tumor tissue compared to tumor tissue from BRCA PV carriers suggesting that BRCA2 promoter methylation in the phenocopy tumor tissues (from familial BRCA2 backgrounds) is consistently higher in phenocopy tumor DNA than in non-malignant and tumor tissue from the control germline BRCA PV carriers. A more thorough analysis of the BRCA promoter regions in tumor and matched non-malignant tissue will confirm the presence of the increased methylation and studies of directed methylation in cell lines would demonstrate the actual effect.
A complex cancer phenotype is probably not driven solely by a single genetic or epigenetic variant, even in the presence of a highly penetrant familial pathogenic variant [5, 30, 38]. The standard polygenic model of carcinogenesis proposes phenotypes produced by multiple loci acting independently and additively [13, 26]. The contribution of multiple loci could explain observed genetic inheritance characteristics, such as phenotypic variability, penetrance and anticipation. A combination of single nucleotide polymorphisms (SNPs), which singularly may not produce a malignant phenotype, may establish a genetic context within which PVs in high-penetrance cancer genes (or other variants not classified as such) can produce it [7, 15]. In the current study, ROS1 PVs were repeatedly observed. Either the genetic background promotes ROS1 to a driver mutation, or ROS1 is a passenger to a yet undiscovered driver mutation in another gene. Theoretically, then, a somatic or germline BRCA variant may be a driver only in the context of particular combinations of other variants. This idea is consistent with results of an exploratory study by Agarwal et al. which identified cancer gene germline-somatic mutation pairs that co-occurred more frequently than would be expected by chance. The authors concluded that germline polymorphisms might function as pre-existing driver “hits”, which together with acquired complementary somatic mutations would act to dysregulate key pathways in malignant transformation [1].
A familial genetic context would provide both conditions, with inheritance of a familial BRCA PV being manifested when present. In the absence of germline BRCA PVs, a combination of variants in other genes (or BRCA promoter methylation) may take this role. The polygenic inheritance might also affect the penetrance of the driver mutation through generations, as observed with anticipation phenomenon where cancer develops at a younger age in subsequent generations in some BRCA-positive families [19]. Once established, the polygenic background may select for additional variants or variant losses which increase the “malignant context,” i.e. establish an environment with greater cancer risk.
Conclusions
An initial hypothesis that BRCA phenocopies were secondary to chimerism was not confirmed in our previous study. This prompted further analysis by extensive sequencing of DNA derived from tumor cells, to look for further insight into the pathogenesis of these tumors. The sequencing results confirmed that none of the familial pathogenic variants were present in the tumors of BRCA phenocopies. It also revealed several presumably somatic variants with potential oncologic significance. At least one variant in each case was previously reported in annotated databases. Somatic mutations in ROS1 were the most frequently represented in this small case group, but their significance in breast and ovarian cancer is unknown at this time. Several presumably somatic variants were found in DNA repair genes which share homologous DNA repair function with BRCA1 and BRCA2 and are in the same Fanconi anemia pathway. Epigenetic silencing through increased DNA methylation and/or polygenic background context can be other underlying mechanisms explaining BRCA phenocopies.
Availability of data and materials
Deidentified complete sequence data analyzed during the current study are available from the corresponding author on reasonable request.
References
Agarwal D, Nowak C, Zhang NR, Pusztai L, Hatzis C. Functional germline variants as potential co-oncogenes. NPJ Breast Cancer. 2017;3:46. https://doi.org/10.1038/s41523-017-0051-5.
Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Ee. 2003Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117–30.
Azzariti DR, Riggs ER, Niehaus A, Rodriguez LL, Ramos EM, Kattman B, Landrum MJ, Martin CL, Rehm HL. Points to consider for sharing variant-level information from clinical genetic testing with ClinVar. Cold Spring Harbor Mol Case Stud. 2018;4(1). https://doi.org/10.1101/mcs.a002345.
Azzollini J, Pesenti C, Ferrari L, Fontana L, Calvello M, Peissel B, et al. Revertant mosaicism for family mutations is not observed in BRCA1/2 phenocopies. PLoS One. 2017;12(2):e0171663.
Baquero JM, Benítez-Buelga C, Fernández V, Urioste M, García-Giménez JL, Perona R; CIMBA Consortium, Benítez J, Osorio A. 2019 “A common SNP in the UNG gene decreases ovarian cancer risk in BRCA2 mutation carriers.” Mol Oncol. 13(5):1110–1120.
Brose MS, Rebbeck TR, Calzone KA, Stopfer JE, Nathanson KL, Weber BL. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst. 2002;94(18):1365–72.
Carter H, Marty R, Hofree M, Gross AM, Jensen J, Fisch KM, Wu X, DeBoever C, Van Nostrand EL, Song Y, Wheeler E, Kreisberg JF, Lippman SM, Yeo GW, Gutkind JS, Ideker T. Interaction landscape of inherited polymorphisms with somatic events in cancer. Cancer Discovery. 2017;7(4):410-23.
Chen S, Iversen ES, Friebel T, Finkelstein D, Weber BL, Eisen A, et al. Characterization of BRCA1 and BRCA2 mutations in a large United States sample. J Clin Oncol. 2006;24(6):863–71.
Comai L, Li B. The Werner syndrome protein at the crossroads of DNA repair and apoptosis. Mech Ageing Dev. 2004;125(8):521–8.
Drilon A, Hu ZI, Lai GGY, Tan DSW. Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol. 2018;15(3):151–67.
Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast cancer linkage consortium. Am J Hum Genet. 1995;56(1):265–71.
Engel C, Fischer C, Zachariae S, Bucksch K, Rhiem K, Giesecke J, Herold N, Wappenschmidt B, Hübbel V, Maringa M, Reichstein-Gnielinski S, Hahnen E, Bartram CR, Dikow N, Schott S, Speiser D, Horn D, Fallenberg EM, Kiechle M, Quante AS, Vesper AS, Fehm T, Mundhenke C, Arnold N, Leinert E, Just W, Siebers-Renelt U, Weigel S, Gehrig A, Wöckel A, Schlegelberger B, Pertschy S, Kast K, Wimberger P, Briest S, Loeffler M, Bick U, Schmutzler RK; German Consortium for Hereditary Breast and Ovarian Cancer (GC-HBOC). 2019. “Breast cancer risk in BRCA1/2 mutation carriers and noncarriers under prospective intensified surveillance.” Int J Cancer. doi: 10.1002/ijc.32396
Evans DG, Brentnall A, Byers H, Harkness E, Stavrinos P, Howell A, et al. The impact of a panel of 18 SNPs on breast cancer risk in women attending aUK familial clinic: a case-control study. J Med Genet. 2017;54(2):111–3.
Fischer C, Engel C, Sutter C, Zachariae S, Schmutzler R, Meindl A, et al. BRCA1/2 testing: uptake, phenocopies, and strategies to improve detection rates in initially negative families. Clin Genet. 2012;82(5):478–83.
Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The breast cancer linkage consortium. Am J Hum Genet. 1998;62(3):676–89.
Goldgar D, Venne V, Conner T, Buys S. BRCA phenocopies or ascertainment bias? J Med Genet. 2007;44(8):e86 author reply e88.
Gronwald J, Cybulski C, Lubinski J, Narod SA. Phenocopies in breast cancer 1 (BRCA1) families: implications for genetic counselling. J Med Genet. 2007;44(4):e76.
Illum LRH, Bak ST, Lund S, Nielsen AL. DNA methylation in epigenetic inheritance of metabolic diseases through the male germ line. J Mol Endocrinol. 2018;60(2):R39–56.
Kuchenbaecker KB, McGuffog L, Barrowdale D, Lee A, Soucy P, Dennis J, et al. Evaluation of polygenic risk scores for breast and ovarian cancer risk preditions in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2017;107(5). https://doi.org/10.1093/jnci/djw302.
Kurian AW, Gong GD, John EM, Johnston DA, Felberg A, West DW, et al. Breast cancer risk for noncarriers of family-specific BRCA1 and BRCA2 mutations: findings from the breast cancer family registry. J Clin Oncol. 2011;29(34):4505–9.
Lee WP, Stromberg MP, Ward A, Stewart C, Garrison EP, Marth GT. MOSAIK: a hash-based algorithm for accurate next-generation sequencing short-read mapping. PLoS One. 2014;9(3):e90581.
Li MM, Datto M, Duncavage EJ, Kulkarni S, Lindeman NI, Roy S, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer. J Mol Diagn. 2017;19:4–20.
Li H. Toward better understanding of artifacts in variant calling from high-coverage samples. Bioinformatics. 2014;30(20):2843–51.
Mangold KA, Wang V, Weissman SM, Rubenstein WS, Kaul KL. Detection of BRCA1 and BRCA2 Ashkenazi Jewish founder mutations in formalin-fixed paraffin-embedded tissues using conventional PCR and heteroduplex/amplicon size differences. J Mol Diagn. 2010;12:20–6.
Nones K, Johnson J, Newell F, Patch AM, Thorne H, Kazakoff SH, de Luca XM, Parsons MT, Ferguson K, Reid L, McCart Reed AE, Srihari S, Lakis V, Davidson AL, Mukhopadhyay P, Holmes O, Xu Q, Wood S, Leonard C; Kathleen Cuningham Foundation Consortium for Research into Familial Aspects of Breast Cancer (kConFab); Australian Breast Cancer Tissue Bank (ABCTB); Brisbane Breast Bank (BBB), Beesley J, Harris J, Barnes D, Degasperi A, Ragan MA, Spurdle AB, Khanna KK, Lakhani SR, Pearson JV, Nik-Zainal S, Chenevix-Trench G, Waddell N, Simpson PT. 2019. “Whole-genome sequencing reveals clinically relevant insights into the aetiology of familial breast cancers.” Ann Oncol. https://doi.org/10.1093/annonc/mdz132.
Mavaddat N, Pharoah PD, Michailidou K, Tyrer J, et al. Prediction of breast cancer risk based on profiling with common genetic variants. J Natl Cancer Inst. 2015;107(5ii). https://doi.org/10.1093/jnci/djv036.
Metcalfe KA, Finch A, Poll A, Horsman D, Kim-Sing C, Scott J, et al. Breast cancer risks in women with a family history of breast or ovarian cancer who have tested negative for a BRCA1 or BRCA2 mutation. Br J Cancer. 2009;100(2):4.
Mitchell R, Buckingham L, Cobleigh M, Rotmensch J, Burgess K, Usha L. Can chimerism explain breast/ovarian cancers in BRCA non-carriers from BRCA-positive families? PLoS One. 2018;13(4):e0195497.
Northstone K, Golding J, Davey Smith G, Miller LL, Pembrey M. Prepubertal start of father's smoking and increased body fat in his sons: further characterisation of paternal transgenerational responses. Eur J Hum Genet. 2014;22:1382–6.
Osorio A, Milne RL, Kuchenbaecker K, Vaclová T, Pita G, Alonso R, Peterlongo P, Blanco I, de la Hoya M, Duran M, Díez O, Ramón Y Cajal T,Konstantopoulou I, Martínez-Bouzas C, Andrés Conejero R, Soucy P, McGuffog L, Barrowdale D, Lee A, Swe-Brca, Arver B, Rantala J, Loman N, Ehrencrona H, Olopade OI, Beattie MS, Domchek SM, Nathanson K, Rebbeck TR, Arun BK, Karlan BY, Walsh C, Lester J, John EM, Whittemore AS, Daly MB, Southey M, Hopper J, Terry MB, Buys SS, Janavicius R, Dorfling CM, van Rensburg EJ, Steele L, Neuhausen SL, Ding YC, Hansen TV, Jønson L, Ejlertsen B, Gerdes AM, Infante M, Herráez B, Moreno LT, Weitzel JN, Herzog J, Weeman K, Manoukian S, Peissel B, Zaffaroni D, Scuvera G, Bonanni B, Mariette F, Volorio S, Viel A, Varesco L, Papi L, Ottini L, Tibiletti MG, Radice P, Yannoukakos D, Garber J, Ellis S, Frost D, Platte R, Fineberg E, Evans G, Lalloo F, Izatt L, Eeles R, Adlard J, Davidson R, Cole T, Eccles D, Cook J, Hodgson S, Brewer C, Tischkowitz M, Douglas F, Porteous M, Side L, Walker L, Morrison P, Donaldson A, Kennedy J, Foo C, Godwin AK, Schmutzler RK, Wappenschmidt B, Rhiem K, Engel C, Meindl A, Ditsch N, Arnold N, Plendl HJ, Niederacher D, Sutter C, Wang-Gohrke S, Steinemann D, Preisler-Adams S, Kast K, Varon-Mateeva R, Gehrig A, Stoppa-Lyonnet D, Sinilnikova OM, Mazoyer S, Damiola F, Poppe B, Claes K, Piedmonte M, Tucker K, Backes F, Rodríguez G, Brewster W, Wakeley K, Rutherford T, Caldés T, Nevanlinna H, Aittomäki K, Rookus MA, van Os TA, van der Kolk L, de Lange JL, Meijers-Heijboer HE, van der Hout AH, van Asperen CJ, Gómez Garcia EB, Hoogerbrugge N, Collée JM, van Deurzen CH, van der Luijt RB, Devilee P, Hebon, Olah E, Lázaro C, Teulé A, Menéndez M, Jakubowska A, Cybulski C, Gronwald J, Lubinski J, Durda K, Jaworska-Bieniek K, Johannsson OT, Maugard C, Montagna M, Tognazzo S, Teixeira MR, Healey S, Investigators K, Olswold C, Guidugli L, Lindor N, Slager S, Szabo CI, Vijai J, Robson M, Kauff N, Zhang L, Rau-Murthy R, Fink-Retter A, Singer CF, Rappaport C, Geschwantler Kaulich D, Pfeiler G, Tea MK, Berger A, Phelan CM, Greene MH, Mai PL, Lejbkowicz F, Andrulis I, Mulligan AM, Glendon G, Toland AE, Bojesen A, Pedersen IS, Sunde L, Thomassen M, Kruse TA, Jensen UB, Friedman E, Laitman Y, Shimon SP, Simard J, Easton DF, Offit K, Couch FJ, Chenevix-Trench G, Antoniou AC, Benitez J. 2014. “DNA glycosylases involved in base excision repair may be associated with cancer risk in BRCA1 and BRCA2 mutation carriers.” PLoS Genet. 10(4):e1004256.
Opresko PL, Calvo JP, von Kobbe C. Role for the Werner syndrome protein in the promotion of tumor cell growth. Mech Ageing Dev. 2007;128(7–8):423–36.
Robson M. Do women remain at risk even if they do not inherit a familial BRCA1/2 mutation? J Clin Oncol. 2011;29(34):4477–8.
Satagopan JM, Offit K, Foulkes W, Robson ME, Wacholder S, Eng CM, et al. The lifetime risks of breast cancer in Ashkenazi Jewish carriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomark Prev. 2001;10(5):467–73.
Schorge JO, Modesitt SC, Coleman RL, Cohn DE, Kauff ND, Duska LR, et al. SGO white paper on ovarian cancer: etiology, screening and surveillance. Gynecol Oncol. 2010;199(1):7–17.
Smith A, Moran A, Boyd MC, Bulman M, Shenton A, Smith L, et al. Phenocopies in BRCA1 and BRCA2 families: evidence for modifier genes and implications for screening. J Med Genet. 2007;44(1):10–5.
Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336(20):1401–8.
Sy SM, Huen MS, Chen J. PALB2 is an integral component of the BRCA complex required for homologous recombination repair. Proc Natl Acad Sci U S A. 2009;106(17):7155–60.
Thorlacius S, Struewing JP, Hartge P, Olafsdottir GH, Sigvaldason H, Tryggvadottir L, et al. Population-based study of risk of breast cancer in carriers of BRCA2 mutation. Lancet. 1998;352(9137):1337–9.
Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164.
Wysoker A, Tibbetts K, Fennell T. Picard tools version 1.107; 2013. Accessed 2013
Xu H, Valerio DG, Eisold ME, Sinha A, Koche RP, Hu W, et al. NUP98 fusion proteins interact with the NSL and MLL1 complexes to drive Leukemogenesis. Cancer Cell. 2016;30(6):863–87.
Yunis EJ, Zuniga J, Romero V, Yunis EJ. Chimerism and tetragametic chimerism in humans: implications in autoimmunity, allorecognition and tolerance. Immunol Res. 2007;38(1–3):213–36.
Acknowledgements
We would like to acknowledge the generous contributions of Mr. and Mrs. Eugene and Shirley Deutsch, Mr. and Mrs. Mark and Maha Halabi Ditsch, Mr. George Ruwe, and Mr. and Mrs. Douglas and Sarah Criner, which made this work possible.
Funding
This study is funded by the philanthropic Metastatic Breast Cancer Research Fund at Rush (Dr. Lydia Usha).
Author information
Authors and Affiliations
Contributions
Authors LU, RM, MC, JR provided medical services to patients, obtained consent and participated in study design. Genetic background and inheritance data were collected and provided by KB. Nucleic acid isolation and molecular testing was performed by LB. MM-C, VH and SG performed NGS and bioinformatic analysis. All authors contributed to writing and editing of the article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Rush University Medical Center Institutional Review Board, Office of Research Affairs. Patients have provided informed consent for use of their archival tissue. Patients were made aware that the data collected is available on an individual basis. The IRB human subjects’ protection division designated this study as low risk to subjects with deidentification of data, once recorded so that subjects cannot be linked directly or by an identifier to the data reported.
Consent for publication
The authors warrant that the manuscript does not infringe upon any copyright or other right(s), and that we are the sole and exclusive owners of the rights conveyed to Hereditary Cancer in Clinical Practice. The authors guarantee that the contribution to the work has not been previously published elsewhere.
Competing interests
The authors have disclosed that they have no financial interests, arrangements, affiliations, or commercial interests with the manufacturers of any products discussed in this article or their competitors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Buckingham, L., Mitchell, R., Maienschein-Cline, M. et al. Somatic variants of potential clinical significance in the tumors of BRCA phenocopies. Hered Cancer Clin Pract 17, 21 (2019). https://doi.org/10.1186/s13053-019-0117-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13053-019-0117-5