Abstract
Background
Intraosseous (IO) access is a recommended method when venous access cannot be rapidly established in an emergency. Experimental data suggest that major hemorrhage and catecholamine administration both reduce bone marrow blood flow. We studied the uptake of gentamicin as a tracer substance administered IO following adrenaline administration in hemorrhagic shock and in cardiac arrest.
Methods
Twenty anesthetized pigs underwent hemorrhage corresponding to 50% of the blood volume. They then received injections of either; adrenaline IO (n = 5), saline IO n = 5), adrenaline IO during cardiac arrest and cardiopulmonary resuscitation (CPR, n = 5), or intravenous adrenaline. The injections were followed by an injection of gentamicin by the same route. Doses and volumes were equivalent among the groups. In all animals, mixed venous antibiotic concentrations were analyzed at 5, 15 and 30 min after administration.
Results
Mean (SD) plasma gentamicin concentrations (mg x L− 1) at 5 min were 26.4 (2.3) in the group with previous IO adrenaline administration, 26.6 (4.5) in the IO saline group, 31. 2 (12) in the IO adrenaline + CPR group and 23 (4.5) in the IV group. Concentrations in the CPR group were significantly higher than the others.
Conclusions
No impairment of drug uptake with IO administration after recent IO adrenaline exposure was demonstrable in this shock model.
Similar content being viewed by others
Background
Intraosseous (IO) cannulation is indicated when intravenous access is not quickly established in a medical emergency and evidence indicates that this method is relatively straightforward with a high success rate and a low frequency of serious complications in emergency medicine and prehospital settings [1,2,3,4,5]. Among other situations, it is frequently used in military and civilian trauma, and in cardiopulmonary resuscitation (CPR) where it is the recommended route for administration of adrenaline and other drugs if venous access is unavailable [6, 7]. There is however experimental evidence indicating that bone marrow blood pressure and flow decreases in hypovolemia and circulation may be substantially reduced both during hemorrhagic shock and following cardiac arrest and systemic catecholamine administration [8, 9]. Therefore, it could be hypothesized that the central delivery of substances administered during hypovolemia and after IO adrenaline administration might be impaired.
We have previously demonstrated a good uptake of gentamicin when administered IO in the tibia in a porcine sepsis model, with a concentration-time curve very similar to that seen after intravenous administration [10].
The aim of the present study was to evaluate drug uptake with IO administration after major hemorrhage and repeated doses of adrenaline, both of which have been demonstrated to reduce bone marrow blood flow and will likely often be relevant factors in situations where IO access is used. Uptake was also studied after IO adrenaline administration during CPR in hypovolemia. Based on previous experience we chose gentamicin as a model substance for drug uptake.
Methods
Animals
Twenty healthy pigs with a mean (SD) weight of 25.1 (1.8) kg were included in the study. The animals were treated according to the guidelines of the Swedish Board of Agriculture and the European Convention on Animal Care. The Animal Ethics Committee of Uppsala University, Sweden, approved the experiment (C155/14, date of approval 24-10-2014).
Anesthesia and preparation
All animals were anesthetized with an injection of 6 mg x kg− 1 tilétamin-zolezepam and 2.2 mg x kg− 1 xylazin intramuscularly in the neck. General anesthesia was maintained with an infusion of 8 mg x kg− 1 x h− 1 of pentobarbital mixed with 1.6 mg x kg− 1 x h− 1 of rocuronium bromide and 0.48 mg x kg− 1 x h− 1 of morphine.
After bolus doses of 20 mg of morphine and 100 mg of ketamine the animals were tracheotomized and mechanical ventilation was initiated with a Servo I® ventilator (Maquet Critical Care, Solna, Sweden). The animals continuously received 8 mL x kg− 1 x h− 1 of a solution containing 25 mg x mL− 1 of glucose, and 7 mL x kg− 1 x h− 1 of Ringer’s Acetate. An arterial catheter was placed into a right cervical artery and a central venous catheter was introduced through a right cervical vein into the superior caval vein. A Swan-Ganz catheter was introduced through a right cervical vein into the pulmonary artery for monitoring purposes. In order, not to compromise the cervical circulation, we avoid both the carotids and the internal jugular veins. Instead we catheterize the central vessels through a branch of the thyrocervical trunk and the right external jugular vein. A 15G IO cannula (EZ-IO®, Teleflex corp. Morrisville, NJ) was inserted into the proximal tibia. Correct placement was verified by the needle standing firmly in the bone, by aspiration and by an incision to the bone after finishing the experiment. A 13.5 F catheter was inserted into a left cervical vein. A small vesicotomy was made and a urinary catheter was inserted into the urinary bladder.
Protocol
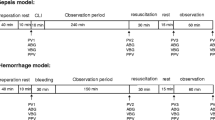
Hemorrhagic shock was induced in all animals by removal of 50% of the calculated (67 ml x kg− 1) blood volume through the 13.5 F venous catheter followed by resuscitation with the same volume of Ringer Acetate [11].
Animals were randomly divided in 4 groups with n = 5/group. Based on data from previous experiments, with an α of 0.05, a sample size of 20 animals was calculated to give an 80% power of detecting a 25% difference in mean concentration between groups, which was considered acceptable in this exploratory study.
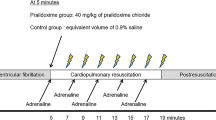
In group 1, three bolus doses of adrenaline, 0.01 mg x kg− 1 in dilution 1:10000, were administered in the IO catheter with an interval of 4 min in accordance with current CPR practice guideline recommendations [7]. Each dose was followed by a 10 ml 0.9% saline bolus. Immediately after the third adrenaline dose a bolus injection of gentamicin, 7 mg x kg− 1, was administered, also followed by a 10 ml 0.9% saline bolus.
Animals in group 2 received, through the IO cannula, three bolus doses of 10 ml 0.9% saline with 4 min interval followed by gentamicin as described above, but no adrenaline.
In group 3, anesthesia was deepened with further injections of ketamine and morphine after which a left sided thoracotomy was performed. After this, cardiac arrest was induced by IV potassium injection and internal heart massage was commenced. This procedure prolonged the preparation phase by approximately 30 min. During CPR, adrenaline and gentamicin was administered IO in a manner identical to that in group 1. Internal heart massage was performed manually by two operators with change over every few minutes upon agreement.
In group 4, adrenaline and gentamicin were administered in the same manner and dose as in group 1, but through an 18G IV cannula sited in an ear vein instead of IO.
CPR operators were not blinded to any experimental aspects but the laboratory performing the gentamicin analyses were blinded to group allocation and mode of administration.
In all animals, blood was sampled from the pulmonary artery catheter at 5, 15 and 30 min after gentamicin administration, after which the experiment was terminated.
Laboratory analysis
Gentamicin concentrations were analyzed on an Architect Ci8200 analyzer (Abbott Laboratories, Abbott Park, IL) using reagent from the same manufacturer (1P31). The total coefficients of variation (CV) for the gentamicin assay were 1.7% at 3.0 mg x L− 1 and 2.2% at 5.5 mg x L− 1.
Data analysis
Plasma gentamicin concentrations were assessed for normality. Concentrations at 5, 15 and 30 min were compared among the groups using one-way ANOVA and Tukey HSD test for post-hoc analysis. The area under the plasma concentration-time curve (AUC) was also calculated for each individual, using the trapezoidal rule, and compared among groups with ANOVA and Tukey HSD. Statistica 13 software (Statsoft Inc. Tulsa, OK) was used for analyses and graphics.
Results
Hemorrhage with partial resuscitation of the animals resulted in a lowered mean arterial pressure while the cardiac index was less affected. During CPR, both parameters were very low. Hemodynamic data at baseline and at the time of drug administration for all groups are presented in Figs. 1 and 2. After IO adrenaline administration, within 10–20 s a marked increase in heart rate and blood pressure was noted in the groups with preserved cardiac activity.
Mean (SD) gentamicin plasma concentrations at 5, 15 and 30 min for all groups are presented in Fig. 3. Mean (SD) concentrations at 5 min were 26.4 (2.3) mg x L− 1 in group 1, 26.6 (4.5) mg x L− 1 in group 2, 31.2 (12) mg x L− 1 in group 3 and 23 (4.5) mg x L− 1 in group 4. At 5 min, concentrations were not significantly different among the groups, but at 15 and 30 min they were higher in group 3 compared with the others (p < 0.05).
The AUC (mg x h x L− 1) was also significantly larger in group 3; mean (SD) 10.5 (2.9), while the other groups were similar; IO 6.9 (1.2), IO Adrenaline 7.1 (0.7), IV 7.2 (0.4).
Discussion
In this experimental study, intraosseous administration yielded clinically relevant concentrations of gentamicin after administration of either adrenaline or saline. Concentrations were equivalent to those after intravenous administration.
The rationale for using a porcine model of hemorrhagic shock is mainly due to the suitable size of the pig, which allowed us to bleed the animals, perform CPR, monitor, administer drugs IO and IV, and to easily take blood samples. Although the proximal humerus may be preferred for IO access [4], we used the proximal tibial route, in order to reduce the risk of interference between the IO needle and practical management of open-chest cardiac massage. Tracheotomy, instead of oro-tracheal intubation, was performed, since we, in previous, long-term (> 30 h), experiments have had several animals that developed pneumonia. Our assumption is that the source of these infections was contaminated debris in the mouth of the animals. Hence, we tracheotomize the animals due to hygiene reasons. Since this is a model of hemorrhagic shock induced cardiac arrest, internal heart massage was performed. In hypovolemic cardiac arrest, such as in trauma, internal heart massage is not unusual, wherefore we chose this setup.
IO access is commonly used in clinical emergencies e.g. cardiopulmonary resuscitation and shock of hypovolemic or other origin. In this setting it is likely that the IO cannula will be used for the administration of adrenaline or other catecholamines. In this situation, it is important to know if the uptake of subsequently administered drugs is adequate. Examples of this could be antiarrhythmics in a CPR scenario, antibiotics in a case of septic shock or hemostatics in trauma. In this experiment, gentamicin was studied as a model substance because of a previously demonstrated good uptake when administered IO. In the hypovolemic animals with preserved cardiac activity, we found no significant difference between plasma concentrations of gentamicin administered IO with or without previous adrenaline injections, even though the antibiotic was not given with a large volume of fluid. Gentamicin levels were also not significantly different in samples taken after IO and IV administration during similar hemodynamic conditions. This indicates that the experimentally demonstrated impairment of bone marrow circulation after catecholamine exposure and hypovolemia may not pose a serious clinical problem in this context. In the group where adrenaline and gentamicin were administered during CPR in hypovolemic animals, plasma concentrations were significantly higher than in the other groups. Since samples were taken relatively early after administration, when redistribution predominates over elimination [12], this is more likely an effect of a reduction in cardiac output than of decreased excretion. However, this still indicates that uptake from the bone marrow has occurred.
This study has several limitations. Although the staff performing the laboratory analyses were blinded to group allocation, the people performing CPR were not, which may be considered a limitation. Also, the thoracotomy and induction of cardiac arrest delayed the start of the experimental procedure by approximately 30 min, compared with the other groups. However, we do not believe that this limitation significantly affects our results. It should be noted that this is an experimental study performed in animals, and that the results therefore may not be directly translated to human conditions. Further, the number of subjects is limited and the study will not detect minor differences. In an emergency however, it may be sufficient to know that adequate central concentrations may quickly be reached after an IO bolus injection following repeated adrenaline administration. In this study, gentamicin was used as a model substance. We have, however, no reason to believe that previous adrenaline administration would have a different effect on the uptake of other substances.
Conclusions
In this model of hemorrhagic shock, IO administration of adrenaline did not impair the subsequent uptake of gentamicin administered through the same cannula. The plasma concentrations of gentamicin were equivalent to those after IV administration.
References
Ngo AS, Oh JJ, Chen Y, Yong D, Ong ME. Intraosseous vascular access in adults using the EZ-IO in an emergency department. Int J Emerg Med. 2009;2:155–60.
Hallas P, Brabrand M, Folkestad L. Complication with intraosseous access: scandinavian users' experience. West J Emerg Med. 2013;14:440–3.
Reades R, Studnek JR, Garrett JS, Vandeventer S, Blackwell T. Comparison of first-attempt success between tibial and humeral intraosseous insertions during out-of-hospital cardiac arrest. Prehosp Emerg Care. 2011;15:278–81.
Paxton JH, Knuth TE, Klausner HA. Proximal humerus intraosseous infusion: a preferred emergency venous access. J Trauma. 2009;67:606–11.
Lewis P, Wright C. Saving the critically injured trauma patient: a retrospective analysis of 1000 uses of intraosseous access. Emerg Med J. 2015;32:463–7.
Soar J, Nolan JP, Bottiger BW, et al. European resuscitation council guidelines for resuscitation 2015: section 3. Adult advanced life support. Resuscitation. 2015;95:100–47.
Maconochie IK, Bingham R, Eich C, et al. European resuscitation council guidelines for resuscitation 2015: section 6. Paediatric life support. Resuscitation. 2015;95:223–48.
Voelckel WG, Lurie KG, McKnite S, et al. Comparison of epinephrine with vasopressin on bone marrow blood flow in an animal model of hypovolemic shock and subsequent cardiac arrest. Crit Care Med. 2001;29:1587–92.
Frascone RJ, Salzman JG, Adams AB, Bliss P, Wewerka SS, Dries DJ. Evaluation of intraosseous pressure in a hypovolemic animal model. J Surg Res. 2015;193:383–90.
Strandberg G, Larsson A, Lipcsey M, Michalek J, Eriksson M. Intraosseous and intravenous administration of antibiotics yields comparable plasma concentrations during experimental septic shock. Acta Anaesthesiol Scand. 2015;59:346–53.
Hannon JP, Bossone CA, Wade CE. Normal physiological values for conscious pigs used in biomedical research. Lab Anim Sci. 1990;40:293–8.
Lipcsey M, Carlsson M, Larsson A, et al. Effect of a single dose of tobramycin on systemic inflammatory response-induced acute kidney injury in a 6-hour porcine model. Crit Care Med. 2009;37:2782–90.
Acknowledgements
We thank Mariette Andersson and Kerstin Ahlgren for excellent laboratory assistance.
Funding
The study was supported by Uppsala University.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
All authors contributed to literature search and study design. GS and ML collected the laboratory data. GS performed primary data analysis and drafted the manuscript. AL carried out the laboratory analyses. All authors contributed to data interpretation and critical revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Animal Ethics Committee of Uppsala University, Sweden, approved the experiment (C155/14, date of approval 24-10-2014).
Consent for publication
Not applicable.
Competing interests
Dr. Mats Eriksson has received travel grants from Vidacare/Teleflex Corporation for Congress participations. Teleflex Corporation has previously financially supported our research on intraosseous access.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Eriksson, M., Larsson, A., Lipcsey, M. et al. The effect of hemorrhagic shock and intraosseous adrenaline injection on the delivery of a subsequently administered drug - an experimental study. Scand J Trauma Resusc Emerg Med 27, 29 (2019). https://doi.org/10.1186/s13049-018-0569-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13049-018-0569-z