Abstract
Background
Choriocarcinoma of the ovary is rare. This tumor can arise from gestational tissue or pure germ cells of the ovary, the former results in gestational choriocarcinoma. The clinical characteristics and histology of both tumor types are identical, differentiation of these tumors is necessary for effective treatment. One strategy for the differentiation of these tumors types is to identify the presence of paternal DNA by DNA polymorphic analysis.
Case presentation
In the present case, a 27-year-old patient with a history of amenorrhea, lower abdominal pain and vaginal bleeding received a laparoscopic dissection of cystic mass of the right ovary according to an initial diagnosis of ectopic pregnancy. Primary choriocarcinoma of the ovary was diagnosed by pathology, but its origin was uncertain. DNA polymorphic analysis was then performed and a gestational origin was confirmed. The patient subsequently exhibited an excellent response to chemotherapy, achieved complete remission and gave birth to a healthy baby.
Conclusion
Differentiation between two etiologies of primary choriocarcinoma can be achieved with DNA polymorphic analysis and it is necessary to distinguish between them to approach to an appropriate treatment of a patient.
Similar content being viewed by others
Background
Primary choriocarcinoma of the ovary can arise from gestational tissue or pure germ cells of the ovary. They are referred to as gestational choriocarcinoma (GCO) or non-gestational choriocarcinoma (NGCO). The estimated incidence of GCO of the ovary is 1:369,000,000 pregnancies, while non-gestational choriocarcinomas correspond to less than 0.6% of ovarian germ cell tumors [1, 2], making this neoplasm very rare. Moreover, both gestational and non-gestational diseases exhibit identical clinical manifestations and histology. The clinical history of pregnancy, amenorrhea, or gestational trophoblastic disease may help to determine the diagnosis, but difficult cases often need DNA analysis, which has not often been performed in the previous reported cases. Saito et al. first described the diagnostic criteria for NGCO in 1963. These include absence of disease in the uterine cavity, pathological confirmation of disease, and exclusion of molar pregnancy and of intrauterine pregnancy [3]. All the criteria were fulfilled in this case, but the presence of paternal DNA revealed the final diagnosis of GCO, indicating that clinical diagnostic criteria are not reliable, except in patients who are unable to conceive or who have never had sexual intercourse [4]. These tumor types should be considered distinct entities with distinct therapeutic approaches, chemotherapy regimens, and prognosis associated with each disease. We summarized 48 cases (ours included) of primary ovarian choriocarcinoma published since 1982. Sixteen more cases reported from 1937 to 1982 are not listed in this article. Although most of the authors declared the reported cases were NGCO, we reanalyzed the information and only 24 NGCO and 2 GCO could be confirmed.
Case presentation
A 27-year-old married woman (gravida 0) was admitted to a local hospital with a history of 51 days of amenorrhea, lower abdominal pain and vaginal bleeding for 5 days. Her previous menstrual cycles were regular. Her medical history and family history were unremarkable. The general condition of the patient appeared to be good, and pelvic examination revealed a mass in the right adnexal area with tenderness. The urine test showed she was pregnant, and serum β-hCG level was more than 200,000 mIU/ml. Transvaginal ultrasound (TVS) revealed a right adnexal mass and profuse abdominal fluid accumulation.
According to an initial diagnosis of ectopic pregnancy, laparoscopic exploration was performed. The right ovary was 5*6 cm, partially cystic, ruptured and surrounded by a hematoma. The left ovary and both fallopian tubes were intact. Approximately 500 ml of intraperitoneal blood was noted. The cystic mass of the right ovary was dissected and sent to pathological diagnosis. On the fifth postoperative day, serum β-hCG levels was 14,510 mIU/ml. The patient then transferred to our hospital six days after the surgery. The pathological consult confirmed a pure choriocarcinoma of the right ovary, and an immunohistochemical panel was performed and the samples analyzed were positive for Pan Cytokeratin (AE1/AE3), hCG, human placental lactogen (hPL) and Ki-67(60%), and negative for p53. (Fig. 1).
At the 7th and 10th postoperative day, the serum β-hCG levels fell to 5907 and 2000 mIU/ml, respectively. Further imaging examination was proceeded ten days after the surgery. The contrast pelvic MRI showed the right ovary was 2.1*2.9*3.2 cm, at the front of which a mass of 1.2 cm*1.0 cm was observed. PET-CT showed bilateral ovarian nodules with hypermetabolism, physiological uptake considered, no other specific abnormalities were observed. Other related tests were examined: CA125 (cancer antigen 125): 70.81 U/ml, AFP (alpha fetoprotein): 2.28 ng/ml. As the endometrium thickness was only 5 mm, endometrial biopsy had not been performed.
The patient received five courses of EP-EMA chemotherapy, including cisplatin (80 mg/m2) and etoposide (100 mg/m2), D1; etoposide (100 mg/m2), methotrexate (100 mg/m2 iv and 200 mg/m2 ivgtt), and actinomycin-D (0.5 mg), D7–8, at two-week intervals. Goserelin (3.6 mg) was injected before the beginning of chemotherapy and at four-week intervals during the treatment to protect the ovarian function. During the chemotherapy, the patient was monitored weekly for serum levels of β-hCG, and a rapidly decrease was detected. We observed normalization of the CA125 serum level after one course of chemotherapy. The β-hCG level decreased to normal after two and a half courses of chemotherapy and remained normal thereafter. The contrast pelvic MRIs performed once a month during the chemotherapy showed reduced lesion which became undetectable during the fourth course. The patient remains without evidence of disease 32 months after chemotherapy, her menstruation recovered 12 months after chemotherapy, and gave birth to a healthy baby 25 months after chemotherapy.
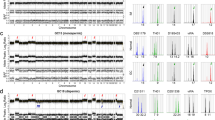
Individual DNA polymorphic analysis was used to verify the presence or absence of paternal genetic material. DNA from paraffin-embedded tumor tissue was compared to the patients’ and her husband’s peripheral blood DNA. Manual microdissection of the tumor cells was performed to eliminate the contamination of maternal DNA. Following extraction of DNA from the formalin-fixed and paraffin wax embedded material (QIAamp DNA FFPE Tissue Kit, Qiagen, Valencia, CA, USA), and from blood samples (ZR Genomic DNA-Tissue MiniPrep Kit, Zymo Research, CA, USA) all samples were quantified by NanoDrop (Thermo Scientific, Wilmington, USA), and MicroreaderTM 21 ID system, MicroreaderTM 23sp ID system (Beijing Microread Genetics Co., Ltd., Beijing, China) were respectively used to amplify 10 ng DNA from each biopsy and blood samples. Amplified products were then detected using an ABI 3730xl Genetic Analyzer (Applied Biosystems, CA, USA). Electrophoresis results were analyzed using GeneMapper® ID v.3.2 (Applied Biosystems, CA, USA), and the genetic profiles of the biopsy and peripheral blood were compared.
We studied the genetic profiles of 43 highly polymorphic short tandem repeats (STRs) in DNA samples prepared from the patient, spouse and tumor. At 25/43 loci examined, the tumor specimen was shown to contain the paternal allele but not the maternal DNA (D21S11, D18S51, D6S1043, D3S1358, D7S820, D16S539, Penta D, D2S441, vWA, TPOX, TH01, FGA, D18S535, D19S253, D20S470, D22-GATA198B05, D16S539, D8S1132, D4S2366, D13S325, D9S925, D3S3045, D10S1435, D17S1290, D5S2500). At 18/43 loci examined, it could not be determined whether the tumor contained paternal allele because the patient and spouse shared one or two identical alleles (D19S433, D5S818, AMEL, D13S317, CSF1PO, D8S1179, Penta E, D12S391, D2S1338, D6S477, D15S659, D11S2368, D1S1656, D7S3048, D21S1270, D14S608, D12S391, D2S1338). Therefore, none of the loci could be proved to contain maternal allele only. At 20/43 loci examined, the tumor was triploid, which was in accord with the nuclear-heteromorphism of tumor cells. Twelve representative loci from these analyses were summarized in Table 1 and Fig. 2. In more than half (25/43) of the loci studied we were able to demonstrate the presence of paternal DNA in the tumor, indicating a gestational origin for the tumor.
Discussion and conclusions
We summarized 48 cases of primary ovarian choriocarcinoma published since 1982 in Table 2 (ours included). Although most of the authors declared the reported cases were non-gestational choriocarcinoma, we reanalyzed the information and only 24 non-gestational choriocarcinoma and 2 gestational choriocarcinoma could be confirmed. The origin of other 22 cases was uncertain.
Of 26 cases with confirmed origin, 19 were diagnosed with NGCO because they were young women with no intercourse [1, 4–18], one was diagnosed with NGCO because of XY gonadal dysgenesis (Swyer syndrome) [19], four were confirmed non-gestational [20–23] and two gestational [24] by DNA analysis. Of patients assigned uncertain etiology, one was deduced GCO because of the presence of a corpus luteum [25], which can be suggestive, but not pathognomonic of gestational etiology; three patients were diagnosed with NGCO because of no intercourse in 10 years (G5P3) [26], long duration from the antecedent pregnancy(G1P1) [2], or husband’s undergoing vasectomy(G4P2) [27]. None of them can be excluded from gestational etiology since GCO has been reported to arise many years after an abortion or molar pregnancy, even in postmenopausal woman [28–31]. Other cases were diagnosed with NGCO simply according to pathology.
How to define the origin of a primary choriocarcinoma of the ovary is difficult by clinical characteristics or traditional methods. The etiology of choriocarcinoma has been ascribed to four different sources: from maternal germ cell; from an ovarian pregnancy; from metastases from a regressed or occult uterine primary; or, in infants, from metastases of the placenta [32]. Choriocarcinoma of the ovary can arise from gestational tissue or pure germ cells of the ovary, and it would be useful to discriminate between tumors of different origins because of distinct therapeutic approaches, chemotherapy regimens, and prognosis [33]. Unfortunately, it is extremely difficult. Both gestational and non-gestational diseases exhibit identical clinical manifestations and histology. Histologically, combining with other germ cell elements such as embryonal carcinoma or dysgerminoma in the tumor imply a non-gestational etiology. When sole choriocarcinoma is present, it is difficult to distinguish the etiology by routine histologic examination, even no significant ultrastructural differences are displayed between non-gestational and gestational choriocarcinoma [5]. HCG level does not distinguish between two types of tumor. The absence of primary lesion in the uterus and the presence of a proliferative endometrium do not imply a primary choriocarcinoma either.
The clinical histology is helpful in assigning the etiology. A patient who is sexually immature, unable to conceive, or who has not engaged in sexually intercourse, must have NGCO. Postpubertal women who have been sexually active or have ever been pregnant, gestational origin is a strong possibility. However, they are assigned uncertain etiology unless the presence of paternal DNA in the tumor was determined. It is considered a non-gestational choriocarcinoma rather than a gestational one with an interval of 15 years or longer between the previous pregnancy and the presentation of choriocarcinoma [2], but this is still controversial.
Molecular diagnostic method has been described long time ago that paternal HLA antigens have been identified in GCO [34]. Short tandem repeats (STRs) are general existed DNA polymorphic loci in human genome, which are of highly specificity, genetic and somatic stability. It is very helpful in diagnosing ovarian choriocarcinoma by detecting paternal alleles of the tumor using STRs analysis. Lorigan was the first reported to diagnose choriocarcinoma by analyze DNA polymorphism [24]. More developed and automated techniques are utilized nowadays and become the golden standard of diagnosis of choriocarcinoma. With the increase of polymorphic loci involved in this analysis (43 loci in this report), a higher accuracy of diagnosis as GCO is concluded for the present case.
Treatment of primary ovary choriocarcinoma should be carefully chosen according to the situation of the patients. In a woman who desires further child-bearing, conservative surgery may be employed if the tumor does not involve the uterus or the other ovary. One patient was pregnant one year after the completion of chemotherapy, and gave birth to a healthy baby [34], and our patient also had the same good outcome. If the tumor is extensive, especially if the etiology is non-gestational, intensive cytoreductive surgery should be performed. Most of the patients under 30 years old (23/34) received conservative surgery, seven underwent radical surgery of total abdominal hysterectomy and salpingo-oophorectomy with or without pelvic lymph node dissection. In our case, the patient’s β-hCG level decreased rapidly after the cystectomy, and became negative during chemotherapy, no lesion was seen in MRI or ultrasound, so we didn’t perform any further surgery.
Advances in chemotherapy significantly promote the survival rate of ovarian choriocarcinoma, and make determinations of the etiology of an ovarian choriocarcinoma important. It is generally accepted that GCO can be treated with methotrexate, actinomycin D or etoposide as a single agent, or with combined agents such as EMA-CO (etoposide, methotrexate, actinomycin D, cyclophosphamide, vincristine) when high risk factors are present. However, NGCO are generally treated with BEP (bleomycin, etoposide, cisplatin) regimen. We assigned an EP-EMA regimen to our patient before the DNA analysis results came out hoping to cover both trophoblastic and germ cell tumor, and received satisfactory results.
It is generally believed that non-gestational choriocarcinoma has a worse outcome than a gestational one. We did not find any differences in prognosis between these two types of tumor probably because of the inadequacy of cases. Most of the patients (20/25) who underwent conservative surgery remained no evidence of disease for 1–16 years. Considering the early onset of non-gestational choriocarcinoma and the sensitivity to chemotherapy of gestational one, we recommend all patients who desire for future pregnancy can receive conservative surgery as long as the contralateral ovary and the uterus are intact.
In conclusion, Ovarian choriocarcinoma is very rare and aggressive. However, it has the potential to be cured by surgery followed by chemotherapy. Differentiation between two etiologies of the tumor can be achieved with DNA polymorphic analysis to detect the presence of paternal DNA, and it is necessary to distinguish between them to approach to an appropriate treatment, and better prognosis of a patient. Conservative surgery should be first considered in nonparous women, and distinguished regimens of chemotherapy are recommended in different etiology of the tumor. The protection of the ovarian function from the chemotherapy should be highly valued for young patients especially for who desire future pregnancy.
Abbreviations
- GCO:
-
Gestational choriocarcinomas
- NGCO:
-
Non-gestational choriocarcinoma
- STRs:
-
Short tandem repeats
- β-hCG:
-
β-human chorionic gonadotropin
- L/ROC:
-
Left/right ovarian cystectomy
- L/RO:
-
Left/right oophorectomy
- UO:
-
Unilateral oophorectomy
- L/RSO:
-
Left/right salpingo-oophorectomy
- USO:
-
Unilateral salpingo-oophorectomy
- BSO:
-
Bilateral salpingo-oophorectomy
- TAH:
-
Total abdominal hysterectomy
- PLND:
-
Pelvic lymph node dissection
- PaLND:
-
Paraaortic lymph node dissection
- A:
-
Actinomycin-D
- B:
-
Bleomycin
- C:
-
Cyclophosphamide
- E:
-
Etoposide
- I:
-
Ifosfamide
- M:
-
Methotrexate
- O:
-
Vincristine
- P:
-
Cisplatin
- T:
-
Paclitaxel
- V:
-
Vincristine
References
Axe SR, Klein VR, Woodruff JD. Choriocarcinoma of the ovary. Obstet Gynecol. 1985;66:111–4.
Lv L, Yang K, Wu H, Lou J, Peng Z. Pure choriocarcinoma of the ovary: a case report. J Gynecol Oncol. 2011;22:135–9.
Saito M, Azuma T, Nakamura K. On ectopic choriocarcinoma. World Obstet Gynecol. 1963;17:459–84.
Kong B, Tian YJ, Zhu WW, Qin YJ. A pure nongestational ovarian choriocarcinoma in a 10-year-old girl: case report and literature review. J Obstet Gynaecol res. 2009;35:574–8.
Vance RP, Geisinger KR. Pure nongestational choriocarcinoma of the ovary: report of a case. Cancer. 1985;56:2321–5.
Raju GC, Woo J, Marchack D, Naraynsingh V. Primary nongestational choriocarcinoma of the ovary. Postgrad med J. 1985;61:757–8.
Sengupta SK, Everett VJ. Ovarian neoplasms in children and adolescents in Papua New Guinea. Aust N Z J Obstet Gynaecol. 1987;27:335–8.
Gribbon M, Ein SH, Mancer K. Pediatric malignant ovarian tumors: a 43-year review. J Pediatr Surg. 1992;27:480–4.
Brown MF, Hebra A, McGeehin K, Ross AJ III. Ovarian masses in children: a review of 91 cases of malignant and benign masses. J Pediatr Surg. 1993;28:930–2.
Trigueros VM, Sereno Colo JA, Villagran UJ. Pure form of primary ovarian choriocarcinoma. Report of a case. Ginecol Obstet Mex. 1995;63:341–5.
Gungor T, Ekin M, Zergeroglu S, Gokmen O. Primary choriocarcinoma of the ovary with coexisting dysgerminoma of the contralateral ovary. J Obstet Gynaecol. 1999;19:325–6.
Inaba H, Kawasaki H, Hamazaki M, Okugawa T, Uchida K, Honzumi M, et al. A case of metastatic ovarian non-gestational choriocarcinoma: successful treatment with conservative type surgery and myeloablative chemotherapy. Pediatr Int. 2000;42:383–5.
Goswami D, Sharma K, Zutshi V, Tempe A, Nigam S. Nongestational pure ovarian choriocarcinoma with contralateral teratoma. Gynecol Oncol. 2001;80:262–6.
Ozdemir I, Demirci F, Yucel O, Demirci E, Alper M. Pure ovarian choriocarcinoma: a difficult diagnosis of an unusual tumor presenting with acute abdomen in a 13-year-old girl. Acta Obstet Gynecol Scand. 2004;83:504–5.
Heo EJ, Choi CH, Park JM, Lee JW, Bae DS, Kim BG. Primary ovarian choriocarcinoma mimicking ectopic pregnancy. Obstet Gynecol Sci. 2014;57:330–3.
Hayashi S, Abe Y, Tomita S, Nakanishi Y, Miwa S, Nakajima T, et al. Primary non-gestational pure choriocarcinoma arising in the ovary: a case report and literature review. Oncol Lett. 2015;9:2109–11.
Xin L, Beier A, Tiede S, Pfiffer T, Kohler C, Favero G. Laparoscopic fertility-preserving treatment of a pure Nongestational Choriocarcinoma of the ovary: case report and review of current literature. J Minim Invasive Gynecol. 2015;22:1095–9.
Wang Q, Guo C, Zou L, Wang Y, Song X, Ma Y, et al. Clinicopathological analysis of non-gestational ovarian choriocarcinoma: report of two cases and review of the literature. Oncol Lett. 2016;11:2599–604.
Spingler H, Albert PJ, Schmid M, Müller J. Malignant germ cell tumor in XY gonadal dysgenesis (Swyer syndrome). Geburtshilfe Frauenheilkd. 1990;50:488–90.
Tsujioka H, Hamada H, Miyakawa T, Hachisuga T, Kawarabayashi T. A pure nongestational choriocarcinoma of the ovary diagnosed with DNA polymorphism analysis. Gynecol Oncol. 2003;89:540–2.
Koo HL, Choi J, Kim KR, Kim JH. Pure non-gestational choriocarcinoma of the ovary diagnosed by DNA polymorphism analysis. Pathol Int. 2006;56:613–6.
Yamamoto E, Ino K, Yamamoto T, Sumigama S, Nawa A, Nomura S, et al. A pure nongestational choriocarcinoma of the ovary diagnosed with short tandem repeat analysis: case report and review of the literature. Int J Gynecol Cancer. 2007;17:254–8.
Exman P, Takahashi TK, Gattás GF, Cantagalli VD, Anton C, Nalesso F, et al. Primary ovary choriocarcinoma: individual DNA polymorphic analysis as a strategy to confirm diagnosis and treatment. Rare Tumors. 2013;5:89–92.
Lorigan PC, Grierson AJ, Goepel JR, Coleman RE, Goyns MH. Gestational choriocarcinoma of the ovary diagnosed by analysis of tumour DNA. Cancer Lett. 1996;104:27–30.
Gerson RF, Lee EY, Gorman E. Primary extrauterine ovarian choriocarcinoma mistaken for ectopic pregnancy: sonographic imaging findings. AJR am J Roentgenol. 2007;189:280–3.
Park SH, Park A, Kim JY, Kwon JH, Koh SB. A case of non-gestational choriocarcinoma arising in the ovary of a postmenopausal woman. J Gynecol Oncol. 2009;20:192–4.
Choi YJ, Chun KY, Kim YW, Ro DY. Pure nongestational choriocarcinoma of the ovary: a case report. World J Surg Oncol. 2013; doi:10.1186/1477-7819-11-7.
Guvener S, Kazancigil A, Eroz S. Long latent development of trophoblastic disease. Am J Obstet Gynecol. 1972;114:679–84.
Lathrop JC, Wachtel TJ, Meissner GF. Uterine choriocarcinoma 14 years following bilateral tubal ligation. Obsfet Gynecol. 1978;51:477–82.
Dougherty CM, Cunningham C, Mickai A. Choriocarcinorna with metastasis in a postmenopausal woman. Am J Obstet Gynecol. 1978;132:700–1.
Brown AF, Snodgrass W, Pratt OB. Latent choriocarcinoma. Am J Cancer. 1940;38:564–8.
Witzleben C, Bruninga G. Infantile choriocarcinoma: a characteristic syndrome. J Pediatr. 1963;73:374–8.
Jacobs AJ, Newland JR, Green RK. Pure choriocarcinoma of the ovary. Obstet Gynecol Surv. 1982;37:603–9.
Corakci A, Ozeren S, Ozkan S, Gurbuz Y, Ustun H, Yucesoy I. Pure nongestational choriocarcinoma of ovary. Gynecol Obstet. 2005;271:176–7.
Kim PS, Kim SC, Kim JH, Choi YM, Lee HP. Pure choriocarcinoma of ovary. Korean J Obstet Gynecol. 1990;33:1607–11.
Shin YS, You HI, Lim OR, Park SY, Kim YT, Lee KW. A case of primary ovarian choriocarcinoma. Korean J Obstet Gynecol. 1994;37:592–6.
Byeun TS, Byeun C, Chung DY, Park DC, Ahn WS, Lee JW, et al. A case of primary ovarian carcinoma. Korean J Obstet Ol. 1995;38:1713–7.
Balat O, Kutlar I, Ozkur A, Bakir K, Aksoy F, Ugur MG. Primary pure ovarian choriocarcinoma mimicking ectopic pregnancy: a report of fulminant progression. Tumor. 2004;90:136–8.
Bazot M, Cortez A, Sananes S, Buy JN. Imaging of pure primary ovarian choriocarcinoma. AJR am J Roentgenol. 2004;182:1603–4.
Hirabayashi K, Yasuda M, Osamura RY, Hirasawa T, Murakami M. Ovarian nongestational choriocarcinoma mixed with various epithelial malignancies in association with endometriosis. Gynecol Oncol. 2006;102:111–7.
Roghaei MA, Mahzuni P, Rezaei F. A pure nongestational choriocarcinoma of the ovary. J Res Med Sci. 2007;12:269–70.
Mood NI, Samadi N, Rahimi-Moghaddam P, Sarmadi S, Eftekhar Z, Yarandi F. Pure ovarian choriocarcinoma: report of two cases. J Res Med Sci. 2009;14:327–30.
Gon S, Majumdar B, Barui G, Karmakar R, Bhattacharya A. Pure primary non-gestational ovarian choriocarcinoma: a diagnostic dilemma. Indian J Pathol Microbiol. 2010;53:178–80.
Haruma T, Ogawa C, Nishida T, Kusumoto T, Nakamura K, Seki N, et al. Pure Choriocarcinoma of the ovary in silver-Russell syndrome. Acta med Okayama. 2015;69:183–8.
Rao KV, Konar S, Gangadharan J, Vikas V, Sampath S. A pure non-gestational ovarian choriocarcinoma with delayed solitary brain metastases: case report and review of the literature. J Neurosci Rural Pract. 2015;6:578–81.
Acknowledgements
We thank Xianrong Zhou (Department of Pathology, Obstetrics and Gynecology Hospital of Fudan University, Shanghai, China) for carrying out the pathological diagnosis and his assistance in the development of treatment plan of this case.
Funding
This study was supported by National Natural Science Foundation of China (No. 81572836).
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
NJ collected the clinical data, carried out the manual microdissection and drafted the manuscript. YC and EO carried out the experimental procedures and DNA polymorphic analysis. XT carried out the pathological diagnosis and immunohistochemical staining. XL participated in the treatment and reviewed the manuscript. WF conceived of the study and reviewed the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors have no competing interest, including relevant, financial interests, activities, and affiliations.
Consent for publication
Written informed consent was obtained from the patient and her spouse for blood specimen collection, DNA analysis, publication of this report and accompanying images. A copy of this written consent is available for review by the Editor-in Chief of this journal.
Ethics approval and consent to participate
This work has been approved by the ethics committee of Obstetrics and Gynecology Hospital of Fudan University (committee’s reference number No.2014–37).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Jia, N., Chen, Y., Tao, X. et al. A gestational choriocarcinoma of the ovary diagnosed by DNA polymorphic analysis: a case report and systematic review of the literature. J Ovarian Res 10, 46 (2017). https://doi.org/10.1186/s13048-017-0334-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13048-017-0334-3