Abstract
Background
Stress-Inducible Protein-1 (STIP1) is a co-chaperone that associates directly with heat shock proteins, and regulates motility of various types of cancer. In the present study, we investigated the role of STIP1 on metastasis of gastric cancer (GC).
Methods
In vivo metastatic experimental model was employed to investigate the effect of STIP1 on metastasis of GC cells. Loss-of-function and gain-of-function experiments were performed to examine the role of STIP1 on metastasis of GC cells. Western blot, immunofluorescence staining, migration and invasion assays, microarray and KEGG pathway analysis were applied to explore the underlying mechanism.
Results
In current study, we demonstrated that STIP1 promoted lung metastasis of GC cells in vivo. Furthermore, STIP1 significantly enhanced migration and invasion abilities of GC cells. In contrast, knock-down of STIP1 yielded the opposite effects on these phenotypes in vitro. STIP1 promoted tumor metastasis through inducing epithelial-to-mesenchymal transition in GC cells. Mechanistically, STIP1 promoted GC metastasis via up-regulation of targeted genes in Wnt/β-catenin signaling pathway, including c-Myc and Cyclin D1, and accompanied with nuclear translocation of β-catenin.
Conclusions
Our findings indicate that elevated expression of STIP1 exhibited a metastasis-promoting effect in GC cells through activation of Wnt/β-catenin signaling pathway. STIP1 may be served as a potential therapeutic target for preventing GC metastasis.
Similar content being viewed by others
Background
Gastric cancer (GC) is a common and lethal cancer over the world [1], with marked morbidity and mortality in China [2]. In spite of some developments in the treatment of GC, the overall survival of GC patients remains poor because most GC patients have local and distant metastases at diagnosis [3,4,5]. Thus, it is of vital importance to investigate the molecular mechanisms underlying metastasis to provide novel biomarkers for treatment of GC.
Tumor growth and metastasis require a series of events within the tumor microenvironment, which involve proliferation, loss of cellular adhesion, degradation of the extracelluar matrix, invasion into host stroma, cell migration, and angiogenesis [6, 7]. Epithelial-to-mesenchymal transition (EMT) is a key event in tumor aggression and metastasis [8]. At molecular level of EMT, cancer cells lose their cell-to-cell contacts by inhibiting the epithelial marker E-cadherin and acquiring the mesenchymal markers like N-cadherin and vimentin [9]. However, the mechanisms and pathways underlying EMT in cancers are not comprehensively understood [10].
Stress-Inducible Protein-1 (STIP1), initially reported as a co-chaperone that associates directly with Hsp70/Hsp90 heat shock proteins, participates in a large number of cellular processes such as RNA splicing, transcription, viral replication, protein folding and translocation, signal transduction, and cell cycle regulation [11]. STIP1 was previously considered to be a protein functioning in intracellular compartments as it lacks transmembrane domain or signal peptide [12]. However, studies indicate that STIP1 could be secreted out of the cells, and then bound to prion protein on cell surface and transduced signals to affect cell proliferation and apoptosis [13,14,15]. In the last decade, a large number of functions of STIP1 have been reported, which includes the protection of cells in the nervous system, development, cellular maintenance, and tumor proliferation [16]. STIP1 is located at 11q13, and copy number gain of this region has been found in cancers and linked to poor prognosis [17,18,19,20]. STIP1 has been reported to be up-regulated in various types of cancer, including hepatocellular carcinoma [21], pancreatic cancer [22], ovarian cancer [23, 24], colon cancer [25], and cholangiocellular carcimoma [26]. Whether STIP1 involved in the regulation of GC metastasis remains unknown. In the present study, we have sought to investigate the roles of STIP1 on migration and invasion of GC cells through both in vitro and in vivo experiments, and further explored the potential mechanism.
Methods
Cell culture
Human gastric cell lines (AGS, BGC823, MGC803, MKN28, MKN45 and SGC7901) were obtained from the Chinese Academy of Science Committee Type Culture Collection Cell Bank (Shanghai, China). All cells lines were cultured in Roswell Park Memorial Institute 1640 (RPMI-1640) medium with supplementation of 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA) and appropriate amounts of penicillin (100 U/ml) and streptomycin (100 mg/ml) in a humidified atmosphere of 5% CO2 at 37 °C.
Western blot
Protein was electrophoretically separated by 10% SDS-PAGE and transferred to PVDF membranes (Millipore, Billerica, MA, USA). The membranes were blocked for 2 h with 5% skim milk in TBST, and incubated with specific primary antibodies (Additional file 1: Table S1) overnight at 4 °C followed by incubation with rabbit or mouse radish peroxidase-coupled secondary antibodies for 2 h. Antibody binding was detected using the enhanced chemiluminescence reagent (Millipore, Billerica, MA, USA).
Small interfering RNA transfection
Small interfering RNA (siRNA) targeting STIP1 (siSTIP1#1: 5’-GCAAGACTGTCGACCTAAA-3′; siSTIP1#2: 5’-CGATGAAGGACTACACCAA-3′) and a negative control (NC) RNA were synthesized by RiboBio (Guangzhou, China). Cells were seeded in six-well plates and starved overnight, and then transfected with 50 nM siRNA using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Plasmid transfection
Cells were seeded in six-well plates and starved overnight, and then pReceiver-120-STIP1 or vector was transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Wound scratch assay
Cells were seeded into six-well plates and then cultured to 90% confluence. The confluent cell monolayer was wounded using a sterile 100 μl pipette tip. The suspended cells were washed using normal growth medium. The scratch wound was captured after 24 h and 48 h in three fields. The area of the open wound was quantified using Photoshop (Adobe).
Migration and invasion assay
Cell migration assays were performed using 24-well transwell chambers (Costar-Corning, New York, USA) with 8.0-μm pore polycarbonate filter. The lower chamber was filled with RPMI-1640 with 10% FBS, and cells (5 × 104 cells/well) serum-starved overnight and pretreated with siRNA or plasmid of STIP1 were added into the upper chamber. After incubation, the non-invading cells were gently removed by scraping with a cotton swab, and the cells migrated to the lower membrane were fixed with methanol, and stained with crystal violet (Beyotime, Nantong, China), photographed, and counted. Cell invasion assay was performed similarly, except that transwell inserts were precoated with matrigel.
Immunofluorescence (IF) staining
Cells were seeded onto glass cover slips placed in six-well plates, fixed with 4% paraformaldehyde permeabilized in phosphate buffered saline (PBS), which contained 0.1% Triton-X 100, and blocked with 5% bovine serum albumin. The cells were incubated with primary antibodies (Additional file 1: Table S1) at 4 °C overnight. After three times washing with PBS, cells were incubated with secondary antibody for 1 h at room temperature. Cells were stained with DAPI (1μg/mL) for 5 min, and washed in PBS. Images were recorded with a fluorescence microscope.
Immunohistochemical (IHC) staining
Slides were dried at 37 °C overnight, deparaffinized in xylene, rehydrated through graded alcohol, washed with PBS, immersed in 3% hydrogen peroxide for 15 min to inhibit endogenous peroxidase activity, antigen-retrieved by pressure cooking for 7 min in 10 mM sodium citrate buffer for antigen unmasking (pH 6.0), blocked in normal serum (Vectastain ABC kit), incubated with specific antibodies (Additional file 1: Table S1) overnight in a moist chamber at 4 °C, and incubated with secondary antibodies (Vectastain ABC kit). Slides were stained with 3, 3-diaminobenzidine and counterstained with hematoxylin.
Construction of stable STIP1 knock-down cells
A short hairpin RNA (shRNA) targeting STIP1 (shSTIP1) and the negative control shRNA were cloned into the pGMLV-SC1 vector. The transduction was performed in SGC7901 cells according to the manufacturer’s recommended protocol. Puromycin (Sigma-Aldrich, St. Louis, MO) was used to select for stably transduced cells.
RNA extraction and microarray analysis
Total RNA was isolated using RNA plus reagent (TaKaRa, Japan). To identify potential STIP1-targeted genes and related pathways, total RNA from SGC7901- recombinant human STIP1 (SGC7901-rhSTIP1) and SGC7901-IgG cells was analyzed using Illumina microarray (HumanHT-12_V4). Raw data were processed using the manufacturer’s standard protocol and further analyzed using KEGG pathway analysis.
In vivo metastatic experimental model
For tail vein injection experiment, 1 × 106 cells were injected intravenously through tail vein into the tested nude mice (n = 12, 6 for each group). All of the mice were euthanized 6 weeks after injection. The lungs excised from the mice were then fixed in 4% paraformaldehyde and embedded in paraffin, and sections of those tissues were used for histologic study. Tumor nodules that formed on the lung were macroscopically detected.
Statistical analysis
Data were analyzed for statistical significance using the t test (SPSS Inc., Chicago, IL). In these analyses, p value < 0.05 was considered to be significant. All the experiments in vitro had been confirmed for three times.
Results
Basic expression level of STIP1 in GC tissues and cells
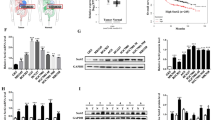
Firstly we investigated the expression level of STIP1 in 8 pairs of GC specimens and adjacent noncancerous tissues. The result showed that STIP1 expression was higher in tumors than that in adjacent noncancerous tissues (Fig. 1a), which indicated that STIP1 was up-regulated in GC tissues.
STIP1 promoted lung metastasis of GC cells in vivo. a The expression of STIP1 was up-regulating in GC tissues than that in adjacent noncancerous tissues. b The basic expression of STIP1 in 6 GC cell lines. c The STIP1 expression of SGC7901 NC and SGC7901 shSTIP1 was assessed by Western blot. d The representative images of lung metastasis model in nude mice. e The representative images of lung metastatic nodules. f Compared with control group, mice in SGC7901-shSTIP1 group had significantly fewer lung metastatic nodules. g Representative IHC staining of STIP1 expressions in lung of nude mice injected with cells of SGC7901 NC or SGC7901 shSTIP1
In order to explore the impact of STIP1 on the biological characteristics of GC cells, we assessed expression level of STIP1 in a panel of 6 GC cells (AGS, BGC823, MGC803, MKN28, MKN45 and SGC7901) by Western blot (Fig. 1b). AGS and SGC7901 cells showed moderate STIP1 expression level and therefore were selected for conducting both loss-of-function and gain-of-function experiments.
STIP1 promoted lung metastasis of GC cells in nude mice model
To investigate the role of STIP1 on GC cells metastasis in vivo, STIP1 stable knock-down cells SGC7901-shSTIP1 and control cells (Fig. 1c) were injected into tail vein of nude mice. 6 weeks later, the mice were sacrificed, and the lung metastatic nodules were counted. Obviously, mice in SGC7901-shSTIP1 group had significantly fewer lung metastatic nodules compared with those in the control group (p < 0.01) (Fig. 1d-g), which indicated STIP1 could promote lung metastasis of GC cells.
STIP1 was associated with migration and invasion of GC cells
To examine the effect of STIP1 on migration and invasion abilities in GC cells, we observed cell morphological changes and carried out wound scratch assay, Transwell assays with/without matrigel in SGC7901 and AGS cells.
We first evaluated cellular phenotype with STIP1 overexpression and found that the mesenchymal morphological changes were stimulated in both AGS and SGC7901 cells (Fig. 2a). The wound scratch assay showed that when knock-down of STIP1, the wound healing ability was weaker than that of control cells (all p < 0.05) (Fig. 2b). We observed consistent results with STIP1 overexpression (all p < 0.05) (Fig. 2b). In order to further validate the migration and invasion abilities of STIP1 in SGC7901 and AGS cells, we performed Transwell assays with/without matrigel. These results confirmed that when STIP1 was knocked down, number of cells transferred to the lower chamber was significantly less than that of control cells (all p < 0.05) (Fig. 2c). Consistently, STIP1 overexpression cells had enhanced migration and invasion abilities than those of control cells (all p < 0.05) (Fig. 2c). Taken together, these results indicated that STIP1 was associated with migration and invasion of GC cells.
STIP1 promoted cells migration and invasion. a SGC7901 and AGS cells were treated with STIP1 or not for 48 h, and the mesenchymal morphological alterations were observed in both cells with overexpression of STIP1. b After transfected with siRNA targeted STIP1 (siSTIP1#1, siSTIP1#2), the distance was longer than that in control cells (siCtrl). After overexpression of STIP1, the distance was shorter than that in control cells (Vector). c After transfected with siRNA targeted STIP1, the migration and invasion abilities were significantly decreased than that in control cells. After overexpression of STIP1, the migration and invasion abilities were significantly increased than that in control cells. d After transfected with siRNA targeted STIP1, the expressions of MMPs (MMP2, MMP7, and MMP9) were reduced than that in control cells. After overexpression of STIP1, the expressions of MMPs (MMP2, MMP7, and MMP9) were elevated than that in control cells. *p < 0.05, **p < 0.01, ***p < 0.001
STIP1 was correlated with matrix metalloproteinases (MMPs)
Based on the fact that MMPs were closely related with migration and invasion abilities of cells, Western blot was applied to determine the MMPs (MMP2, MMP7, and MMP9) expression levels in STIP1 knock-down cells and STIP1 overexpression cells. Protein expression levels of these three MMPs were significantly decreased in STIP1 knock-down group than that in control group (Fig. 2d), while the expression levels of these three MMPs were increased in STIP1 overexpression group than that in control group (Fig. 2d). These data were consistent with the results of migration and invasion experiments in both AGS and SGC7901 cells with different expression levels of STIP1.
STIP1 was involved in the EMT process
It is well-known that EMT plays an important role in tumor metastasis. Since we found that STIP1 was associated with migration and invasion abilities of GC cells, we further investigated the relationship between STIP1 and EMT.
Western blot results showed that the mesenchymal markers (N-cadherin, vimentin) were decreased and the epithelial marker (E-cadherin) was elevated by knock-down of STIP1 (Fig. 3a). Similar results were observed by IF staining in both AGS and SGC7901 cells (Fig. 3b).
STIP1 was associated with the expressions of EMT markers. a Knock-down of STIP1, the expressions of mesenchymal markers (N-cadherin, vimentin) were decreased and the expression of epithelial marker (E-cadherin) was increased by Western blot (Left), while overexpression of STIP1, it yielded the opposite results (Right). b Knock-down of STIP1, the mesenchymal marker (vimentin) was decreased and the epithelial marker (E-cadherin) was increased (Upper panel) by IF. In contrary, overexpression of STIP1 yielded the opposite results (Lower panel)
Consistently, when STIP1 was overexpressed, mesenchymal markers were upregulated and the epithelial marker was downregulated by both Western blot (Fig. 3a) and IF staining (Fig. 3b).
These data suggested STIP1 was involved in the EMT process to promote metastasis of GC.
STIP1 promoted metastasis of GC cells via Wnt/β-catenin signaling pathway
To explore the potential mechanism of STIP1 on metastasis of GC cells, we treated SGC7901 cells with rhSTIP1, using IgG as control. Total RNA from SGC7901-rhSTIP1 and SGC7901-IgG cells was analyzed using gene expression microarray. We identified 1683 genes differentially expressed (Fig. 4a). The microarray data were uploaded to Gene Expression Omnibus database and No. is GSE107819.
STIP1 was correlated with Wnt signaling pathway. a Differentially expressed genes between SGC7901-rhSTIP1 and SGC7901-IgG cells in microarray analyses. b KEGG pathway analyses between SGC7901-rhSTIP1 and SGC7901-IgG cells. c GSEA revealed that STIP1 significantly correlated with Wnt signaling pathway
Through KEGG pathway analysis, Wnt/β-catenin signaling pathway was found to be significantly affected (Fig. 4b, c). It is well-known that Wnt/β-catenin signaling pathway cascade is a key pathway in regulating metastasis of GC. To investigate the possible involvement of STIP1 in Wnt/β-catenin signaling pathway, we determined the expression of important genes in Wnt/β-catenin signaling pathway and its targeted genes (such as c-Myc, and CyclinD1). As shown in Fig. 5a, knock-down of STIP1 markedly reduced the expression levels of phosphor-Glycogen synthase kinase-3β (p-GSK-3β)(Ser9), β-catenin, p-β-catenin(Tyr654), c-Myc, and CyclinD1 in both SGC7901 and AGS cells, while overexpression of STIP1 significantly increased the expression levels of p-GSK-3β(Ser9), β-catenin, p-β-catenin(Tyr654), c-Myc, and CyclinD1. These results suggested that STIP1 might play an important role on the activation of Wnt/β-catenin signaling pathway.
STIP1 was associated with the activation of Wnt/β-catenin signaling pathway. a Knock-down of STIP1 markedly reduced the expressions of p-GSK-3β(Ser9), β-catenin, p-β-catenin(Tyr654), c-Myc, and CyclinD1 by Western blot (Left). Overexpression of STIP1 significantly increased the expressions of p-GSK-3β(Ser9), β-catenin, p-β-catenin(Tyr654), c-Myc, and CyclinD1 by Western blot (Right). b Knocked down of STIP1 decreased nuclear β-catenin expression and increased cytosolic β-catenin expression by Western blot (Left). Overexpression of STIP1 increased nuclear β-catenin expression and decreased cytosolic β-catenin expression by Western blot (Left). c β-catenin nuclear translocation was detected by IF staining
As Glycogen synthase kinase-3β (GSK-3β) prevents β-catenin from translocating into the nucleus, we tested the sub-cellular distribution of β-catenin. The results showed that decreased β-catenin was detected in the nucleus of GC cells with knock-down of STIP1, while increased β-catenin was detected in the nucleus of GC cells with overexpression of STIP1 (Fig. 5b). At the same time, expression of β-catenin in cytoplasm had contrary trend (Fig. 5b). To confirm β-catenin translocation, we performed IF staining and found that β-catenin was decreased in nucleus of GC cells with knock-down of STIP1, while β-catenin was increased in nucleus of GC cells with overexpression of STIP1 (Fig. 5c).
To further determine whether the metastasis-promoting effect of STIP1 was through activation of Wnt/β-catenin pathway, β-catenin inhibitor PNU-74654 was applied. SGC7901 and AGS cells with/without overexpression of STIP1 were treated with/without 50 μmol/L of PNU-74654 for 6 h prior to conduct Western blot, Transwell assays with/without matrigel. The results of Western blot analysis showed that PNU-74654 could effectively decrease the expression levels of p-β-catenin(Tyr654), c-Myc, Cyclin D1, as well as the mesenchymal markers (N-cadherin, and vimentin), and increase the expression level of epithelial marker (E-cadherin) (Fig. 6a). Furthermore, the migration and invasion abilities of STIP1-overexpressed cells could not be totally inhibited by PNU-74654 (all p < 0.05) (Fig. 6b, c).
Inhibition of Wnt/β-catenin signaling pathway could not totally abolish STIP1-mediated cells’ migration and invasion abilities. a PNU-74654 effectively decreased the expression levels of p-β-catenin(Tyr654), c-Myc, Cyclin D1, as well as N-cadherin and vimentin, and increased the expression level of E-cadherin by Western blot. b Treated with/without PNU-74654, the migration number of cells in STIP1-overexpression group was increased than that of control group. c Treated with/without PNU-74654, the invasion number of cells in STIP1-overexpression group was increased than that of control group. *p < 0.05
Taken together, these results indicated that STIP1 promoted metastasis of GC cells via Wnt/β-catenin signaling pathway.
Discussion
It is well known that cell migration and invasion is required for cancer metastasis, which accounts for majority of cancer deaths [27]. Matrix meralloproteinases was reported to play an important role in cell migration and invasion since cell invasion involves degradation of basement membrane extracellular matrix proteins [28]. Previous studies have showed that STIP1 was associated with disease progression and poor prognosis in various types of cancers, particularly for those with advanced lymph node metastasis [21,22,23,24,25,26]. In this study, we found that STIP1 increased invasiveness and metastatic potential by in vitro and in vivo assays.
Firstly, we observed that STIP1 was up-regulated in GC tissues than that in adjacent noncancerous tissues. From in vivo metastatic experiment, we found that STIP1 could promote GC cells metastasis to lung. We further investigated the relationship between STIP1 and EMT. IF staining results showed that knock-down of STIP1 promoted mesenchymal-to-epithelial transition while overexpression of STIP1 promoted EMT. Moreover, knock-down of STIP1 reduced migration and invasion abilities of AGS and SGC7901 cells, which was associated with decreased expressions of MMP2, MMP7 and MMP9 in both cells. In contrast, overexpression of STIP1 stimulated the migration and invasion of AGS and SGC7901 cells, and increased the expression levels of MMP2, MMP7 and MMP9. Down-regulation of E-cadherin is regarded as one of the critical molecular features involved in the loss of cell-to-cell adhesion, which promotes cancer invasion and metastasis [29]. We observed loss of epithelial cell phenotype and decrease of E-cadherin with STIP1 overexpression. N-cadherin and vimentin are markers of the mesenchymal phenotype. Our study showed that the expression levels of N-cadherin and vimentin were up-regulated in AGS and SGC7901 cells with STIP1 overexpression. These data supported the hypothesis that STIP1 induced EMT in GC cells.
To gain insights into the molecular mechanisms by which STIP1 promoted GC cells migration and invasion, we performed gene expression microarray using rhSTIP1-treated and control SGC7901 cells. The KEGG pathway analysis nominated the Wnt signaling pathway, which was a well-established cell migrated and invaded pathway associated with activation of EMT [30]. GSK-3β was a dual kinase differentially regulated by tyrosine and serine/threonine phosphorylation, and phosphorylation of serine 9 inhibited its kinase activity [31, 32]. β-catenin can be phosphorylated at serine, threonine and tyrosine sites, and phosphorylation of tyrosine 654 promoted translocation of β-catenin from cytoplasm into nucleus, affecting its subcellular location [33,34,35].
In Wnt/β-catenin signaling pathway, GSK-3β is widely acknowledged for its essential role in phosphorylation, degradation and translocation of β-catenin [36]. Once Wnt/β-catenin signaling pathway is activated, the activity of GSK-3β is inhibited [37]. In our study, we found that overexpression of STIP1 significantly increased the levels of p-GSK-3β (Ser9) and p-β-catenin (Tyr654), and the levels of total β-catenin and nuclear β-catenin were also increased. The expression of c-Myc and Cyclin D1, which are downstream genes of Wnt/β-catenin signaling pathway, were consistent with the expression pattern of β-catenin in STIP1-knockdown and STIP1-overexpression cells. We further confirmed that STIP1 promoted metastasis through the activation of Wnt/β-catenin signaling pathway using β-catenin inhibitor-PNU-74654. Our data demonstrated that PNU-74654 could effectively decrease expressions of key genes of Wnt/β-catenin pathway but could not totally abolish STIP1-medited migratory and invasive ability, implying that the metastasis-promoting function of STIP1 in GC cells was partially dependent on Wnt/β-catenin signaling pathway.
Phosphorylated GSK-3β is a negative regulator of the Wnt/β-catenin activation in gastric cancer. Since STIP1 has no intrinsic phosphatase, it is unlikely that STIP1 directly mediates the phosphorylation of GSK-3β [38, 39]. It is possible that STIP1 regulates the Wnt/β-catenin pathway by indirectly affecting the activity of p-GSK-3β in gastric cancer.
Conclusions
In summary, we demonstrated that STIP1 promoted cell migration and invasion via Wnt/β-catenin pathway in GC. A better understanding of the oncogenic mechanism of STIP1 in GC may lead to develop novel therapeutic strategy in GC treatment.
Abbreviations
- EMT:
-
Epithelial-to-mesenchymal transition
- GC:
-
Gastric cancer
- GSK-3β:
-
Glycogen synthase kinase-3β
- IF:
-
Immunofluorescence
- IHC:
-
Immunohistochemical
- MMPs:
-
Matrix metalloproteinases
- NC:
-
Negative control
- PBS:
-
Phosphate buffered saline
- p-GSK-3β:
-
Phosphor-Glycogen synthase kinase-3β
- SGC7901-rhSTIP1:
-
SGC7901-recombinant human STIP1
- shRNA:
-
Short hairpin RNA
- shSTIP1:
-
Short hairpin RNA targeting STIP1
- siRNA:
-
Small interfering RNA
- STIP1:
-
Stress-Inducible Protein-1
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
Jiang C, Chen X, Alattar M, Wei J, Liu H. MicroRNAs in tumorigenesis, metastasis, diagnosis and prognosis of gastric cancer. Cancer Gene Ther. 2015;22:291–301.
Robertson-Tessi M, Gillies RJ, Gatenby RA, Anderson AR. Impact of metabolic heterogeneity on tumor growth, invasion, and treatment outcomes. Cancer Res. 2015;75:1567–79.
Zhao J, Liu Y, Huang G, Cui P, Zhang W, Long ZY. Non-coding RNAs in gastric cancer: versatile mechanisms and potential for clinical translation. Am J Cancer Res. 2015;5:907–27.
Shim HJ, Kim KR, Hwang JE, Bae WK, Ryu SY, Park YK, et al. A phase II study of adjuvant S-1/cisplatin chemotherapy followed by S-1-based chemoradiotherapy for D2-resected gastric cancer. Cancer Chemother Pharmacol. 2016;77:605–12.
Chambers AF, Groom AC, IC MD. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72.
Crissman JD. Tumor-host interactions as prognostic factors in the histologic assessment of carcinomas. Pathol Annu. 1986;21(Pt 1):29–52.
Salvesen HB, Haldorsen IS, Trovik J. Markers for individualised therapy in endometrial carcinoma. LANCET ONCOL. 2012;13:e353–61.
Wik E, Raeder MB, Krakstad C, Trovik J, Birkeland E, Hoivik EA, et al. Lack of estrogen receptor-alpha is associated with epithelial-mesenchymal transition and PI3K alterations in endometrial carcinoma. Clin Cancer Res. 2013;19:1094–105.
Li Y, Xie Y, Cui D, Ma Y, Sui L, Zhu C, et al. Osteopontin promotes invasion, migration and epithelial-mesenchymal transition of human endometrial carcinoma cell HEC-1A through AKT and ERK1/2 signaling. Cell Physiol Biochem. 2015;37:1503–12.
Odunuga OO, Longshaw VM, Blatch GL. Hop: more than an Hsp70/Hsp90 adaptor protein. BioEssays. 2004;26:1058–68.
Lassle M, Blatch GL, Kundra V, Takatori T, Zetter BR. Stress-inducible, murine protein mSTI1. Characterization of binding domains for heat shock proteins and in vitro phosphorylation by different kinases J BIOL CHEM. 1997;272:1876–84.
Tsai CL, Tsai CN, Lin CY, Chen HW, Lee YS, Chao A, et al. Secreted stress-induced phosphoprotein 1 activates the ALK2-SMAD signaling pathways and promotes cell proliferation of ovarian cancer cells. Cell Rep. 2012;2:283–93.
Li J, Sun X, Wang Z, Chen L, Li D, Zhou J, et al. Regulation of vascular endothelial cell polarization and migration by Hsp70/Hsp90-organizing protein. PLoS One. 2012;7:e36389.
Chen Z, Xu L, Su T, Ke Z, Peng Z, Zhang N, et al. Autocrine STIP1 signaling promotes tumor growth and is associated with disease outcome in hepatocellular carcinoma. Biochem Biophys Res Commun. 2017;493:365–72.
de Souza LE, Moura CM, Bilek ES, Lopes MH, Martins VR, Puschel AW, et al. STI1 antagonizes cytoskeleton collapse mediated by small GTPase Rnd1 and regulates neurite growth. Exp Cell Res. 2014;324:84–91.
Shadeo A, Lam WL. Comprehensive copy number profiles of breast cancer cell model genomes. Breast Cancer Res. 2006;8:R9.
Gerami P, Jewell SS, Pouryazdanparast P, Wayne JD, Haghighat Z, Busam KJ, et al. Copy number gains in 11q13 and 8q24 [corrected] are highly linked to prognosis in cutaneous malignant melanoma. J MOL DIAGN. 2011;13:352–8.
van Kempen PM, Noorlag R, Braunius WW, Moelans CB, Rifi W, Savola S, et al. Clinical relevance of copy number profiling in oral and oropharyngeal squamous cell carcinoma. Cancer Med. 2015;4:1525–35.
Sapkota Y, Ghosh S, Lai R, Coe BP, Cass CE, Yasui Y, et al. Germline DNA copy number aberrations identified as potential prognostic factors for breast cancer recurrence. PLoS One. 2013;8:e53850.
Sun W, Xing B, Sun Y, Du X, Lu M, Hao C, et al. Proteome analysis of hepatocellular carcinoma by two-dimensional difference gel electrophoresis: novel protein markers in hepatocellular carcinoma tissues. Mol Cell Proteomics. 2007;6:1798–808.
Walsh N, O'Donovan N, Kennedy S, Henry M, Meleady P, Clynes M, et al. Identification of pancreatic cancer invasion-related proteins by proteomic analysis. Proteome Sci. 2009;7:3.
Chao A, Lai CH, Tsai CL, Hsueh S, Hsueh C, Lin CY, et al. Tumor stress-induced phosphoprotein1 (STIP1) as a prognostic biomarker in ovarian cancer. PLoS One. 2013;8:e57084.
Wang TH, Chao A, Tsai CL, Chang CL, Chen SH, Lee YS, et al. Stress-induced phosphoprotein 1 as a secreted biomarker for human ovarian cancer promotes cancer cell proliferation. Mol Cell Proteomics. 2010;9:1873–84.
Kubota H, Yamamoto S, Itoh E, Abe Y, Nakamura A, Izumi Y, et al. Increased expression of co-chaperone HOP with HSP90 and HSC70 and complex formation in human colonic carcinoma. Cell Stress Chaperones. 2010;15:1003–11.
Padden J, Megger DA, Bracht T, Reis H, Ahrens M, Kohl M, et al. Identification of novel biomarker candidates for the immunohistochemical diagnosis of cholangiocellular carcinoma. Mol Cell Proteomics. 2014;13:2661–72.
Willmer T, Contu L, Blatch GL, Edkins AL. Knockdown of hop downregulates RhoC expression, and decreases pseudopodia formation and migration in cancer cell lines. Cancer Lett. 2013;328:252–60.
Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006;11:1696–701.
Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008;27:6920–9.
MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26.
Wang QM, Fiol CJ, AA DP-r, Roach PJ. Glycogen synthase kinase-3 beta is a dual specificity kinase differentially regulated by tyrosine and serine/threonine phosphorylation. J Biol Chem. 1994;269:14566–74.
Medina M, Wandosell F. Deconstructing GSK-3: the fine regulation of its activity. Int J Alzheimers Dis. 2011;2011(479249)
Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–6.
Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J Biol Chem. 2006;281:9971–6.
van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem. 2002;277:17901–5.
Wei A, Fan B, Zhao Y, Zhang H, Wang L, Yu X, et al. ST6Gal-I overexpression facilitates prostate cancer progression via the PI3K/Akt/GSK-3beta/beta-catenin signaling pathway. Oncotarget. 2016;7:65374–88.
Qian HY, Zhang DG, Wang HW, Pei DS, Zheng JN. Tyrosine phosphorylation of beta-catenin affects its subcellular localization and transcriptional activity of beta-catenin in Hela and Bcap-37 cells. Bioorg Med Chem Lett. 2014;24:2565–70.
Choi CH, Lee BH, Ahn SG, Oh SH. Proteasome inhibition-induced p38 MAPK/ERK signaling regulates autophagy and apoptosis through the dual phosphorylation of glycogen synthase kinase 3beta. Biochem Biophys Res Commun. 2012;418:759–64.
Zhu Y, Xu L, Zhang J, Xu W, Liu Y, Yin H, et al. Klotho suppresses tumor progression via inhibiting PI3K/Akt/GSK3beta/snail signaling in renal cell carcinoma. Cancer Sci. 2013;104:663–71.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81502078, 81372341, and 81772579), the Science and Technology Foundation of Guangzhou (201707010125), the Natural Science Foundation of Guangdong (2017A030313513, 2017A030313893), the Medical Science and Technology Research Foundation of Guangdong Province (A2017058), Postdoctoral Science Foundation of China (2017 M622878).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding authors on reasonable request.
Author information
Authors and Affiliations
Contributions
SP, LXX and ZRZ conceived and designed the experiments; LLH, ETZ, YL, JBL and HLJ conducted the experiments; LLH and ETZ performed the statistical analysis; SP, SRC and MHC supported the experiments. LLH and LXX wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies were in accordance with the ethical standards of the Ethics Committee of the Institutional Ethical Review Board of the First Affiliated Hospital, Sun Yat-sen University. All animal procedures and care were conducted in accordance with institutional guidelines and in compliance with national and international laws and policies.
Consent for publications
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1: Table S1.
Antibodies Used. (DOCX 17 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Huang, L., Zhai, E., Cai, S. et al. Stress-inducible Protein-1 promotes metastasis of gastric cancer via Wnt/β-catenin signaling pathway. J Exp Clin Cancer Res 37, 6 (2018). https://doi.org/10.1186/s13046-018-0676-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13046-018-0676-8