Abstract
Background
Semaphorin 3 F (Sema3F) is a secreted type of the Semaphorin family of axon guidance molecules. Sema3F and its receptor neuropilin-2 (Npn-2) are expressed in a mutually exclusive manner in the embryonic mouse brain regions including olfactory bulb, hippocampus, and cerebral cortex. Sema3F is thought to have physiological functions in the formation of neuronal circuitry and its refinement. However, functional roles of Sema3F in the brain remain to be clarified. Here, we examined behavioral effects of Sema3F deficiency through a comprehensive behavioral test battery in Sema3F knockout (KO) male mice to understand the possible functions of Sema3F in the brain.
Results
Male Sema3F KO and wild-type (WT) control mice were subjected to a battery of behavioral tests, including neurological screen, rotarod, hot plate, prepulse inhibition, light/dark transition, open field, elevated plus maze, social interaction, Porsolt forced swim, tail suspension, Barnes maze, and fear conditioning tests. In the open field test, Sema3F KO mice traveled shorter distance and spent less time in the center of the field than WT controls during the early testing period. In the light/dark transition test, Sema3F KO mice also exhibited decreased distance traveled, fewer number of transitions, and longer latency to enter the light chamber compared with WT mice. In addition, Sema3F KO mice traveled shorter distance than WT mice in the elevated plus maze test, although there were no differences between genotypes in open arm entries and time spent in open arms. Similarly, Sema3F KO mice showed decreased distance traveled in the social interaction test. Sema3F KO mice displayed reduced immobility in the Porsolt forced swim test whereas there was no difference in immobility between genotypes in the tail suspension test. In the fear conditioning test, Sema3F KO mice exhibited increased freezing behavior when exposed to a conditioning context and an altered context in absence of a conditioned stimulus. In the tests for assessing motor function, pain sensitivity, startle response to an acoustic stimulus, sensorimotor gating, or spatial reference memory, there were no significant behavioral differences between Sema3F KO and WT mice.
Conclusions
These results suggest that Sema3F deficiency induces decreased locomotor activity and possibly abnormal anxiety-related behaviors and also enhances contextual memory and generalized fear in mice. Thus, our findings suggest that Sema3F plays important roles in the development of neuronal circuitry underlying the regulation of some aspects of anxiety and fear responses.
Similar content being viewed by others
Background
Semaphorins are a family of secreted or membrane-bound molecules containing an around 500-amino acid extracellular domain termed semaphorin domain. The semaphorins are subdivided into eight classes, of which classes 3-7 are found in vertabrates [1]. The class 3 subfamily of semaphorins, originally identified as axon guidance molecules, is expressed throughout the brain and has crucial roles in synapse formation and plasticity [2]. Despite the advances in the understanding of some semaphorins and their downstream signaling pathways that are implicated in neural circuit development [2], there is relatively limited information about contributions of those molecules to brain function and behavior [3–5].
Semaphorin 3 F (Sema3F), one of the class 3 semaphorins, is a secreted protein that binds to a transmembrane receptor protein Neuropilin-2 (Npn-2) [6, 7]. The Sema3F/Npn-2 interaction evokes intracellular signals for axon guidance via the Plexin family protein(s) [2]. The mRNAs for Sema3F and its receptor neuropilin-2 (Npn-2) are expressed in a mutually exclusive manner in the embryonic mouse brain regions including olfactory bulb, hippocampus, and cerebral cortex [8]. To examine physiological roles of Sema3F during developing brain, we and others investigated physiological properties in postnatal brain of Sema3F knockout (KO) mice or Npn-2 KO mice. It was demonstrated that Sema3F KO and Npn-2 KO mice showed various neuroanatomical defects in neuronal wiring and/or its synaptic transmission in brain regions such as olfactory bulb, hippocampus, cortex, and habenula [8–14]. It is reported that Sema3F and Npn-2 KO mice exhibit increased spine number and size in the granule cells in the dentate gyrus (DG) of the hippocampus and cortical layer V pyramidal neurons and also show increased frequency of miniature excitatory postsynaptic current, indicating that Sema3F/Npn-2 signaling is a negative regulator of spine development and synaptic structure [12]. Taken together, Sema3F/Npn-2 signaling has essential roles in neuronal circuitry formation during brain development. However, it remains to be examined whether the neuroanatomical aberrations induced by abnormal Sema3F expression lead to changes in behavior.
In the present study, to reveal the functional and behavioral roles of Sema3F in brain, we examined various behaviors of Sema3F KO mice through a battery of behavioral tests, including neurological screen, light/dark transition, open field, elevated plus maze, hot plate, social interaction, rotarod, prepulse inhibition, Porsolt forced swim, fear conditioning, tail suspension, and Barnes maze tests. Our results of behavioral analysis indicate that Sema3F KO mice show abnormal anxiety-related behaviors in the open field and light/dark transition tests and enhanced freezing in the conditioning context and altered context of the fear conditioning test, suggesting that Sema3F is involved in regulation of innate and learned fear.
Results
Sema3F KO mice exhibit normal sensorimotor function
We compared general health and neurological characteristics of Sema3F KO mice with those of WT control mice. The appearance of fur and whiskers and the neurological reflexes were normal in Sema3F KO mice. Sema3F KO mice exhibited significantly lighter body weight (Fig. 1a, t 37 = 4.996, p < 0.0001), weaker grip strength (Fig. 1c, t 37 = 3.955, p = 0.0003), and shorter wire-hang latency (Fig. 1d, t 37 = 3.103, p = 0.0037) than WT control mice. There was no significant difference between the genotypes in body temperature (Fig. 1b, t 37 = 0.361, p = 0.7204). These results show that Sema3F is required for physical and neuromuscular development.
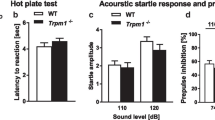
There were no significant differences between Sema3F KO and WT control mice in rotarod performance (Fig. 2a, F 1, 37 = 0.07, p = 0.7921), hot plate latency (Fig. 2b, t 37 = 0.708, p = 0.4832), startle responses to 110 and 120 dB stimuli (Fig. 2c, F 1, 37 = 0.394, p = 0.5338), or prepulse inhibition of the startle response (Fig. 2d, for 110 dB startle, F 1, 37 = 2.561, p = 0.118; for 120 dB startle, F 1, 37 = 0.031, p = 0.8604). These observations indicate that Sema3F KO mice have no deficits in sensory or motor functions.
Normal motor coordination, pain sensitivity and sensorimotor function in Sema3F KO mice. a The latency to fall from an accelerating rotarod was measured by three trials per day for two consecutive days in the rotarod test. There was no significant difference in the latency to fall between Sema3F KO mice and WT controls. b The hot plate test was used to evaluate sensitivity to a painful stimulus. Mice were placed on a hot plate and latency to the first hindpaw response was recorded. There was no significant difference in the latency between the genotypes. c, d The startle response/prepulse inhibition tests were performed to examine startle responses to loud stimuli (110 or 120 dB) and inhibition of the startle response by prepulse stimulus (74 or 78 dB). c The startle response and (d) the prepulse inhibition were not significantly different between Sema3F KO mice and WT controls. Data indicate means ± SEM (n = 19 for Sema3F KO mice; n = 20 for WT controls)
Sema3F KO mice exhibit increased anxiety-related behavior
Sema3F KO and WT control mice were compared in behavioral tests for assessing anxiety-related behavior, including light/dark transition, open field, elevated plus maze, and social interaction tests. In the light/dark transition test, Sema3F KO mice showed decreased distance traveled, fewer number of transitions between the light and dark chambers, and shorter latency to enter the light chamber compared with WT mice (Fig. 3a, for distance traveled in the light and dark chambers, Genotype effect, F 1,33 = 11.871, p = 0.0016; Genotype × Chamber interaction, F 1,33 = 0.00005, p = 0.9944; Fig. 3b, for number of transitions, t 33 = 3.763, p = 0.0007; Fig. 3c, for latency to enter the light chamber, t 33 = 2.597, p = 0.0139). Moreover, Sema3F KO mice showed a trend to stay shorter time in the light chamber than WT controls (Fig. 3d, t 33 = 2.001, p = 0.0537). These results indicate that Sema3F deficiency induces increased anxiety-related behavior.
Decreased locomotor activity and increased anxiety-related behavior in Sema3F KO mice. a-d Light/dark transition test. a Distance traveled, (b) number of transitions between the light and dark chambers, (c) latency to enter the light chamber, and (d) stay time in the light chamber (n = 17 for Sema3F KO mice; n = 18 for WT controls). e-h Open field test. e Distance traveled, (f) vertical activity, (g) time spent in center area, and (h) stereotypic behavior counts (n = 19 for Sema3F KO mice; n = 20 for WT controls). i-m Elevated plus maze test. i Number of total entries into arms, (j) distance traveled, (k) percentage of entries into open arms, (l) percentage of time spent in open arms, and (m) time spent in open arms, center area, and closed arms (n = 19 for Sema3F KO mice; n = 20 for WT controls). n-r Social interaction test in a novel environment. n Number of contacts, (o) total duration of contacts, (p) total duration of active contacts, (q) mean duration per contact, and (r) distance traveled (n = 9 for each genotype)
In the open field test, there were no significant genotype differences in the total distance traveled, vertical activity, center time, or stereotypic counts during 120-min test period (Fig. 3e, for total distance traveled, Genotype effect, F 1,37 = 0.023, p = 0.8811; Genotype × Time interaction, F 23,851 = 1.845, p = 0.0093; Fig. 3f, for vertical activity, Genotype effect, F 1, 37 = 0.168, p = 0.6841; Genotype × Time interaction, F 23, 851 = 0.807, p = 0.7254; Fig. 3g, for center time, Genotype effect, F 1, 37 = 0.044, p = 0.8358; Genotype × Time interaction, F 23, 851 = 1.321, p = 0.143; Fig. 3h, for stereotypic counts, Genotype effect, F 1, 37 = 0.549, p = 0.4634; Genotype × Time interaction, F 23, 851 = 1.891, p = 0.007). However, in the first 5-min period of the test, during which anxiety-like behavior has been generally assessed [15], Sema3F KO mice traveled a shorter distance and stayed less time in the center area compared with WT controls (Fig. 3e, for distance traveled, p = 0.0223; Fig. 3g, for center time, p = 0.0221).
In the elevated plus maze test, Sema3F KO mice showed a significantly smaller number of total entries into arms and traveled a significantly shorter distance than WT control mice (Fig. 3i, for number of total arm entries, t 37 = 2.039, p = 0.0486; Fig. 3j, for distance traveled, t 37 = 2.98, p = 0.0051). The Sema3F KO mice spent longer time in closed arms and less time in center area than WT controls although the difference did not reach a statistical significance (Fig. 3m, for time spent in closed arms, t 37 = 1.693, p = 0.0988; for time spent in center area, t 37 = 1.902, p = 0.065). These results indicate decreased locomotor activity in Sema3F KO mice in novel environment, which appear to be consistent with the findings of the light/dark transition test and open field test. However, there were no significant differences between the genotypes in time spent in open arms (Fig. 3m, t 37 = 0.295, p = 0.77), percentage of entries into open arms (Fig. 3k, t 37 = 0.914, p = 0.3667), or percentage of time spent in open arms (Fig. 3l, t 37 = 0.295, p = 0.7697).
In the social interaction test in a novel environment, total duration of active social contacts and distance traveled were significantly decreased in Sema3F KO mice compared with WT control mice (Fig. 3p, for total duration of active contacts, t 16 = 2.726, p = 0.015; Fig. 3r, for distance traveled, t 16 = 2.249, p = 0.039). The mean duration per contact was increased in Sema3F KO mice (Fig. 3q, t 16 = 2.128, p = 0.0493). There were no significant differences in total number of contacts (Fig. 3n, t 16 = 1.36, p = 0.1925) or total duration of contacts (Fig. 3o, t 16 = 0.309, p = 0.761). These results show an altered locomotor activity under this novel environment with a stranger mouse, but failed to show abnormality in social behavior per se in Sema3F KO mice.
Together, these results from the different types of tests for assessing anxiety-related behavior indicate that Sema3F KO mice exhibit decreased locomotor activity and abnormal anxiety-related behaviors in novel environments.
Sema3F KO mice show hyperactivity in forced swim test
Depression-related behavior was evaluated by the Porsolt forced swim and tail suspension tests. In the Porsolt forced swim test, Sema3F KO mice displayed significantly less immobility and traveled significantly longer distances than their WT controls (Fig. 4a, for immobility on Day 1, F 1, 37 = 24.035, p < 0.0001; for immobility on Day 2, F 1, 37 = 23.318, p < 0.0001; Fig. 4b, for distance traveled on Day 1, F 1, 37 = 13.735, p = 0.0007; for distance traveled on Day 2, F 1, 37 = 11.053, p = 0.002). In the tail suspension test, there was no significant difference in immobility between Sema3F KO and WT control mice (Fig. 4c, F 1, 31 = 0.32, p = 0.5758). These findings suggest that Sema3F deficiency may induce hyperactivity in forced swim tests. The discrepancy between forced swim and tail suspension tests indicate that the results may not simply reflect decreased depression-related behavior (see Discussion below).
Hyperactivity in forced swim test in Sema3F KO mice. a, b Porsolt forced swim test. Mice were placed in a water-filled cylinder, and immobility time was measured every 1 min for a 10-min period on the first and second days. Sema3F KO mice spent significantly less time in immobility (a) and traveled significantly longer distances (b) than their WT controls. N = 19 for Sema3F KO mice; N = 20 for WT controls. c Tail suspension test. There was no significant difference in immobility between Sema3F KO mice and WT controls. All data indicate means ± SEM (n = 18 for Sema3F KO mice; n = 15 for WT controls)
Sema3F KO mice exhibit increased freezing in the conditioning context and altered context
To assess memory performance in Sema3F KO and WT control mice, mice were conditioned with three pairs of auditory cue (conditioned stimulus, CS) and footshock (unconditioned stimulus, US) in the conditioning session. Twenty-four hours after the conditioning, they were tested to assess fear memory by exposure to the conditioning context (context test) followed by the altered context without and with the auditory cue (cued test). There was no significant difference in the percentage of freezing between the genotypes during the conditioning session (Fig. 5a, F 1, 31 = 1.453, p = 0.2371). In the context test, Sema3F KO mice showed a significantly more freezing behavior than WT controls in the re-exposure to the same context of the conditioning session (Fig. 5b, F 1, 31 = 9.163, p = 0.0049). In the cued test with altered context, Sema3F KO mice exhibited significantly more freezing than WT controls in the absence of the CS (Fig. 5c, F 1, 31 = 6.447, p = 0.0163). During the cued test, Sema3F KO mice also showed more freezing than WT controls in presence of the CS although the difference failed to reach a statistical significance (Fig. 5c, F 1, 31 = 3.514, p = 0.0703). These results suggest that Sema3F deficiency causes increased generalized fear as well as increased contextual fear memory.
Increased fear memory in Sema3F KO mice. Freezing behavior was measured in the contextual and cued fear conditioning test to assess fear memory in Sema3F KO mice. a There were no significant differences in freezing between Sema3F KO mice and WT controls during the conditioning session. b, c Sema3F KO mice exhibited more freezing than WT controls in the re-exposure to the same context approximately 24 hours after the conditioning session and in the altered context with absence or presence of the auditory cue. Data indicate means ± SEM (n = 18 for Sema3F KO mice; n = 15 for WT controls)
Sema3F KO mice show normal spatial reference memory
Spatial reference memory in Sema3F KO and WT control mice was tested in the Barnes circular maze. In the training session, there were no significant differences between the genotypes in the number of error to reach the correct target hole or latency to reach the correct target hole (Fig. 6a, F 1, 30 = 0.995, p = 0.3266; Fig. 6b, F 1, 30 = 2.249, p = 0.1441, respectively), indicating normal spatial learning in Sema3F KO mice. One day and 10 days after the last training session, probe tests were performed to evaluate the retention of spatial memory. There were no significant differences between Sema3F KO and WT control mice in the time spent around the target hole in either 1- or 10-day retention probe tests (Fig. 6c, for 1 day later, t 30 = 0.209, p = 0.8361; for 10 days later, t 30 = 0.406, p = 0.6878). These findings indicate that spatial learning and memory in Sema3F KO mice are normal.
Normal spatial reference memory in Sema3F KO mice. Spatial reference memory was tested in the Barnes circular maze. a, b During the training session, there were no significant differences in the number of errors and latency to reach the correct target hole between Sema3F KO mice and WT controls. c, d In the retention probe tests, 1 day and 10 days after the last training session, Sema3F KO mice and WT controls spent similar time in the correct target hole. Data indicate means ± SEM (n = 18 for Sema3F KO mice; n = 15 for WT controls)
Discussion
The present study examined behaviors of Sema3F KO male mice using a battery of behavioral tests. To our knowledge, this is the first report on the comprehensive behavioral analysis of mutant mice lacking the class 3 semaphorin. Our physical and behavioral assessments revealed that Sema3F KO mice showed no differences in the hot plate latency, rotarod latency, acoustic startle response, and prepulse inhibition of the startle response compared with WT mice, indicating normal sensory and motor functions and sensorimotor gating. Decreased body weight, grip strength, and wire hang latency were observed in Sema3F KO mice, indicating that the KO mice exhibited reduced neuromuscular strength. However, Sema3F KO and WT mice showed no differences in total distance traveled and vertical activity during the entire period of the open field test in addition to rotarod performance. Thus, it is not likely that the decreased neuromuscular strength affect motor function and general locomotor activity in Sema3F KO mice. This conclusion seems to be consistent with the previous study showing that there are no significant differences between Sema3F KO and WT control mice in anatomical structures in the somatosensory cortex or cerebellum [8], underlying the control of motor functions. These results seem to be consistent with the findings of a recent study reporting that mice lacking Npn-2, a Sema3F receptor, exhibited normal locomotor activity and hot plate latency, compared with their control mice [5]. However, Npn-2 KO mice showed impaired rotarod performance at the trial 8 and 10, while there were no significant differences in the performances from trial 1 to 7 compared with heterozygous control mice [5]. What causes slightly different results of rotarod test between Sema3F KO mice and Npn-2 KO mice? The Sema3F receptor is considered to be a heteromeric complex containing Npn-2 and Plexin A [16]. Npn-2, when ectopically expressed in COS cells, binds to Sema3B, Sema3C, and Sema3G as well as Sema3F [16]. The observed differences between behavioral phenotypes of Sema3F KO mice in this study and that of Npn-2 KO mice [5] may reflect the loss of binding of Sema3B, Sema3C, and Sema3G to Npn-2 in Npn-2 KO mice.
The light/dark transition, open field, and elevated plus maze tests have been widely used to assess anxiety-related behavior [15, 17, 18]. In the light/dark transition test, Sema3F KO mice exhibited increased latency to enter the light chamber, decreased distance traveled in the light and dark chambers, reduced number of transitions between the chambers, and stayed decreased time in the light chamber. Sema3F KO mice showed decreased distance traveled and reduced time spent in the center area in the open field during the early testing period, and also displayed decreased locomotor activity in the elevated plus maze. These data suggest that Sema3F KO mice show an innate aversion to a centain type of novel situations or increased anxiety to novelty. In addition, in the social interaction test, which has also been used to assess anxiety [19, 20], Sema3F KO mice showed decreased distance traveled and reduced duration of active social contact with a novel environment with a stranger mouse compared with WT controls. However, in the elevated plus maze test, there were no significant differences in the percentages of open time or open arm entries between Sema3F KO mice and WT controls. The absence of genotype effects on the behavioral indices in the elevated plus maze may be presumably explained by so called “floor effect” [21–23]. Overall, the results of the different types of tests for assessing anxiety-related behavior except for the elevated plus maze test suggest that Sema3F deficiency may induce abnormal anxiety-related behavior in novel environments, although further studies are needed to confirm whether Sema3F plays a critical role in the regulation of anxiety-related behavior in various situations.
In contrast to the reduced behavioral responses to the novel environments, Sema3F KO mice showed decreased immobility behavior in the Porsolt forced swim test, suggesting an antidepressant-like effect. However, this effect was not supported by the result of the tail suspension test. Similar findings were reported in mice deficient in phosphodiesterase 4 B, an enzyme that catalyzes hydrolysis of cyclic AMP [24], which is a potential downstream signaling molecule of class 3 Semaphorin [2]. It is reported that treatment of a specific agonist of corticotropin-releasing factor receptor subtype 1, stimulating the release of cyclic AMP, induced decrease in immobility in the forced swim test and anxiogenic-like effects in mice [25]. Since Sema3F KO mice exhibited abnormal anxiety-related behavior in some of tests, the ‘antidepressant-like’ effect observed in the forced swim test might be resulted from anxiety-related struggling, escape behavior, or heightened agitation in stressful situation, resulting in the decreased immobility. However, given that the two types of test are used as a measurement of depression-related behavior, further studies are needed to clarify the role of Sema3F in the regulation of depression-related behavior.
Sema3F KO mice showed no deficits in spatial reference learning or the long-term retention memory assessed by the Barnes maze test. On the other hand, in the contextual and cued fear conditioning test, we found that Sema3F KO mice displayed increased freezing responses in re-exposure to the conditioning context, indicating enhanced contextual fear memory. Moreover, Sema3F KO mice exhibited increased freezing when they were exposed to the altered context. This suggests that Sema3F deficiency induces enhanced generalized fear, reflecting impairment in the ability to distinguish two contexts, which is thought to be associated with a process known as pattern separation [26–28]. Our results seem to be consistent with those of Shiflett et al., reporting that Npn-2 KO mice exhibited behavioral impairments in the two discrimination tasks based on object novelty and object or context association [5]. Thus, our findings suggest that Sema3F deficiency induces increased fear-related behavior or strengthens consolidation and generalization of fear memories for aversive experiences.
Sema3F/Npn-2 signaling is thought to contribute to development of neuronal circuit formation in the brain regions including hippocampus, cortex, olfactory bulb, and amygdala [8–14]. Tang et al. reported that mice deficient for COUP-TFII transcription factor showed abnormality of amygdala patterning, presumably due to defects in neuronal migration caused by decreased Npn-2 expression [29]. Sahay et al. demonstrated that mice deficient for Npn-2 or Sema3F showed severely disorganized and defasciculated axons of amygdala efferents projecting to the forebrain including the hypothalamus and the bed nucleus of the stria terminalis (BNST) [11]. Lesion and pharmacological studies have suggested that BNST is involved in the modulation of innate fear responses [30, 31]. These findings suggest that these anatomical defects, especially the abnormal targeting of amygdala efferents to BNST, may be associated with abnormalities in anxiety- and fear-related behaviors in Sema3F KO mice found in the present study. During synaptogenesis, Sema3F and its receptor Npn-2 are strongly expressed in the hippocampal DG [8, 12, 32]. In particular, Sema3F is abundantly expressed in the hilus of the DG and in the CA3 to which mossy and infrapyramidal tract (IPT) of the DG granule cells project, and Npn-2 is enriched in the molecular layer of the DG [12, 32]. Consistent with these observations, the hippocampal IPT projects abnormally to CA3 in Sema3F KO and Npn-2 KO mice [11, 13]. It is reported that Sema3F and Npn-2 KO mice exhibit increased spine number and size in the DG granule cells and cortical layer V pyramidal neuron and also increase frequency of miniature excitatory postsynaptic current, which indicate that Sema3F/Npn-2 signaling is a negative regulator of spine development and synaptic structure [12]. The density and size of spines in the hippocampus and cortex have been found to change after behavioral paradigms [33–38]. Increased spine morphogenesis and abnormal development of neural circuit induced by loss of Sema3F/Npn-2 signaling in various brain regions may induce increases in fear memory and generalized fear.
Disruptions of synaptic formation and function are associated with development of neurological and psychiatric disorders [39–41]. Recent human genetic studies have discovered associations between Semaphorins-neuropilins/plexins and various disorders, such as autism [42, 43], bipolar disorder [44], and schizophrenia [45–47]. However, so far it remains to be explored how these mutations lead to neurodevelopmental and functional outcomes of the brain. Sema3F KO mice may be served as a model to investigate the neural basis underlying some behavioral abnormalities related to the neurodevelopmental disorders.
Conclusions
The present study provides novel findings on the behavioral effects of Sema3F deficiency through comprehensive behavioral analysis of Sema3F KO mice. We demonstrated that Sema3F KO mice show increased anxiety- and fear-related behaviors and enhanced fear memory, suggesting an important role of Sema3F in regulating innate and learned fear responses and memory functions. Sema3F/Npn-2 signaling is known to be crucial for hippocampal and cortical synaptic formation and organization during postnatal development. Thus, Sema3F can serve as a potential target to understand the neural mechanisms underlying neurodevelopment disorders and to treat the behavioral abnormalities.
Methods
Animals and experimental design
Sema3F KO mice were generated as previously described [8]. In brief, Sema3F loxP/+ mice were obtained by crossing Sema3F loxP-neo/+ mice with EIIa-cre transgenic mice [48]. Male Sema3F KO mice and the wild-type (WT) control littermates were generated from a cross between the heterozygous Sema3F loxP/+ mice with a C57BL/6 J background. Subjects were group-housed (four per cage, two KO and two WT) in a room with a 12-hr light/dark cycle (lights on at 07:00 hr) with access to food and water ad libitum. Behavioral testing was performed as previously described [49, 50]. The animals were 10–11 weeks old at the start of the testing. The tests were conducted between 09:00 and 17:00 hr. After the tests, all of the apparatus were cleaned with 70 % ethanol and super hypochlorous water to prevent a bias based on olfactory cues. All animal experiments were performed in accordance with the Guidelines for Animal Experimentation at Kobe University Graduate School of Medicine and Kyoto University Graduate School of Medicine. Behavioral tests were performed according to the test order as described below.
General health and neurological screen
Physical characteristics, including body weight, rectal temperature, presence of whiskers or bald hair patches, were recorded. The righting, whisker twitch, and ear twitch reflexes were also evaluated. The neuromuscular strength was examined by the grip strength and wire hang tests as described previously [51]. The grip strength meter (O’Hara & Co., Tokyo) was used to assess forelimb grip strength. Mice were lifted and held by their tail so that their forepaws could grasp a wire grid, and then they were gently pulled backward until they release the grid. The peak force applied by mouse forelimbs was recorded in Newton. In the wire hang test, mice were placed on a wire mesh (O’Hara & Co., Tokyo), which was then inverted and waved gently, so that the subject grasped the wire. Latency to fall from the wire was recorded with a 60 sec cut-off time.
Light/dark transition test
The light/dark transition test, developed by Crawley and colleagues [52], was performed as described previously [53]. The apparatus consisted of a cage (21 × 42 × 25 cm) divided into two sections of equal size by a partition with door (O’Hara & Co., Tokyo). One chamber was brightly illuminated (390 lux), whereas the other chamber was dark (2 lux). Mice were placed into the dark chamber, and allowed to move freely between the two chambers for 10 min after the door was open. The distance traveled (cm), total number of transitions, latency to first enter the light chamber (sec), and time spent in the light chamber (sec) were recorded automatically using ImageLD software (see ‘Image analysis’).
Open field test
In the open field test [15], each subject was placed in the center of an open field apparatus (40 × 40 × 30 cm; Accuscan Instruments, Columbus, OH). Total distance traveled (cm), vertical activity (rearing measured by counting the number of photobeam interruptions), time spent in the center area (sec), and stereotypic counts (beam-break counts for stereotyped behaviors) were recorded. The center area was defined as 20 cm × 20 cm area located at the center of the field. Data were collected over a 120-min period.
Elevated plus maze test
The elevated plus maze test, which has been widely used for evaluation of anxiety-related behavior [54, 55], was performed as described previously [56]. The apparatus consisted of two open arms (25 × 5 cm) and two enclosed arms of the same size with 15 cm high transparent walls, which arms were connected by a central square (5 × 5 cm) (O’Hara & Co., Tokyo). The arms and central square were made of white plastic plates and were elevated to a height of 55 cm above the floor. The open arms were surrounded by a raised ledge (3-mm thick and 3-mm high) to avoid mice falling off the arms. Arms of the same type were located opposite from each other. Each mouse was placed in the central square of the maze, facing one of the closed arms. The number of arm entries, distance traveled (cm), percentage of entries into open arms, and percentage of time spent in open arms were measured during a 10-min test period. Data acquisition and analysis were performed automatically using ImageEP software.
Hot plate test
The hot plate test was performed to evaluate sensitivity to a painful stimulus. Mice were placed on a 55.0 ± 0.3 °C hot plate (Columbus Instruments, Columbus, OH), and latency to first hind-paw response was recorded. The hind-paw response was defined as either a paw lick or a foot shake.
Social interaction test in a novel environment
Social interaction test was conducted to measure social behavior in a novel environment [20, 57]. Weight-matched (within 5 g) mice of the same genotype, which have been housed in different cages, were placed into an acrylic box together (40 × 40 × 30 cm) and allowed to explore freely for 10 min. The total number of contacts, total duration of contacts, total duration of active contacts, mean duration per contact, and total distance traveled were recorded and analyzed automatically using ImageSI software (see ‘Image analysis’). The active contact was defined as follows. Images were captured at 3 frames per second, and distance traveled between two successive frames was calculated for each mouse. If the two mice contacted each other and the distance traveled by either mouse was longer than 5 cm, the behavior was considered as an ‘active contact’.
Rotarod test
Motor coordination and balance were tested with the rotarod test. Mice were placed on a rotating drum (3 cm diameter, UGO Basile Accelerating Rotarod). Time during which each animal was able to maintain its balance on the rod was measured (a cut-off time of 300 sec). The speed of the rotarod accelerated from 4 to 40 rpm over a 300-sec period.
Startle response/prepulse inhibition tests
A startle reflex measurement system was used (O’Hara & Co., Tokyo). A test session began by placing a mouse in a Plexiglas cylinder where it was left undisturbed for 10 min. White noise (40 ms) was used as a startle stimulus. The intensity of startle stimulus was 110 or 120 dB. A prepulse sound was presented 100 msec before the startle stimulus, and its intensity was 74 or 78 dB. A test session consisted of six trial types (i.e. two types for startle stimulus only trials, and four types for prepulse inhibition trials). Four combination of prepulse and startle stimuli were employed (74-110, 78-110, 74-120, and 78-120 dB). Six blocks of the six trial types were presented in pseudorandom order such that each trial type was presented once within a block. The average interval was 15 sec (range: 10-20 sec). A 70 dB white noise was presented as a background noise during the test. Startle responses to the stimuli were recorded for 140 msec (measuring the response every 1 msec) starting with the onset of the prepulse stimulus. The peak startle amplitude recorded during the 140 msec sampling window was used as the dependent variable.
Porsolt forced swim test
The Porsolt forced swim test, developed by Porsolt and colleagues [58, 59], was performed to assess depression-related behavior. Mice were placed into a Plexiglas cylinder (20 cm height × 10 cm diameter, O’Hara & Co., Tokyo) filled with water (23 °C), up to a height of 7.5 cm. Percentage of immobility and distance traveled (cm) were recorded over a 10-min test period. Data acquisition and analysis were performed automatically using ImagePS software (see Image Analysis).
Contextual and cued fear conditioning test
To assess fear memory [60], mice were placed in a conditioning chamber (26 × 34 × 29 cm) in a sound-attenuated room and allowed to explore freely for 2 min. The animals were presented with an auditory cue (60 dB white noise) served as a conditioned stimulus (CS) for 30 sec. During the last 2 sec of the CS, mice were given a mild foot shock (2 sec, 0.5 mA) as an unconditioned stimulus (US). Two more CS-US pairings were presented with 120 sec inter-stimulus interval. Approximately 24 hr after the conditioning session, context test was performed in the conditioning chamber. Cued test in an altered context was performed after the context test using a triangular box (35 × 35 × 40 cm) made of white opaque plexiglas, which was located in a different sound-attenuated room. Following initial 3-min of pre-CS period, the CS was presented for 3 min. Data acquisition, control of stimuli (white noise and footshock), and data analysis were performed automatically using ImageFZ software. Images were captured at 1 frame per second. For each successive frame, the amount of area (pixels) by which the mouse moved was measured. When this area was below a certain threshold (i.e., 20 pixels), the behavior was judged as ‘freezing.’ When the amount of area equaled or exceeded the threshold, the behavior was judged as ‘non-freezing.’ The optimal threshold (the amount of pixels) to judge freezing was determined by adjusting it to the amount of freezing measured by human observation. ‘Freezing’ that lasted less than the defined time threshold (i.e. 2 sec) was not included in the analysis.
Tail suspension test
Tail suspension test was used to evaluate depression-related behavior [61]. Mice were suspended 30 cm above the floor in a visually isolated area by adhesive tape placed approximately 1 cm from the tip of the tail. Immobility behavior was recorded for a 10-min test session. Data acquisition and analysis were performed automatically using ImageTS software.
Barnes maze test
The Barnes circular maze task was conducted to test spatial reference memory [62] on ‘dry land’, a white circular surface, 1.0 m in diameter, with 12 holes equally spaced around the perimeter (O’Hara & Co., Tokyo). The circular open field was elevated 75 cm from the floor. A black Plexiglas escape box (17 × 13 × 7 cm), which had paper cage bedding on its bottom, was located under one of the holes. The hole above the escape box represented the target, analogous to a hidden platform in the Morris water maze task. The location of the target was consistent for a given mouse but randomized across mice. The maze was rotated daily, with the spatial location of the target unchanged with respect to the distal visual cues, to prevent a bias based on an olfactory cue or proximal cues within the maze. Three trials per day were conducted for 9 successive days. In each trial, latency to first reach the target hole (sec) and number of errors to reach the target hole was recorded by ImageBM software. On day 1 and 10, probe trials were performed without the escape box, to confirm that this spatial task was acquired based on navigation by distal environmental room cues. In the probe tests, time spent around each hole (sec) was measured using ImageBM software.
Image analysis
All application softwares used for each behavioral analysis were run on Macintosh computers. The application softwares were based on the public domain NIH Image or Image J program (developed by Wayne Rasband at the National Institute of Mental Health, Bethesda) and were modified for each test by Tsuyoshi Miyakawa (available through O’Hara & Co., Tokyo).
Statistical analysis
Statistical analysis was conducted using StatView (SAS Institute, Cary, NC). Data were analyzed by two-tailed t-tests or two-way repeated-measures ANOVAs. Values in graphs were expressed as mean ± SEM.
References
Semaphorin Nomenclature Committee. Unified nomenclature for the semaphorins/collapsins. Cell. 1999;97(5):551–2.
Pasterkamp RJ. Getting neural circuits into shape with semaphorins. Nat Rev Neurosci. 2012;13(9):605–18.
Yukawa K, Tanaka T, Takeuchi N, Iso H, Li L, Kohsaka A, et al. Sema4D/CD100 deficiency leads to superior performance in mouse motor behavior. Can J Neurol Sci. 2009;36(3):349–55.
Rünker AE, O'Tuathaigh C, Dunleavy M, Morris DW, Little GE, Corvin AP, et al. Mutation of Semaphorin-6A disrupts limbic and cortical connectivity and models neurodevelopmental psychopathology. PLoS One. 2011;6(11):e26488.
Shiflett MW, Gavin M, Tran TS. Altered hippocampal-dependent memory and motor function in neuropilin 2-deficient mice. Transl Psychiatry. 2015;5:e521. doi:10.1038/tp.2015.17.
Chen H, Chédotal A, He Z, Goodman CS, Tessier-Lavigne M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for thesemaphorins Sema E and Sema IV but not Sema III. Neuron. 1997;19(3):547–59.
Giger RJ, Urquhart ER, Gillespie SK, Levengood DV, Ginty DD, Kolodkin AL. Neuropilin-2 is a receptor for semaphorin IV: insight into the structural basis of receptor function and specificity. Neuron. 1998;21(5):1079–92.
Matsuda I, Fukaya M, Nakao H, Nakao K, Matsumoto H, Mori K, et al. Development of the somatosensory cortex, the cerebellum, and the main olfactory system in Semaphorin 3 F knockout mice. Neurosci Res. 2010;66(3):321–9.
Takeuchi H, Inokuchi K, Aoki M, Suto F, Tsuboi A, Matsuda I, et al. Sequential arrival and graded secretion of Sema3F by olfactory neuron axons specify map topography at the bulb. Cell. 2010;141(6):1056–67.
Sahay A, Kim CH, Sepkuty JP, Cho E, Huganir RL, Ginty DD, et al. Secreted semaphorins modulate synaptic transmission in the adult hippocampus. J Neurosci. 2005;25(14):3613–20.
Sahay A, Molliver ME, Ginty DD, Kolodkin AL. Semaphorin 3 F is critical for development of limbic system circuitry and is required in neurons for selective CNS axon guidance events. J Neurosci. 2003;23(17):6671–80.
Tran TS, Rubio ME, Clem RL, Johnson D, Case L, Tessier-Lavigne M, et al. Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature. 2009;462(7276):1065–9.
Chen H, Bagri A, Zupicich JA, Zou Y, Stoeckli E, Pleasure SJ, et al. Neuropilin-2 regulates the development of selective cranial and sensory nerves and hippocampal mossy fiber projections. Neuron. 2000;25(1):43–56.
Giger RJ, Cloutier JF, Sahay A, Prinjha RK, Levengood DV, Moore SE, et al. Neuropilin-2 is required in vivo for selective axon guidance responses to secreted semaphorins. Neuron. 2000;25(1):29–41.
Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463(1-3):3–33.
Sharma A, Verhaagen J, Harvey AR. Receptor complexes for each of the Class 3 Semaphorins. Front Cell Neurosci. 2012;6:28.
Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29(8):1193–205.
Bourin M, Hascoët M. The mouse light/dark box test. Eur J Pharmacol. 2003;463(1-3):55–65.
File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods. 1980;2(3):219–38.
File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463(1-3):35–53.
Miyakawa T, Leiter LM, Gerber DJ, Gainetdinov RR, Sotnikova TD, Zeng H, et al. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100(15):8987–92.
Tsujimura A, Matsuki M, Takao K, Yamanishi K, Miyakawa T, Hashimoto-Gotoh T. Mice lacking the kf-1 gene exhibit increased anxiety- but not despair-like behavior. Front Behav Neurosci. 2008;2:4.
Hashimoto-Gotoh T, Iwabe N, Tsujimura A, Takao K, Miyakawa T. KF-1 Ubiquitin ligase: an anxiety suppressor. Front Neurosci. 2009;3(1):15–24.
Zhang HT, Huang Y, Masood A, Stolinski LR, Li Y, Zhang L, et al. Anxiogenic-like behavioral phenotype of mice deficient in phosphodiesterase 4B (PDE4B). Neuropsychopharmacology. 2008;33(7):1611–23.
Tezval H, Jahn O, Todorovic C, Sasse A, Eckart K, Spiess J. Cortagine, a specific agonist of corticotropin-releasing factor receptor subtype 1, is anxiogenic and antidepressive in the mouse model. Proc Natl Acad Sci U S A. 2004;101(25):9468–73.
Rudy JW, O'Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cogn Affect Behav Neurosci. 2001;1(1):66–82.
McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317(5834):94–9.
Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends Neurosci. 2011;34(10):515–25.
Tang K, Rubenstein JL, Tsai SY, Tsai MJ. COUP-TFII controls amygdala patterning by regulating neuropilin expression. Development. 2012;139(9):1630–9.
Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philos Trans R Soc Lond B Biol Sci. 1997;352(1362):1675–87.
Xu HY, Liu YJ, Xu MY, Zhang YH, Zhang JX, Wu YJ. Inactivation of the bed nucleus of the stria terminalis suppresses the innate fear responses of rats induced by the odor of cat urine. Neuroscience. 2012;221:21–7.
Holtmaat AJ, De Winter F, De Wit J, Gorter JA, da Silva FH, Verhaagen J. Semaphorins: contributors to structural stability of hippocampal networks? Prog Brain Res. 2002;138:17–38.
O'Malley A, O'Connell C, Regan CM. Ultrastructural analysis reveals avoidance conditioning to induce a transient increase in hippocampal dentate spine density in the 6 hour post-training period of consolidation. Neuroscience. 1998;87(3):607–13.
O'Malley A, O'Connell C, Murphy KJ, Regan CM. Transient spine density increases in the mid-molecular layer of hippocampal dentate gyrus accompany consolidation of a spatial learning task in the rodent. Neuroscience. 2000;99(2):229–32.
Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci. 2003;23(2):659–65.
Restivo L, Vetere G, Bontempi B, Ammassari-Teule M. The formation of recent and remote memory is associated with time-dependent formation of dendritic spines in the hippocampus and anterior cingulate cortex. J Neurosci. 2009;29(25):8206–14.
Lai CS, Franke TF, Gan WB. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 2012;483(7387):87–91.
Sanders J, Cowansage K, Baumgärtel K, Mayford M. Elimination of dendritic spines with long-term memory is specific to active circuits. J Neurosci. 2012;32(36):12570–8.
Mann F, Chauvet S, Rougon G. Semaphorins in development and adult brain: Implication for neurological diseases. Prog Neurobiol. 2007;82(2):57–79.
Pasterkamp RJ, Giger RJ. Semaphorin function in neural plasticity and disease. Curr Opin Neurobiol. 2009;19(3):263–74.
Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14(3):285–93.
Wu S, Yue W, Jia M, Ruan Y, Lu T, Gong X, et al. Association of the neuropilin-2 (NRP2) gene polymorphisms with autism in Chinese Han population. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(4):492–5.
Butler MG, Rafi SK, Hossain W, Stephan DA, Manzardo AM. Whole exome sequencing in females with autism implicates novel and candidate genes. Int J Mol Sci. 2015;16(1):1312–35.
Lydall GJ, Bass NJ, McQuillin A, Lawrence J, Anjorin A, Kandaswamy R, et al. Confirmation of prior evidence of genetic susceptibility to alcoholism in a genome-wide association study of comorbid alcoholism and bipolar disorder. Psychiatr Genet. 2011;21(6):294–306.
Mah S, Nelson MR, Delisi LE, Reneland RH, Markward N, James MR, et al. Identification of the semaphorin receptor PLXNA2 as a candidate for susceptibility to schizophrenia. Mol Psychiatry. 2006;11(5):471–8.
Wedenoja J, Loukola A, Tuulio-Henriksson A, Paunio T, Ekelund J, Silander K, et al. Replication of linkage on chromosome 7q22 and association of the regional Reelin gene with working memory in schizophrenia families. Mol Psychiatry. 2008;13(7):673–84.
O'Donovan MC, Craddock N, Norton N, Williams H, Peirce T, Moskvina V, et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet. 2008;40(9):1053–5.
Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, et al. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci U S A. 1996;93(12):5860–5.
Miyakawa T, Yamada M, Duttaroy A, Wess J. Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J Neurosci. 2001;21(14):5239–50.
Yamasaki N, Maekawa M, Kobayashi K, Kajii Y, Maeda J, Soma M, et al. Alpha-CaMKII deficiency causes immature dentate gyrus, a novel candidate endophenotype of psychiatric disorders. Mol Brain. 2008;1:6.
Nakatani J, Tamada K, Hatanaka F, Ise S, Ohta H, Inoue K, et al. Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell. 2009;137(7):1235–46.
Crawley JN, Goodwin FK. Preliminary report of a simple animal behavior for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–70.
Takao K, Miyakawa T. Light/dark transition test for mice. J Vis Exp. 2006;1:104.
Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl). 1987;92(2):180–5.
Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2(2):322–8.
Komada M, Takao K, Miyakawa T. Elevated plus maze for mice. J Vis Exp. 2008;22. doi:10.3791/1088.
de Angelis L, File SE. Acute and chronic effects of three benzodiazepines in the social interaction anxiety test in mice. Psychopharmacology (Berl). 1979;64(2):127–9.
Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229(2):327–36.
Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl). 2005;177(3):245–55.
Shoji H, Takao K, Hattori S, Miyakawa T. Contextual and cued fear conditioning test using a video analyzing system in mice. J Vis Exp. 2014:85.
Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl). 1985;85(3):367–70.
Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93(1):74–104.
Acknowledgements
We thank all the colleagues in Kobe University, Kyoto University, and Fujita Health University for their technical support and helpful discussions. This work is supported by a Grant-in-Aid for Scientific Research on Innovative Areas (Comprehensive Brain Science Network) and a Grant-in Aid for Scientific Research on Priority Areas –Integrative Brain Research- from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
IM, NY, TM, and AA designed the research. IM, HS, and NY performed the behavioral analysis. IM, HS, TM, and AA wrote the paper. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Matsuda, I., Shoji, H., Yamasaki, N. et al. Comprehensive behavioral phenotyping of a new Semaphorin 3 F mutant mouse. Mol Brain 9, 15 (2016). https://doi.org/10.1186/s13041-016-0196-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13041-016-0196-4