Abstract
Background
The non-invasive prenatal testing that evaluates circulating cell free DNA, and has been established as an additional pregnancy test for detecting the common fetal trisomies 21, 18 and 13 is rapidly revolutionizing prenatal screening as a result of its increased sensitivity and specificity. However, false positive and false negative results still exist.
Case presentation
We presented a case in which the non-invasive prenatal testing results were normal at 15 gestational age (GA), but an ultrasound examination at 30GA showed that the fetus had heart abnormalities, and the third trimester ultrasound at 33GA noted multiple anomalies including a 3.0 mm ventricular septal defect. Along with cordocentesis at 33GA, the cord blood sample cytogenetics analysis showed a mos 47,XN,+18[61]/46,XN[39] T18 karyotype. Six placental biopsies confirmed that the chromosome 18 placenta chimerism ratio had changed from 33% to 72%. Ultimately, the pregnancy was interrupted at 34GA.
Conclusions
We presented this case to highlight the need to clearly explain false positive or false negative results to patients. We believe that this information will also influence the development of future diagnostic test methodologies.
Similar content being viewed by others
Background
Non-invasive prenatal testing (NIPT) which was established as an additional pregnancy test for detecting of the common fetal trisomies 21 (T21), 18 (T18) and 13 (T13), is rapidly becoming a common clinical practice [1]. It evaluates circulating cell-free DNA (cfDNA) as early as 9 gestational age (GA). These DNA fragments are derived from apoptotic placental cytotrophoblast cells [2]. Cell-free fetal DNA (cffDNA) can be described quantitatively as the fetal DNA fraction, and is determined by the ratio of the absolute concentration of cffDNA to the absolute concentration of total (maternal and fetal) cfDNA [3]. NIPT has used for several years as part of prenatal care to screen high-risk patients for fetal aneuploidy, and it has been used increasingly in clinical practice. The pooled sensitivities in the selected high-risk pregnant population were 0.998 (95% CI 0.981 to 0.999), 0.977 (95% CI 0.958 to 0.987) for T21 and T18, respectively. Pooled sensitivity for T13 sensitivity was closer to 0.900. The pooled specificity in the high-risk population for trisomies 21, 18, and 13 is 0.999 (95% 0.998 to 0.999) [4, 5]. However, false positive and false negative results till exist, as cffDNA comes from apoptotic placental trophoblast cells [6]. Therefore, the results may not always represent the actual fetal karyotype in all cases. In the vast majority of pregnancies, although the genetic component between the placental and fetal tissue is identical, false positive or false negative results still exist due to confined placental mosaicism (CPM) [7, 8]. Some positive NIPT results were finally confirmed to be false positive, and common reasons include placental mosaicism, vanishing twin or cotwin demise, fetal chromosome rearrangement, and maternal chromosome abnormalities or malignancy [9, 10]. In contrast, there is a small chance of a false negative result. The fact that cffDNA in the maternal plasma fraction originates from the cytotrophoblast explains a part of the discrepancies between NIPT results and the actual fetal karyotype. A low level of cffDNA fraction in maternal plasma can also result a false negative NIPT result [11].
Herein, we presented one case of a patient whose fetus tested “negative” for T18 by NIPT but was diagnosed as mos 47,XN,+18[61]/46,XN[39]. Our report suggests that some pregnant women display regional placental mosaicism, which is sufficient to cause a discrepancy between the NIPT and karyotyping results.
Methods
The NIPT test was performed at 15 and 34GA by sequencing cfDNA from the maternal peripheral blood. Blood collection, cfDNA extraction, library construction and sequencing were performed according to the instructions of JingXin Fetal Chromosome Aneuploidy (T21, T18, T13) Testing Kits (CFDA registration permit No. 0153400300) [12].
Based on our previous study, we developed a technique that uses the read length to estimate the concentration of fetal cfDNA in maternal plasma by sequencing [12]. The fetal DNA concentration was calculated as a quality control, as described in Yin’s paper [12]. Combined GC-correction and Z-score testing methods were used to identify fetal autosomal aneuploidy for trisomy as described in Liao’s paper [13]. Z score range from −3 to 3 was considered to indicate a low risk for a trisomy chromosome [14]. A cord blood sample was taken at 33GA. A cord blood sample and six placental biopsies (three from the maternal side and three from the fetal side) were taken for analysis. DNA sequencing, cfDNA extraction, library construction and sequencing were performed through bioconductor sequencing platform [12]. The speculated chimeric proportion of T18 was calculated from the ratio of the samples to the control. For example, for sample 1, the speculated chimeric proportion = 2*(1–3.327%/2.856%) =33%.
Case presentation
A 32-year-old healthy pregnant woman was referred to the Medical Genetic Centre of Guangdong Women and Children Hospital. Maternal serum screening at 12GA combined nuchal translucency measurement (2.4 mm) showed a high risk of fetal T21 at 1 in 190. The NIPT at 15GA showed that the fetal DNA fraction in the maternal plasma sample was 7.8% (normal NIPT result) and the Chromosome18 Z scores were 1.889 (Table 1). NIPT provided at 15GA gave a low risk result.
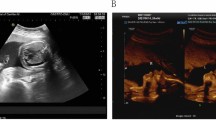
As routine practice, ultrasound was conducted to monitor the developmental status of the fetus. The ultrasound examination at 24GA showed that the fetus displayed a defect in the ventricular compartment, which was confirmed by the ultrasound examination at 30 GA (Figure 1A). The ultrasound examination showed a 2.2 mm ventricular septal defect. At 33GA (Figure 1B), the patient was referred for further evaluation after a third trimester ultrasound revealed a 3.0 mm ventricular septal defect, and all four limbs were smaller than is observed at normal gestational weeks. For further counseling, the patient consented to have a cord blood sample taken by cordocentesis at 33GA. Cytogenetics analyses reported a karyotyping of mos 47,XN,+18[61]/46,XN[39] indicated that 61% of cells had trisomy 18 even though both parental karyotypes were normal. After genetic counseling and communicating with families, the pregnant woman opted to terminate her pregnancy at 34GA.
Ultrasound examination images. a Ultrasound examination result at 30wk. A ventricular septal defect for 2.2 mm was shown as the arrow in the image. Abbreviations: LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; VSD, ventricular septal defect. b Ultrasound examination result at 33wk. A ventricular septal defect (VSD) for 3.0 mm was shown as the arrow in the image
Maternal peripheral blood was collected for a confirmatory NIPT test before the pregnancy was interrupted at 34GA. Placental tissues were also retained. Six placental biopsies (three from the maternal side and three from the fetal side) were taken for sequencing. Placental biopsies confirmed that it was placental chimerism of chromosome18, with a chimeric ratio from 33% to 72% (Table 2). The chimeric ratios of the placental biopsies were consistently around 61%, which was the T18 mosaicism fetal umbilical cord blood type result. The NIPT reported Z-scores were −1.491, 5.500 and −0.016 for chromosome21, 18 and 13 respectily (Table 1), and the fetal fraction was 16.6%. These results confirmed that the fetus displayed T18 mosaicism, which indicated a false negative result for NIPT at 15GA.
Discussion and conclusions
Increasing amounts of evidence have shown that the circulating fetal DNA in maternal blood during pregnancy mainly originates from placental trophoblastic cells, although there are still small contributions from fetal tissues [2]. Since cfDNA was identified, it has been widely promoted for the development of NIPT [15]. However, many factors can still affect NIPT results; for example, the maternal weight can significantly decrease the fetal DNA fraction [16], which usually leads to a false negative result. Otherwise, 0.8–1% of cases are confirmed as placental mosaicism according to a large-scale evaluation of chorionic villi sampling (CVS) [17]. Placental mosaicism means that the cffDNA from cytotrophoblast cells has a different karyotype than that of the true fetal DNA [18]. Thus, in this study, we provided a false negative NIPT case caused by both fetal and placental mosaicism. Clinicians should be aware of this situation and patients should be informed of the possibility of discordant NIPT results.
In this case, of placental mosaicism, the fetal DNA fraction in the maternal plasma sample at 15GA was 7.8% (normal NIPT result) with a chromosome18 Z-score of 1.889. Examination of the placental tissue at six sites showed that T18 mosaicism, as measured by the ratio of trisomy to disomy 18, averaged approximately 42%. Further, the chimeric ratio varied from 32% to 72% at different sites, which suggested that there were significant regional variations in the T18 mosaicism in this placenta. However, this was consistent with the fetal umbilical cord blood karyotype results, which showed a T18 mosaicism ratio of 61%. Furthermore, at 15GA, the fetal DNA percentage was 7.8% due to the mosaicism, and the effective/perceived fetal fraction observed by the algorithm on the trisomy chromosome was (100%–42%)*7.8% = 4.52%. However, a trisomy with an actual fetal fraction of 4.52% is mathematically insufficient to detect the 42% placental T18 when using our NIPT test. The fetal DNA concentration increases with gestational weeks [19]. At 35GA, the increased fetal DNA fraction was sufficient to detect the mosaicism. Similar patterns of mosaicism were also reported in cases with false negative cfDNA screening results [8, 9, 20].
This report describes a case of a false negative cell-free DNA result for trisomy 18 due to fetal and placental mosaicism. To date, few NIPT reports have demonstrated that placental mosaicism [14, 21, 22] manifests quite differently across individual pregnancies in pregnant women. The existence of false negatives due to mosaicism is not unique, and information pertaining to these cases is still limited. Multicenter studies have determined the frequency of mosaicism to be approximately 1% [23].In most situations, the mosaic cell line is only found in the placenta and will lead to a normal fetal outcome [24]. However the frequency of false negative fetal test results with maternal serum cffDNA testing due to placental mosaicism or other fetal-placental discrepancies is still unknown [7]. How should unexpected false negative NIPT results be handled in clinical practice? On the one hand, it is necessary that patients receive pretest counseling and informed consent prior to NIPT screening. Patients should be aware of the potential for false positive and false negative results as well as discordant results due to differences between the fetally and parentally derived analyzed samples [7]. We hope to give clinicians a reference with this case. Clinicians should be cognizant of false negative results when the fetal DNA concentrations are relatively low. In contrast, as the frequency and level of various types of mosaicism are still limited, new and accurate documents are valuable to the diagnostic and medical community. It is necessary for the NIPT field to improve its sensitivity and specificity and reduce the incidence of discordant results. This information can not only contribute to the development of future screening test methodologies and algorithms, but can also help optimize the counseling and medical decision applied by medical practitioners.
Abbreviations
- cfDNA:
-
Cell free DNA
- cffDNA:
-
Cell-free fetal DNA
- CPM:
-
Confined placental mosaicism
- CVS:
-
Chorionic villi sampling
- GA:
-
Gestational age
- NIPT:
-
Non-invasive prenatal testing
- T18:
-
Trisomy 18
References
Norwitz ER. Noninvasive prenatal testing: the future is now. Rev Obstet Gynecol. 2013;6(2):48–62.
Liao GJW, Gronowski AM, Zhao Z. Non-invasive prenatal testing using cell-free fetal DNA in maternal circulation. Clin Chim Acta. 2014;428:44–50. doi: 10.1016/j.cca.2013.10.007.
Bissell MG. Noninvasive prenatal diagnosis of fetal chromosomal Aneuploidies by maternal plasma nucleic acid analysis. Yearb Pathol Lab Med. 2009;2009:301–3. doi: 10.1016/s1077-9108(08)79012-1.
Kim S, Zhao C, Bombard AT, et al. Comment on “clinical application of massively parallel sequencing-based prenatal non-invasive fetal trisomy test for trisomies 21 and 18 in 11 105 pregnancies with mixed risk factors”. Prenat Diagn. 2013;33(13):1310–3. doi: 10.1002/pd.4231.
Iwarsson E, Jacobsson B, Dagerhamn J, et al. Analysis of cell-free fetal DNA in maternal blood for detection of trisomy 21, 18 and 13 in a general pregnant population and in a high risk population - a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2017;96(1):7–18. doi: 10.1111/aogs.13047.
Chen EZ, Chiu RWK, Sun H, et al. Noninvasive prenatal diagnosis of fetal Trisomy 18 and Trisomy 13 by maternal plasma DNA sequencing. PLoS One. 2011;6(7):e21791. doi:10.1371/journal.pone.0021791.g001.
Hall AL, Drendel HM, Verbrugge JL, et al. Positive cell-free fetal DNA testing for trisomy 13 reveals confined placental mosaicism. Genet Med. 2013;15(9):729–32. doi: 10.1038/gim.2013.26.
Wang Y, Zhu J, Chen Y, et al. Two cases of placental T21 mosaicism: challenging the detection limits of non-invasive prenatal testing. Prenat Diagn. 2013;33(12):1207–10. doi: 10.1002/pd.4212.
Gao Y, Stejskal D, Jiang F, et al. False-negative trisomy 18 non-invasive prenatal test result due to 48,XXX,+18 placental mosaicism. Ultrasound Obstet Gynecol. 2014;43(4):477–8. doi: 10.1002/uog.13240.
Osborne CM, Hardisty E, Devers P, et al. Discordant noninvasive prenatal testing results in a patient subsequently diagnosed with metastatic disease. Prenat Diagn. 2013;33(6):609–11. doi: 10.1002/pd.4100.
Smith M, Lewis KM, Holmes A, et al. A case of false negative NIPT for down syndrome-lessons learned. Case Rep Genet. 2014;2014:1–3. doi: 10.1155/2014/823504.
A-h Y, C-f P, Zhao X, et al. Noninvasive detection of fetal subchromosomal abnormalities by semiconductor sequencing of maternal plasma DNA. Proc Natl Acad Sci. 2015;112(47):14670–5. doi: 10.1073/pnas.1518151112.
Liao C, Yin A, Cf P, et al. Noninvasive prenatal diagnosis of common aneuploidies by semiconductor sequencing. Proc Natl Acad Sci. 2014;111(20):7415–20. doi: 10.1073/pnas.1321997111.
Chiu RWK, Akolekar R, Zheng YWL, et al. Non-invasive prenatal assessment of trisomy 21 by multiplexed maternal plasma DNA sequencing: large scale validity study. BMJ. 2011;342(jan11 1):c7401. doi: 10.1136/bmj.c7401.
Langlois S, Brock J-A, Douglas Wilson R, et al. Current status in non-invasive prenatal detection of down syndrome, Trisomy 18, and Trisomy 13 using cell-free DNA in maternal plasma. J Obstet Gynaecol Can. 2013;35(2):177–81. doi: 10.1016/s1701-2163(15)31025-2.
Canick JA, Palomaki GE, Kloza EM, et al. The impact of maternal plasma DNA fetal fraction on next generation sequencing tests for common fetal aneuploidies. Prenat Diagn. 2013;33(7):667–74. doi: 10.1002/pd.4126.
Ledbetter DH, Zachary JM, Simpson JL, Golbus MS, Pergament E, Jackson L, Mahoney MJ, Desnick RJ, Schulman J, Copeland KL, et al. Cytogenetic results from the U.S. collaborative study on CVS. Prenat Diagn. 1992;12(5):317–45.
Flori E, Doray B, Gautier E, Kohler M, Ernault P, Flori J, Costa JM. Circulating cell-free fetal DNA in maternal serum appears to originate from cyto- and syncytio-trophoblastic cells. Case Rep Hum Reprod. 2004;19(3):723–4.
Hu H, Liu H, Peng C, Deng T, Fu X, Chung C, Zhang E, Lu C, Zhang K, Liang Z, Yang Y. Clinical experience of non-invasive prenatal chromosomal Aneuploidy testing in 190,277 patient samples. Curr Mol Med. 2016;16(8):759–66.
Pan Q, Sun B, Huang X, Jing X, Liu H, Jiang F, Zhou J, Lin M, Yue H, Hu P, Ning Y. A prenatal case with discrepant findings between non-invasive prenatal testing and fetal genetic testings. Mol Cytogenet. 2014;7(1):48.
Choi H, Lau TK, Jiang FM, et al. Fetal aneuploidy screening by maternal plasma DNA sequencing: ‘false positive’ due to confined placental mosaicism. Prenat Diagn. 2013;33(2):198–200. doi: 10.1002/pd.4024.
Pan M, Li FT, Li Y, et al. Discordant results between fetal karyotyping and non-invasive prenatal testing by maternal plasma sequencing in a case of uniparental disomy 21 due to trisomic rescue. Prenat Diagn. 2013;33(6):598–601. doi: 10.1002/pd.4069.
Lebo RV, Novak RW, Wolfe K, et al. Discordant circulating fetal DNA and subsequent cytogenetics reveal false negative, placental mosaic, and fetal mosaic cfDNA genotypes. J Transl Med. 2015;13(1). doi:10.1186/s12967-015-0569-y.
Mardy A, Wapner RJ. Confined placental mosaicism and its impact on confirmation of NIPT results. Am J Med Genet C: Semin Med Genet. 2016;172(2):118–22. doi: 10.1002/ajmg.c.31505.
Acknowledgements
Thanks to the CapitalBio Genomics Co., Ltd. and Wei Lei for data analysis and writing help.
Funding
National Key Research and Development Program of China, 2016YFC1000700, 2016YFC1000703.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
All authors have materially participated in the study and manuscript preparation. Jiexia Yang and Yiming Qi carried out all the molecular genetic analysis, and paticipated in the design of the work; Fangfang Guo and Yaping Hou collected all clininal data and participated in conceiving the work; Dongmei Wang and Haoxin OY participated in conceiving the work, and revising the manuscript. Aihua Yin designed the work, drafted and revised the manuscript. All authors have approved the final article.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed with the approval of Medical Ethics Committee of Guangdong Women and Children Hospital, and written informed consent was obtained from the patient.
Consent for publication
The patient in this case report had provided her consent for publication.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Yang, J., Qi, Y., Guo, F. et al. A case of placental trisomy 18 mosaicism causing a false negative NIPT result. Mol Cytogenet 10, 40 (2017). https://doi.org/10.1186/s13039-017-0341-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13039-017-0341-5