Abstract
Background
The Cerrado is the second largest biome in Brazil and the most biodiverse tropical savannah in the world and acts as a great sequester of atmospheric carbon. The lack of studies related to the quantification of its total biomass compromises the understanding of the dynamics of CO2 in this biome. Thus, it is relevant to develop studies aiming at obtaining accurate estimates of the carbon stock in the different phytophysiognomies that make the Cerrado, to include them in appropriate forest management models. Based on the hypothesis that the amount of carbon stored can vary according to the vegetation typology and vegetation compartments, the aerial stock of dry biomass and carbon were estimated in different compartments (arboreal, herbaceous-shrub and litter). The study was developed in open Brazilian savannah and soils on the sandstone and discussed the effect of fire on this phytophysiognomy. For the arboreal compartment were adjusted mathematical models to fit the biomass equations to estimate the individual stock of the trees in this compartment. The results of the stocks were discussed considering the effect of fire on the phytophysiognomy.
Results
Based on the precision and extra distribution measures, the Schumacher-Hall (non-logarithmic) equation presented better results to estimate the individual biomass and carbon stocks of the open Brazilian savannah trees. The aboveground biomass was 12.88 Mg ha−1, corresponding to a total carbon stock of 5.91 Mg ha−1, where most of the stocks are in the herbaceous-shrub compartment (44%). The arboreal compartment accounts for the smallest part of the stocks, followed by the litter.
Conclusions
The observed values are in the interval verified for other areas of savannah studied in Brazil. The values verified for the open Brazilian savannah in sandy soils are at the lower limit of this range, due to the nutrient-poor nature of this type of soil. The distribution of stocks in the different compartments above the ground points to the fragility of this environment to the random fire effect, common in the region. That shows the need for conservation measures for vegetation maintenance and soil protection to preserve adequate nutrient cycling in the ecosystem.
Similar content being viewed by others
Background
Topics that seek to understand the causes and effects of global climate change, as well as the decision-making necessary for the proper management of natural resources, permeate the current scientific discussions. Above all, because the increase in concentrations of atmospheric carbon dioxide and its direct effect on climate variability leads to uncertainties about its influence on the quality of human life on the planet [11]. In Brazil, the conversion of forested areas to other forms of land use accounts for about 77% of carbon dioxide emissions in the atmosphere [4]. This data demonstrates the need to know and conserve remaining forest ecosystems, which, besides providing this environmental service, offer other indispensable ones. So, there are few studies on carbon stock and dynamics in areas of Cerrado [28, 29].
In the case of the Cerrado biome, the change in land use intensified in the 1970s due to the expansion of the agricultural frontier in this biome, which presents favorable relief and soil conditions for mechanization processes of agricultural production, besides low land prices [3, 13]. Still, according to these authors, the deforestation also occurred in an unsustainable way for the production of coal. In this context, in the last 40 years, about 50% of the Cerrado biome has been completely deforested and converted to fullanthropic uses [5]. The degradation of this biome occurred more intensely in the western region of Bahia state, especially in the São Francisco River basin, being this the region that more evolves on the conversion of vegetated areas in the biome [5].
The Cerrado is the second largest biome in Brazil and the most biodiverse tropical savannah in the world [19], it acts as a great sequester of atmospheric carbon [14, 15, 34]. The lack of studies related to the quantification of its total biomass compromises the understanding of the dynamics of CO2 in this biome [28]. Thus, it is relevant to develop studies aiming at obtaining accurate estimates of the carbon stock in the different phytophysiognomies that compose the Cerrado, to include them in appropriate forest management models for this biome considering its different phytophysiognomies [31, 33].
Based on the hypothesis that the amount of carbon stored can vary according to the vegetation typology, this work had the aim to quantify the total above ground stocks of dry biomass and carbon for the arboreal, herbaceous-shrub and litter compartments in an open Brazilian savannah (OBS) phytophysiognomy of the Cerrado biome with occurrence in soils of medium to the sandy texture from the sandstone.
Methods
Study area
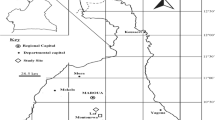
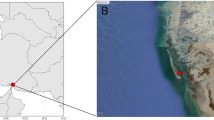
The study was developed in a continuous area of the Cerrado biome of approximately 9570 ha in the central region of Brazil (boundaries of the states of Bahia, Minas Gerais, and Goiás) (Fig. 1). The regional climate is Aw, according to the classification of Köppen, characterized a tropical dry winter, presenting a mean annual temperature of 24 °C and mean annual rainfall of 870 mm [9]. The altitude is approximately 850 m, and the relief is flat.
The area is located in the region of occurrence of the plateau that forms the Urucuia Formation [8], in this lithological substrate, quartz sandstones of varied colors is predominant, whose granulometry varies from thin to medium. The developed soils of these sandstones present a sandy loam texture, dominated by Oxisols and Quartzipsamments [35].
The area is inserted in the Cerrado biome, being almost covered by native vegetation, with a great diversity of environments, including different phytophysiognomies of the savannahs [25]. The OBS, a phytophysiognomy chosen for the study, covers about 1022 ha, which represents 10.7% of the total area of this continuum biome.
The study was conducted in an area < 30 ha covered by open Brazilian savannah. The area was defined based on the real representativeness of the phytophysiognomy and not being designated as a Conservation Unit, Legal Reserve or Permanent Preservation Area, making it possible to get authorization from the competent environmental agency for the suppression of trees, which were used for the destructive sampling of biomass quantification. In this area of 30 ha, the forest inventory was first made to know the floristic composition and the structure of the tree component of the open Brazilian savannah. For this, 20 plots of 1000 m2 (20 m × 50 m) were allocated randomly in the research area. In each of the plots were collected the data of diameter and a total height of the trees that met the criterion of inclusion of the diameter measured at 30 cm from the soil (d30cm) equal to or greater than 5 cm (d30cm ≥ 5 cm), as recommended by Felfili et al. [12]. The trees measured had the botanical material collected to identify the botanical species present.
Sampling of the arboreal, herbaceous-shrub and litter compartment
The biomass stored in the studied physiognomy was quantified by vegetation compartment (arboreal, herbaceous-shrub, and litter). For the sampling of the arboreal compartment were considered the woody individuals (trees) with a diameter at 30 cm from the ground level (d30cm) ≥ 5 cm. According to data from the forest inventory carried out in the area, 60 trees were chosen to compose the sample of trees that were cut for quantification of the biomass by a destructive method for later adjustment of the biomass equations. The selection of these trees was made according to the diametric structure of the studied open Brazilian savannah and the species composition, being sampled those of higher importance value index (IVI), as presented in Oliveira [24]. Later they were cut to 30 cm of the ground, and the total height of the tree was measured. Once the diametric measurements were taken, its components (leaves, thin branches, thick branches, trunk, flowers, and fruits) were weighed. After weighing, samples of each component were collected and conditioned in plastic bags to get the weight of dry matter in the laboratory. From the trunks, disks were removed at the 0.30 m positions, at half the total height, and at the total height, whose samples were dried in a forced circulation oven, with a temperature around 70 °C, until obtaining a constant mass.
From the data obtained from each tree (weight), statistical models were adjusted for the estimation of dry biomass per tree (non-destructive), as a function of the variables diameter (d30cm) and total height (ht), as well as their combinations. For this, different linear and non-linear models were tested according to Scolforo et al. [37].
The linear models were adjusted using regression analysis with the ordinary least squares method to estimate the parameters [36]. The non-linear models were adjusted by iterations. The data were tested for the assumptions of normality and homogeneity, using the Shapiro–Wilk test (α = 0.05) and f-test (α = 0.05). Graphical analysis was performed to verify the correlation between the dependent and independent variables. The choice of the best model was based on the adjusted coefficient of determination (R2adjust.), on the significance of the coefficients at 95% probability, on the standard error of estimate in percentage terms (Sxy%), and the graphical distribution of the residues. For the models with the transformed dependent variable, the residual standard error was corrected in the original scale of the dependent variable, according to Scolforo [36]. The analysis of the difference between the values observed and those estimated by the chosen equation was performed by paired t-test (α = 0.05).
For the herbaceous-shrub compartment sampling, woody individuals with d30cm < 5 cm and total ht < 1 m, and undergrowth, including small herbs, grasses, and palms (Syagrus petrea) were distributed in the area. Sampling for quantification of the biomass in this compartment was performed using 20 fixed sub-plots of 4 m2 (2 m × 2 m). The 4 m2 subplots were allocated in the center of the largest parcels of forest inventory (20 m × 50 m). In each of the 20 subplots, all the wet material was separated into the components and weighed (leaves, twigs and grasses, branches and grasses). Samples of each component were taken to get moisture contents and dry matter weight in the laboratory. A sampling of the biomass present in the litter was carried out by 20 fixed subplots of 1 m2 (1 m × 1 m). These subplots were also allocated within the larger parcels of the forest inventory. The procedure was to allocate the subplot in one of the four corners of the largest part of the forest inventory. In each of the 20 subplots, all the wet material present was weighed, and one sample was collected to get moisture content and dry matter weight in the laboratory.
Estimation of stocks of dry biomass and carbon
For the herbaceous-shrub and litter compartments, the biomass values obtained in the field were converted into dry biomass quantity per hectare from the moisture content determined in the laboratory. The chosen biomass equation was applied to the data collected in the forest inventory to estimate the dry biomass stock of the arboreal compartment. This allowed estimating the stocks per hectare, as well by individuals, species and diametric classes of the Cerrado compartment. Estimates for each compartment were made by confidence interval at 95% probability (α = 0.05). For the arboreal and herbaceous-shrub compartments, the total dry biomass was converted to carbon considering the proportion of 47% [18]. For the litter, the carbon concentration was considered 44.36% [22].
Results and discussion
Adjustment of models to estimate dry biomass and carbon stocks of individual trees
The sample for quantification of the biomass contained in the arboreal compartment of the studied physiognomy, OBS, counted with 60 trees, distributed in five diametric classes (Table 1) and among eight different species: Kielmeyera coriacea (17), Pouteria ramiflora (15), Kielmeyera petiolaris (8), Hancornia speciosa (7), Pouteria torta (5), Eugenia dysenterica (2), Vochysia tucanorum (3), Palicourea rigida (3).
All models adjusted were significant (p < 0.05) (Table 2). The R2adjust. ranged from 71.20 to 94.55%, which represents a high correlation between the dependent variables (biomass and independent variables (d30cm and ht).
The Sxy% varied from 30.50 to 72.89%, demonstrating that the pattern of high heterogeneity of Cerrado samples [10, 31, 38], which is confirmed by the dendrometric characteristics and the stocks per plant compartment of the trees sampled in the OBS to adjust of the models (Table 3). In this table it is observed that the Schumacher-Hall model (non-logarithmic) presented the best measures of precision and also satisfactory residual distribution, indicating trend-free estimates. Thus, it is the model chosen to estimate the individual biomass and, so carbon stocks, of the studied phytophysiognomy trees, since there was no significant difference between the values observed and estimated by the equation when compared by the paired t-test (α = 0.05):
Biomass and carbon stocks in the arboreal compartment
The biomass stock in the arboreal compartment was 3.5 Mg ha−1 ([2.4 Mg ha−1 ≤ μ ≤ 4.6 Mg ha−1] = 0.95), corresponding to a stock carbon mean of 1.7 Mg ha−1 ([1.1 Mg ha−1 ≤ μ ≤ 2.2 Mg ha−1] = 0.95) in this compartment (Table 4). In nine localities of OBS, Ottmar et al. [27] verified dry biomass stock ranging from 3.31 to 29.84 t ha−1. The lowest value (3.31 t ha−1) was observed in an OBS sampled in the Grande Sertão Veredas National Park area (close to the area of this study), corroborating with the result found in this study, which presents a mean of 3.5 t ha−1 of dry biomass stored in its arboreal compartment. According to Castro and Kauffmam [7], total aboveground biomass (including trees, shrubs, undergrowth, and litter) tends to be smaller as vegetation tends to be less dense. Thus, for this same area, Oliveira et al. [26] found values of dry biomass of 10.9 Mg ha−1, for the arboreal component in the typical savannah.
The sampling error for the biomass and carbon variables in the studied physiognomy was higher than 20% (Table 4), indicating that for more efficient sampling of these variables it is necessary to allocate more plots in the field. The high variability and the high sampling error were due to the presence of a plot in which 108 individuals were sampled, giving density much higher than the mean density calculated per plot, which was 55 ind. 0.1 ha−1 on this physiognomy.
About the contribution of species, it was observed that four species accounted for more than 50% of biomass and carbon stocks, being Kielmeyera coriaceae, H. speciosa, P. ramiflora, and K. petiolaris, which were also responsible for the higher IVI in this savannah (Table 5). Salvertia convallareiodora, represented by only three individuals inventoried presented significant stocks due to the presence of a large individual, responsible for the largest diameter recorded.
By relating the biomass production and, so carbon, with the diametric structure of the communities, there was an increase from the first to the second class, from which there was a decrease to the penultimate class. The first three classes account for more than 60% of the biomass and carbon stored in the arboreal component, with the most stock in the second diametric class, presenting 0.9 t ha−1, which corresponds to 28.55% of the total stock of this compartment in this physiognomy.
Although in the higher diametric classes are the largest individual stock, the high number of individuals in the lower classes makes the stocks per hectare higher in this range, revealing that small individuals are an important part for the maintenance of biomass and carbon stocks, as well as the functioning of this ecosystem.
Dry biomass and carbon stocks of the herbaceous-shrub component
The dry biomass mean stock was 5.63 Mg ha−1 ([5.00 Mg ha−1 ≤ µ ≤ 6.28 Mg ha−1] = 0.95), corresponding to a mean stock of carbon of 2.64 Mg ha1 ([2.35 Mg ha−1 ≤ µ ≤ 2.95 Mg ha−1] = 0.95). About the stocks by components of the compartment studied, grasses account for about 96% of the dry biomass and carbon stocks (Table 6).
Ottmar et al. [27] observed biomass of grasses varying from 1.76 to 9.95 Mg ha−1 for nine different locations studied in the OBS, while for typical Brazilian savannah the values ranged from 1.11 to 1.69 Mg ha−1. According to Ribeiro and Walter [32], the OBS represents the lowest and least dense form of the typical Brazilian savannah, where the shrub and herb layer is the most prominent when compared to the dense and typical subtypes, especially the grassy cover.
In the study area, the presence of woody shrubs is almost absent, the grassy layer is predominant, where Syagrus petraea individuals are widely distributed. The presence of natural regeneration individuals also occurred in the sampled plots, with individuals of Kielmeyera coreaceae, P. ramiflora, Connarus suberosus, and Palicourea rígida. According to Silva [39], the grassy layer is characterized by the presence of Echinolaena inflexa, Paspalum gardnerianum, Paspalum polyphyllum, and Eragrostis sp.
Dry biomass and carbon stocks of the litter
In the studied physiognomy a dry biomass mean of 3.75 Mg ha−1 ([2.80 Mg ha−1 ≤ µ ≤ 4.70 Mg ha−1] = 0.95) was stored in the litter, which corresponds to a carbon stock mean of 1.66 Mg ha−1 ([1.24 Mg ha−1 ≤ μ ≤ 2.08 Mg ha−1] = 0.95) in this compartment (Table 7).
When quantifying the dry biomass stocks stored in the litter of a typical Brazilian savannah in the sub-basin of the São Francisco River, in the north of Minas Gerais State, Morais [22] found higher values, on mean, 6.24 Mg ha−1 of dry biomass stored in the litter, which corresponds to a mean stock of carbon of 3.05 Mg ha−1. But, among the five fragments studied by Morais [22], one presented values very close to this study, with means values of dry biomass and carbon present in the litter of 3.17 and 1.04 Mg ha−1, respectively.
Biomass and carbon total stocks
The total aboveground biomass of the study area was 12.88 Mg ha−1, corresponding to the total stock of 5.91 Mg ha−1 of carbon. The biomass estimation methodologies are very varied in the existing studies; few have tried to quantify the stocks in the different compartments. In studies that considered total aboveground biomass (arboreal, herbaceous-shrub and litter compartments) in typical Brazilian savannah values ranged from 12.55 to 76.63 Mg ha−1 [1, 7, 27, 33].
According to Castro and Kauffman [7], the total aboveground biomass (including trees, herbaceous-shrub layer and litter) tends to be larger as vegetation tends to be denser, and found that biomass increased along the gradient of 5.5 Mg ha−1 in the napeadic grassland (“campo limpo”) for 29.4 Mg ha−1 in dense savannah. The values presented in this study are within this limit of variation, tending to the lower limit, since the OBS has more open vegetation, with spaced and lower trees, which will reflect less biomass per area.
The largest stock of total aboveground biomass is in the herbaceous-shrub component, which represents 44.13% of the total, of which 42% are grasses (Fig. 2). The lowest stock is in the arboreal compartment, representing 27% of the total (Fig. 2).
Environmental fire considerations
The highest biomass stock in the herbaceous-shrub compartment of the open Brazilian savannah shows a high fragility of this environment about the fire. Because the predominance of this gramineous stratum in this environment of dry climate with a high risk of burning, when under the effect of fire, burns easily, releasing carbon which changes the nutritional dynamics of the ecosystem [42]. According to Pivello and Coutinho [30], on mean, 95% of the nitrogen and between 42 and 59% of the phosphorus, potassium, calcium, magnesium, and sulfur contained in the biomass of the herbaceous-shrub vegetation of Savannah are released into the atmosphere after the burn, requiring a least interval of 3 years between successive fires to promote cycling without affecting the nutritional balance of the ecosystem.
The litter, responsible for 29% of the biomass stock in the OBS, is also a sensitive material to the fire activity in these natural open environments, especially in the sandy soil areas. Castro and Kauffman [7] observed that litter combustion efficiency is higher in open environments where solar radiation is higher. According to Miranda and Sato [20], the presence of trees and shrubs in the more closed savannah formations influence the local microclimate, changing the characteristics of the fire.
The high frequency of burning in these environments reduces the recruitment of arboreal individuals, favoring the establishment of herbaceous vegetation by reducing the density of trees in the ecosystem [23]. Thus, the indiscriminate action of fire over time can change the dynamics of the tree compartment, decreasing the diversity and number of trees in the ecosystem. Furthermore, the negative impacts on the arboreal compartment in the savannah caused by the indiscriminate fire effect and unsustainable management also reflect the biomass and carbon stocks of the roots and other subterranean organisms, since in this case there is a substitution of a complex and deep root system to another homogeneous and superficial. Concurrent to the reduction of the density of arboreal individuals, there is also the change in the litter stock, due to its strong relationship with the presence of the arboreal layer. Werneck et al. [41], observed that the quantitative differences in litter production in three parts of a deciduous forest occurred due to the structural differences and consequent formation of a more developed canopy.
The litter comprises the compartment responsible for the connection between the aerial and underground matrices, behaving as a largesource of nutrients and carbon in the soil, usually dystrophic in Cerrado biome [16]. It is also known that inadequate management of arboreal vegetation can lead to soil degradation, and more in environments such as these, where soils with high levels of sand and naturally fragile, predominate [40]. Thus, the main carbon drain in the biome, the soil, can be highly altered, whose conservation, as well as the carbon storage capacity, is related to the presence of the arboreal vegetation, occurring, according to Aduan et al. [2], stocks under the trees.
When evaluating the dynamics of the arboreal compartment in typical savannah areas sampled in the Cerrado biome, Miranda [21] observed that areas sampled in conservation units showed differences in the stocks between measurements. In an area with frequent fire, the biomass decreased over time, while in another area that does not experience the fire, there was an increase in biomass, corroborating the fact that the indiscriminate fire changes the structure and stocks in Savannah [21]. Areas where fires occur at least intervals of 5 years, biomass has increased over time. The mean rate of increase in carbon was 0.13 t ha−1 year−1 [21].
According to Bustamante and Oliveira [6], floristic and structural changes of the vegetation caused by fire, concomitant with the increase of the herbaceous-gramineous vegetation, increase the risk of new fires, besides reducing the albedo and the roughness of the vegetation. These changes are, on a larger scale, responsible for increases in temperature and flows of chemical elements to the atmosphere and reductions in rainfall, which in turn increase the chances of new fires are occurring. According to Hoffman and Jackson [17], physical changes in savannah vegetation can reduce evapotranspiration and heat flow into the atmosphere, reducing rainfall and increasing the frequency and intensity of summer. Thus, the intense anthropic pressure added to the natural fragility of the sandy soils of this region and to the particularity of the environmental attributes of this phytophysiognomy, point out the immediate necessity of adopting conservationist measures that promote the sustainable use of these environments, avoiding the indiscriminate conversion of the areas of remaining natural vegetation, causing high environmental damage.
Conclusions
Based on the precision and residual distribution measures, the Schumacher-Hall (non-logarithmic) equation presented better results to estimate the individual biomass of OBS trees. Total aboveground biomass considering all compartments above the ground was 12.88 Mg ha−1, corresponding to a total stock of 5.91 Mg ha−1 of carbon. The arboreal compartment represents the smallest part of the stocks, and the grasses predominate storing a large amount of biomass and carbon.
The observed values are in the interval verified for other areas OBS, showing that the values verified for savannah influenced by the sandstones (Urucuia Formation), under Quartzipsamments and Oxisols, are at the lower limit of this range, due to the nutrient-poor nature of these classes of soils.
The distribution of stocks in the different compartments above the ground points to the fragility of this environment to the random fire effect, common in the region. That shows the need for conservation measures for vegetation maintenance and soil protection to preserve adequate nutrient cycling in the ecosystem.
Abbreviations
- OBS:
-
open Brazilian savannah
- D30cm :
-
diameter at 30 cm from the ground level
- D1.30m :
-
diameter at 1.30 m from the ground level
- IVI:
-
importance value index
- h:
-
total height
- R2 adjust. :
-
adjusted coefficient of determination
- Sxy%:
-
standard error of estimate in percentage terms
References
Abdala GC, Caldas LS, Haridasan M, Eiten G. Above and belowground organic matter and root: shoot ratio in a Cerrado in central Brazil. Braz J Ecol. 1998;2:11–23.
Aduan RE, Vilela MF, Klink CA. Ciclagem de carbono em Ecossistemas Terrestres – O Caso do Cerrado Brasileiro. Planaltina: Embrapa Cerrados; 2003. p. 1–30.
Beuchle R, Grecchi RC, Shimabukuro YE, Seliger R, Eva HD, Sano E, Achard F. Land cover changes in the Brazilian Cerrado and Caatinga biomes from 1990 to 2010 based on a systematic remote sensing sampling approach. Appl Geogr. 2015;58:116–27.
BRASIL. Segunda Comunicação Nacional do Brasil à Convenção-Quadro das Nações Unidas sobre Mudança do Clima. Brasília: Coordenação-Geral de Mudanças Globais do Clima, Ministério da Ciência e Tecnologia; 2010.
Brasil. Ministério do Meio Ambiente. Mapeamento do uso e cobertura do Cerrado. Projeto TerraClass Cerrado. Brasília: MMA; 2015.
Bustamante MMC, Oliveira EL. Impacto das Atividades Agrícolas, Florestais e Pecuárias nos Recursos Naturais. In: Faleiro FG, Farias Neto AL, editors. Savanas: desafios e estratégias para o equilíbrio entre sociedade, agronegócio e recursos naturais. Planaltina: EMBRAPA Cerrados; 2008. p. 647–69.
Castro EA, Kauffman JB. Ecosystem structure in the Brazilian Cerrado: a vegetation gradient of aboveground biomass, root mass and consumption by fire. J Trop Ecol. 1998;14:263–83.
Castro KBD, Martins ÉDS, Gomes MP, Reatto A, Passo DP, Lima LADS, Gomes RAT. Caracterização Geomorfológica do Município de Jaborandi, Oeste baiano, Escala 1:100,000. Planaltina: EMBRAPA Cerrados; 2010.
Climate-Date. Clima de Jaborandi. 2018. https://pt.climate-data.org/america-do-sul/brasil/bahia/jaborandi-312741/. Accessed 20 Nov 2018.
Delliti WB, Meguro M, Pausas JG. Biomass and mineral mass estimates in a “cerrado” ecosystem. Rev Bras Bot. 2006;29:531–40.
Dietz T, Frank KA, Whitley CT, Kelly J, Kelly R. Political influences on greenhouse gas emissions from US states. Proc Natl Acad Sci USA. 2015;112:8254–9.
Felfili JM, Carvalho FA, Haidar RF. Manual para o monitoramento das parcelas permanentes no biomas Cerrado e Pantanal. Brasília: Universidade de Brasília, Departamento de Engenharia Florestal; 2005.
Françoso RD, Brandão R, Nogueira CC, Salmona YB, Machado RB, Colli GR. Habitat loss and the effectiveness of protected areas in the Cerrado Biodiversity Hotspot. Nat Conserv. 2015;13:35–40.
Gmach MR, Dias BO, Silva CA, Nóbrega JC, Lustosa-Filho JF, Siqueira-Neto M. Soil organic matter dynamics and land-use change on Oxisols in the Cerrado, Brazil. Geoderma Reg. 2018;14:e00178.
Gomes LC, Faria RM, de Souza E, Veloso GV, Schaefer CEG, Fernandes Filho EI. Modelling and mapping soil organic carbon stocks in Brazil. Geoderma. 2019;340:337–50.
Haridasan M. Observations on soils, foliar nutrient concentration and floristic composition of cerrado sensu stricto and cerradão communities in central Brazil. In: Furley PA, Proctor J, Ratter JA, editors. Nature and dynamics of forest-savanna boundaries. London: Chapman & Hall Publishing; 1992. p. 171–84.
Hoffmann WA, Jackson RB. Vegetation-climate feedbacks in the conversion of tropical savannah to grassland. J Clim. 2000;13:1593–602.
Intergovernmental panel on climate change. Guidelines for national greenhouse gas inventories, prepared by the National Greenhouse Gas Inventories Programme. Kanagawa: IGES; 2006.
Klink CA, Machado RB. A conservação do Cerrado brasileiro. Megadiversidades. 2005;1:147–55.
Miranda HS, Sato MN. Efeitos do fogo na vegetação lenhosa do Cerrado. In: Scariot A, Sousa-Silva JC, Felfili JM, editors. Cerrado: ecologia, biodiversidade e conservação. Ministério do Meio Ambiente: Brasília; 2005. p. 95–105.
Miranda SC. Variação espacial e temporal da biomassa vegetal em áreas de Cerrado. Ph.D. dissertation, Universidade de Brasília. 2012.
Morais VA. Modelagem e espacialização do estoque de carbono de cerrado sensu stricto em Minas Gerais. M.S. thesis, Universidade Federal de Lavras. 2012.
Moreira AG. Effects of fire protection on savannah structure in Central Brazil. J Biogeogr. 2000;27:1021–9.
Oliveira CP. Caracterização Florística e Estimativa dos Estoques de Biomassa seca e Carbono em duas Fitofisionomias Savânicas do bioma Cerrado sobre a Formação Urucuia, no oeste da Bahia. Ph.D. dissertation, Universidade Federal Rural do Rio de Janeiro. 2014.
Oliveira CP, Francelino MR, Cysneiros VN, Andrade FC, Booth MC. Composição florística e estrutura de um Cerrado sensu stricto no oeste da Bahia. CERNE. 2015;21:545–52.
Oliveira CP, Francelino MR, Leles PSS, Andrade FC, Daher MP. “Comparação de modelos estatísticos para estimativa da biomassa de árvores, e estimativa do estoque de carbono acima do solo em Cerrado. Cienc Flor. 2019;29:255–69.
Ottmar RD, Vihnanek RE, Miranda HS, Sato MN, Andrade SMA. Stereo Photo series for quantifying cerrado fuels in central Brazil—volume I. Portland: Department of Agriculture, Forest Service, Pacific Northwest Research Station; 2001. p. 1–87.
Paiva AO, Rezende AV, Pereira RS. Estoque de Carbono em Cerrado Sensu stricto no Distrito Federal. Rev Árvore. 2011;35:527–38.
Paiva AO, Faria GE. Estoques de carbono do solo sob cerrado sensu stricto no Distrito Federal, Brasil. Revista Trópica-Ciências Agrárias e Biológicas. 2007;1:60–5.
Pivello VR, Coutinho LM. Transfer of macro-nutrients to the atmosphere during experimental burnings in an Open Cerrado (Brazilian Savannah). J Trop Ecol. 1992;8:487–97.
Rezende AV, Vale AD, Sanquetta CR, Figueiredo Filho A, Felfili JM. Comparação de modelos matemáticos para estimativa do volume, biomassa e estoque de carbono da vegetação lenhosa de um cerrado sensu stricto em Brasília, DF. Sci For J. 2006;71:65–76.
Ribeiro JF, Walter BMT. As Principais Fitofisionomias do Bioma Cerrado. In: Sano SM, De Almeida SP, Ribeiro JF, editors. Cerrado: Ecologia e Flora, vol. 1. Brasília: Embrapa; 2008. p. 151–99.
Ribeiro SC, Fehrmann L, Soares CPB, Jacovine LAG, Kleinn C, de Oliveira Gaspar R. Above and below ground biomass in a Brazilian Cerrado. For Ecol Manag. 2011;262:491–9.
Silva JE, Resck DVS, Corazza EJ, Vivaldi L. Carbon storage in clayey Oxisol cultivated pastures in the “Cerrado” region, Brazil. Agric Ecosyst Environ. 2004;103:357–63.
Silva RBM, Francelino MR, Moura PA, Moura TA, Pereira MG, de Oliveira CP. Relação solo/vegetação em ambiente de Cerrado sobre influência do grupo Urucuia. Cienc Florest. 2015;25:363–73.
Scolforo JRS. Biometria Florestal: Parte I: Modelos de regressão linear e não linear; Parte II: Modelos para relação hipsométrica, volume, afilamento e peso de matéria seca. Lavras: UFLA/FAEPE; 2005. p. 1–352.
Scolforo JRS, Oliveira AD, Acerbi Júnior FW. Inventário Florestal de Minas Gerais: Equações de Volume, Peso de Matéria Seca e Carbono para Diferentes Fitofisionomias da Flora Nativa. Lavras: Editora UFLA; 2008.
Scolforo JRS, Mello JM, Oliveira AD. Cerrado: Florística, Estrutura, Diversidade, Distribuição Diamétrica e de Altura, Volumetria, Tendências de Crescimento e Áreas Aptas para Manejo Florestal. Lavras: Editora UFLA; 2008.
Silva RMB. Relação Solo-Vegetação em uma Área de Cerrado sob Influência do Grupo Urucuia. Ph.D. dissertation, Universidade Federal Rural Rio de Janeiro. 2011.
Souza RC, Mendes IC, Reis-Junior FB, Carvalho FM, Nogueira MA, Vasconcelos ATR, Hungria M. Shifts in taxonomic and functional microbial diversity with agriculture: how fragile is the Brazilian Cerrado? BMC Microbiol. 2016;16:42.
Werneck MDS, Pedralli G, Gieseke LF. Produção de serrapilheira em três trechos de um florestasemidecídua com diferentes graus de perturbação na Estação Ecológica de Tripuí, Ouro Preto, MG. Rev Bras Bot. 2001;24:195–8.
Williams PR. Contrasting demographics of tropical savanna and temperate forest eucalypts provide insight into how savannas and forests function. A case study using Corymbia clarksoniana from north-eastern Australia. Austral Ecol. 2009;34:120–31.
Authors’ contributions
CO led the writing of the manuscript. MF designed the study and critically reviewed the paper. MD provided detailed editing of the manuscript. EA, LS, KA and JC collected and analyzed the data. All authors contributing to the writing of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank Trijunção Farm for their assistance, to forest engineer Renata Machado for the opportunity, and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for support.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data generated or analysed during this study are included in this article.
Consent for publication
All authors have approved the manuscript for publication.
Ethics approval and consent to participate
The ethical approval was obtained from the Brazilian environmental agencies responsible for conducting the cuts.
Funding
I certify that no funding has been received for the conduct of this study or preparation of this manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
de Oliveira, C.P., Francelino, M.R., Daher, M. et al. Estimation of the aboveground biomass and carbon stocks in open Brazilian Savannah developed on sandy soils. Carbon Balance Manage 14, 5 (2019). https://doi.org/10.1186/s13021-019-0121-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13021-019-0121-0