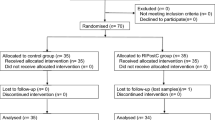
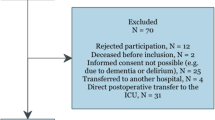
Abstract
Background
Grafting vessel with LIMA to the left anterior descending coronary artery plays a most important role in the long-term prognosis of OPCABG surgery. The aim of this study was to compare the effects of isoflurane preconditioning on miRs and mRNAs levels in the left internal mammary arterie (LIMA) graft with propofol in patients undergoing off-pump coronary artery bypass surgery (OPCABG).
Methods
Patients were randomly assigned to receive either propofol (n = 15), or interrupted isoflurane (n = 15). In group P, propofol administration was continued at 3–5 mg/kg/h intravenous injection for the duration of surgery. Five minutes prior to incision, patients of the isoflurane group (group Iso) received 2 cycles of 1 MAC isoflurane.
Results
miR-221 were significantly lower in group Iso (P < 0 .05). E-selectin mRNA, RhoA mRNA and ROK mRNA were significantly lower at specimens of LIMA in group Iso compared with those in group P patients (P < 0 .05). The expression of NOS3 mRNA was significantly higher in group Iso patients (P < 0 .05).
Conclusion
Our findings provide some insight that prior interrupted isoflurane administration could regulate miR-221, and downstream effectors (mRNAs) and resulted in actual attenuation of inflammation and spasm of LIMA in patients undergoing OPCABG surgery.
Trial registration
NCT No. (ClinicalTrials.gov): NCT02678650; Registration date: January 23, 2016.
Similar content being viewed by others
Background
Coronary artery bypass grafting (CABG) surgery is the standard treatment for patients with 3-vessel coronary artery disease. Reports [1, 2] have showed that autologous arteries rather than veins as bypass graft provide superior long-term outcomes. Moreover, using of LIMA to graft the diseased left anterior descending coronary artery has become the standard method for almost all CABG surgery [3]. However, graft spasm, especially in LIMA with an incidence of at least 0.43% in all CABG surgery [4], and graft stenosis still cause the problem in patients undergoing CABG surgery.
miRs are small non–coding RNAs that regulate the expression of target protein–coding genes by promoting the degradation or suppressing the translation of target mRNAs. Evidence shows that miRs act as key regulators for endothelial biology and function [5].
Recent studies have also shown that volatile anesthetics can influence miRs expression profiles in the liver and cardiomyocytes [6, 7], suggesting that isoflurane preconditioning may influence miRs in LIMA as well. In addition, the regulatory effects of anesthetics on proinflammatory cytokines and NO/eNOS are demonstrated by experimental data [8]. Thus, in this study, we hypothesize that isoflurane-mediated preconditioning could protect LIMA via down-regulation of miRs. To test this, we compare the effects of isoflurane on miRs and mRNAs levels in LIMA graft with propofol in patients undergoing OPCABG surgery.
Methods
Patients
We performed a randomized-, prospective-, controlled clinical study. This investigation conforms to the principles outlined in the Declaration of Helsinki after receiving the Medical Ethics Committee approval at Beijing Anzhen Hospital, Capital Medical University. Written informed consent was obtained from all patients prior to inclusion. This study is registered in the ClinicalTrials.gov database (ID: NCT02678650), Clinical trial date of registration was 23/01/2016, and was performed between January 2016 and December 2016 at Beijing Anzhen Hospital.
Patients with American Society of Anesthesiologists (ASA) scores between class II and III having coronary artery revascularization by OPCAB surgery for 3-vessel disease were included. Exclusion criteria were angina during the previous 72 h, unstable angina, acute myocardial infarction, ejection fraction lower than 40%, the need for inotropic agents or an intra-aortic balloon pump preoperatively, congestive heart failure, emergency procedures, former CABG surgery, concurrent valve repair, severe systemic diseases involving the renal and hepatic systems, respiratory disease (forced vital capacity less than 50% of predicted values), or theophylline therapy.
Anesthesia and surgery
Patients received 10 mg of morphine intramuscularly as premedication one hour prior to entering the operating room. Standard monitoring was achieved in all the patients. Anesthesia was induced with sufentanil (1 μg/kg), etomidate (0.3 mg/kg), cisatracurium (0.2 mg/kg) and maintained with hypnotics (propofol or isoflurane), cisatracurium, and opioid (sufentanil). Patient monitoring included continuous 5-lead electrocadiographic registration with ST-segment analysis, peripheral oxygen saturation by pulse oximetry, radial arterial blood pressure, central venous pressure, capnography, rectal temperature, and urine output. The radial artery catheter was connected to a monitor for pulse contour analysis (MostCare system, Vygon-Vytech, Padova, Italy) and the resulting signal processed for determination of hemodynamic variables. Depth of anesthesia was determined with bispectral index (BIS XPTM, Aspect Medical Systems, Newton, MA, USA) and aimed at BIS values between 40 and 50 during surgery. According to the study protocol, body-position changes, vasoactive drugs (e.g., norepinephrine), and dopamine were used to keep mean arterial pressure (MAP) above 70 mmHg. The nasopharyngeal temperature was held above 36.0 °C. All OPCABG procedures were performed by the same surgical team.
Intervention
According to computer-generated randomization, patients were randomly assigned to receive either propofol (group P, n = 15), or interrupted isoflurane (group Iso, n = 15). In group P, propofol administration was continued at 3–4 mg/kg/h for the duration of surgery. In group Iso, 5 min prior to incision, began to isoflurane wash-in / wash-out operation: isoflurane administration was interrupted for 10 min, by washed out to achieve a MAC value below 0.2(with a high fresh gas flow 8 L.min− 1). Following the interruption, isoflurane to achieve 1 MAC end-tidal concentration as soon as possible (with a high fresh gas flow 6 L.min− 1), and repeated twice periods of 10 min. Discontinuation of isoflurane for at 15 min during the last wash out time. When isoflurane inhaled anesthetic, propofol was stopped infusion. If during this interruption the BIS value increased to > 50, 0.5 mg/kg propofol was administered repeatedly in boluses until the BIS value returned to < 50. The mean flow (MF) and pulsatility index (PI) of the LIMA graft were measured at 10 min after OPCABG when the ultrasonic coupling index (ACI) was ≥90% by an Ultrasonic Blood Flow Detector (Medi-stim, VeriQ 4122, Norway).
Tissue sampling
At 1 h after isoflurane exposure (group P, about 100 min after incision), a segment (1 cm) of LIMA was taken from 30 subjects undergoing OPCABG surgery by sgurgeon. We excluded patients who received papaverine or high-dose vasoactive drugs before the LIMA graft. Before we obtained LIMA, surgeon did not apply any vasodilator such as papaverine. In all patients, LIMA was freed firstly and then the distal end of the internal mammary artery was severed. After we got the pedicled LIMA, LIMA was carefully dissected from their surrounding tissue, and then snap-frozen in liquid nitrogen and kept at − 80 °C until further analyses were carried out.
Quantitative RT-PCR
Total RNA was extracted using the TRIzol reagent kit (BioTeke, China), and RNA purity, integrity and quantity were examined by nanodrop (NANO 2000, Thermo, USA). Purified RNA samples were performed on reverse transcribed cDNA using a reverse-transcription kit (BioTeke, China). Real-time PCR was performed with 2 μ L of diluted RT product in a Exicycler™ 96 (BIONEER, Korea) using SYBR Green PCR Supermix (Solarbio, China) according to the manufacturer’s instructions. U6 was used as reference for miRs and β-actin for mRNAs. Target expression was normalized to the expression of loading control for each sample and the difference between samples was calculated using the 2 - △△ CT method. The designed primers are listed in Table 1.
Western blot (WB)
Biopsies were kept stored at − 80 °C and prepared for analysis on the same day. To measure protein expression, total protein concentration was measured by the BCA Protein Assay Kit (Pierce, Rockford, IL, USA). 40 μg protein samples were loaded and separated on a 5–10% SDS-PAGE gradient gel, and electrically transferred onto a PVDF membrane (Millipore, Billerica, USA). PVDF membranes were blocked with 5% nonfat milk in Tris buffer solution containing 0.1% Tween-20 (TBST) for 1 h at room temperature, followed by incubation with primary antibody. After washing four times with TBST (5 min each, room temperature), the membranes were incubated for 1 h with fluorescent-labeled secondary antibodies (anti-Rabbit IgG at 1:5000). Then, the membranes were washed and proteins were visualized by using an enhanced peroxidase/luminal chemiluminescence reaction (ECL Western blotting detection reagent) (Wanleibio, China), and the final images were analyzed by densitometry using Gel-Pro-Analyzer software (Media Cybernetics, USA). All primary and secondary antibodies were purchased from Wanleibio Biology Ltd., Shenyang, China.
Statistical analysis
Sample size calculation was based on the assumption that the SD of the means of the TnI plasma levels will be 1.5 ng/mL 4 h after surgery, a = 0.05, and β = 0.8. A 40% decrease with isoflurane preconditioning was assumed [9], the power analysis indicated that a minimum sample size of 10 patients was required for each group. In this study we enrolled 15 patients to improve the power of test.
Descriptive analysis was performed using mean ± SD for continuous variables and frequencies (percentages) for categorical data. For comparisons of categorical variables Chi-square analysis was used. Student’s t-test was used to compare the means of two samples. All of the collected data were stored electronically and analyzed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Kolmogorov–Smirnov and normal-quantile plots were used to determine whether the continuous variables were normally distributed. Graphics were produced by use of GraphPad Prism 5 (GraphPad Software, San Diego, CA).
Results
Patient characteristics
Major complications, in-hospital and 30-days mortality did not occur in this study. Table 2 summarizes characteristics and clinical parameters of the perioperative patient (n = 30) in the two study groups. There were no significant differences in terms of age, height, body weight, sex, diabetes mellitus (receive insulin therapy one week before surgery), hypertension, and LVEF, or in other parameters, such as MF, PI values, ventilation duration, ICU stay and hospital stay.
Myocardial injury
The two groups had similar baseline preoperative serum cTnI and CK-MB values. cTnI and CK-MB values were higher in both groups in the periods after surgery and peaked at the 4th hour after surgery in both groups. At 4 h after surgery, the cTnI and CK-MB values in the patients in the Iso group were lower compared to the P group (P < 0.05). (Fig. 1).
Postoperative release of serum cTnI and CK-MB. (a) Serum cTnI levels at different time points. (b) Serum CK-MB levels at different time points. * P < 0.05, ** P < 0.01, compare with T0; + P < 0.05, ++ P < 0.01, between the groups. T0, 5 min prior to incision; T1, 4 h after surgery; T2, 12 h after surgery; T3, 24 h after surgery; T4, 48 h after surgery
Quantitative RT-PCR for miRs
We found that compared with the absence of isoflurane preconditioning, isoflurane pretreatment significantly down-regulated miR-221 expression in LIMA at 1 h after isoflurane exposure(P < 0.05). Further, miR-221 acts as key regulators for endothelial biology and function [10,11,12]. (Fig. 2).
Expression of NO-associated genes
NO, synthesized by endothelial NO synthase (eNOS) which is a putative target gene of miR-221, is very important for vascular function [13]. Our data indicated that NOS3 mRNA and eNOS protein increased significantly at 1 h after isoflurane preconditioning(P < 0.05) and suggested isoflurane pretreatment regulation of eNOS translational efficiency. (Fig. 3).
Expression of inflammatory-associated genes
Nuclear factor-kappa B (NF-κB) is considered to be another putative target gene of miR-221 [14]. We found that the expression of E-selectin mRNA and VCAM-1 mRNA levels decreaced after isoflurane exposure(P < 0.05). However, IκB-a mRNA was not significantly changed in group Iso compared with group P(P = 0.316). (Fig. 4).
Expression of spasm-associated genes
The pathway of RhoA/Rho kinase (RhoA/ROK) is a major cellular target for regulating vasoconstriction [15]. RhoA and ROK were studied at the mRNA and protein levels using quantitative RT-PCR and Western blotting. The results showed that group P had a significantly higher level of mRNA expression of RhoA and ROK than of group Iso, and RhoA protein was also expressed higher in group P(P < 0.05). (Fig. 5).
Discussion
The detailed mechanisms of the influence of anesthetic agents on LIMA are still unknown. The primary findings of the present study were that isoflurane preconditioning during the OPCAB surgery for 3-vessel disease attenuated myocardial injury, and reduced inflammatory response and vasospasm in LIMA. More importantly, results also indicated that miR-221 expression were decreased in group Iso and their common target genes changed accordingly in LIMA. A growing body of evidence shows an organ protective effect by volatile anesthetics, such as isoflurane and sevoflurane [16, 17]. In addition, miRs are known to be involved in the protective effect of volatile anesthetics [18,19,20]. In accordance with our findings, the protective effect of LIMA of isoflurane preconditioning could be at least in part attributed to miR-221.
miR-221 plays a key important role in vascular biology through its effects on vascular smoothmuscle cells and endothelial cells. For example, miR-221 is found to be changed in vascular disease, such as atherosclerosis, coronary artery disease, and post-angioplasty restenosis [21,22,23]. In addition, evidence showed [14] that miR-221 inhibited NO production and abolished the inhibitory effect of adiponectin on E-selectin activation in human umbilical vein endothelial cells, which was in line with our study. Olson and colleagues [20] demonstrated that miR-21 is acting to protect cardiomyocytes after isoflurane exposure. However, our study demonstrated that miR21 was not changed between groups but miR-221 and were down-regulated after isoflurane exposure in LIMA, and LIMA and cardiomyocytes tissue differences may be a major reason for the difference between the two results.
Taken together, eNOS and E-selectin seem to be the common targets of miR-221 to regulate inflammatory. Although the long-term patency of LIMA graft principally depends on the severity of the native coronary artery stenosis and the quality of the distal vascular bed, the formation of atherosclerosis [24] which is caused by inflammation and reduced NO production in the vascular endothelium also plays a key role in the restenosis of LIMA. eNOS which has been proposed as a major factor involved in inflammatory process [25] is expressed mainly in endothelial cells and involved in regulating vascular function. Meanwhile, E-selectin is essential for the expression of inflammatory genes and endothelium pathology [26]. In this study, by quantitative RT-PCR and WB, our results indicated that eNOS was up-regulated and E-selectin mRNA was down-regulated in LIMA in group Iso. Ge et al. [27] also reported that NO functions as both a trigger and a mediator of cardioprotection produced by iso post-conditioning. Smul et al. [28] showed that early preconditioning with desflurane produces a marked reduction in infarct size in an in vivo myocardial infarction rabbit model by desflurane-induced NOS activation. In addition, Huang et al. [29] reported that myocardial tissue eNOS protein expression in a joint isoflurane and propofol anaesthesia was significantly higher than those in the control group. Although regulatory effects of anesthetics on NO/eNOS are demonstrated by experimental data [30], the detailed regulatory mechanisms are still not fully understood. Here, by our study results, we hypothesized that isoflurane exposure inhibits the increase in miR-221 expression in LIMA, which attenuates inflammatory in LIMA. The exactly cause-effect relationship in our hypothesis was not fully supported by our findings. Further detailed regulatory mechanisms studies are thus needed.
Our another finding was that the expression of RhoA/ROK was down-regulated after isoflurane preconditioning. RhoA/ROK-kinase signaling pathways which is a major cellular target for regulating Ca2+ sensitivity of agonist-induced contraction [31] is a most important contraction mechanisms of artery smooth muscle cell, the activation of RhoA leads to stimulation of ROK that can favor myosin light chain (MLC) phosphorylation, actin-myosin interaction and cell contraction [32]. However, whether miRs regulate expression of RhoA/ROK in arterial grafts induced by isoflurane exposure remain unknown. Yang et al. [33] demonstrated that Iso inhibit KCl-induced PI3K-C2α-participation, Rho kinase-mediated MLC phosphorylation, and vasoconstriction in rat aortic smooth muscle. Here, our data indicated that isoflurane preconditioning supressed the expression of RhoA/ROK and activated endothelial eNOS, resulting in vasodilation of LIMA, so we demonstrated that isoflurane preconditioning may be very useful for reperfusion of ischemic myocardium after revascularization for left anterior descending coronary artery. However, cellular mechanistic experiments may be needed in the futuer.
The limitations of the current study are discussed as follows. First, in this study, experimental time under anesthesia was only 1 h after isoflurane exposure. There is a need to verify time-dependent changes of miRs and mRNAs expression. Second, the tissue samples were only taken from LIMA. Given the difference among great saphenous vein, radial artery and LIMA, our results in LIMA may be different with other grafting vessels. However, grafting vessel with LIMA to the left anterior descending coronary artery plays a most important role in the long-term prognosis of OPCABG surgery. Our results at least provide some insight into how isoflurane preconditioning reduces inflammation and relieves vasospasm in LIMA via miR-related pathways.
Conclusions
In conclusion, the results obtained in our study revealed that isoflurane preconditioning applied during OPCABG surgery indicated cardioprotective effects, which can be partly explained by its ability to inhibit inflammatory responses and vasoconstriction in LIMA grafting. Further, isoflurane preconditioning altered the expression of miR-221, which may help better understand the underlying mechanisms of isoflurane preconditioning.
Abbreviations
- ASA:
-
American Society of Anesthesiologists
- CK-MB:
-
Creatinine kinase-MB
- cTnI:
-
Cardiac troponin I
- eNOS:
-
Endothelial NO synthase
- LIMA:
-
Left internal mammary arterie
- MAC:
-
Minimum alveolar concentration
- miRs:
-
MicroRNAs
- NF-κB:
-
Nuclear factor-kappa B
- OPCABG:
-
Off-pump coronary artery bypass surgery
- VCAM-1:
-
Vascular cell adhesion molecule 1
References
Yi G, Shine B, Rehman SM, Altman DG, Taggart DP. Effect of bilateral internal mammary artery on long-term survival: a meta-analysis approach. Circulation. 2014;130:539–45.
Zhang M, Guddeti RR, Matsuzawa Y, Sara JD, Kwon TG, Liu Z, Sun T, Lee SJ, Lennon RJ, Bell MR, Schaff HV, Daly RC, Lerman LO, Lerman A, Locker C. Left internal mammary artery versus coronary stents: impact on DownstreamCoronary Stenoses and conduit patency. J Am Heart Assoc. 2016;24:e003568.
Carneiro Neto JD, Lima Neto JA, Simões RM, Stolf NA. Coronary- artery spasm after coronary artery bypass graft surgery without extracorporeal circulation: diagnostic and management. Rev Bras Cir Cardiovasc. 2010;25:410–4.
He GW, Taggart DP. Spasm in arterial grafts in coronary artery bypass grafting surgery. Ann Thorac Surg. 2016;101:1222–9.
Qin B, Yang H, Xiao B. Role of microRNAs in endothelial inflammation and senescence. Mol Biol Rep. 2012;39:4509–18.
Ishikawa M, Tanaka S, Arai M, Genda Y, Sakamoto A. Differences in microRNA changes of healthy rat liver between sevoflurane and propofol anesthesia. Anesthesiology. 2012;117:1245–52.
Qiao S, Olson JM, Paterson M, Yan Y, Zaja I, Liu Y, Riess ML, Kersten JR, Liang M, Warltier DC, Bosnjak ZJ, Ge ZD. MicroRNA-21 mediates isoflurane-induced cardioprotection against ischemia/reperfusion injury via Akt/nitric oxide synthase/mitochondrial permeability transition pore pathway. Anesthesiology. 2015;123:786–98.
Li JT, Wang H, Li W, Wang LF, Hou LC, Mu JL, Liu X, Chen HJ, Xie KL, Li NL, Gao CF. Anesthetic isoflurane posttreatment attenuates experimental lung injury by inhibiting inflammation and apoptosis. Mediat Inflamm. 2013;2013:e108928.
Frässdorf J, Borowski A, Ebel D, Feindt P, Hermes M, Meemann T, Weber R, Müllenheim J, Weber NC, Preckel B, Schlack W. Impact of preconditioning protocol on anesthetic-induced cardioprotection in patients having coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2009;137(6):1436–42.
Qu K, Wang Z, Lin XL, Zhang K, He XL, Zhang H. MicroRNAs: key regulators of endothelial progenitor cell functions. Clin Chim Acta. 2015;448:65–73.
Pfeifer P, Werner N, Jansen F. Role and function of MicroRNAs in extracellular vesicles in cardiovascular biology. Biomed Res Int. 2015;2015:161393.
Santulli G. MicroRNAs and Endothelial (Dys) Function. J Cell Physiol. 2016;231:1638–44.
Suárez Y, Fernández-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100:1164–73.
Chen CF, Huang J, Li H, Zhang C, Huang X, Tong G, Xu YZ. MicroRNA-221 regulates endothelial nitric oxide production and inflammatory responseby targeting adiponectin receptor 1. Gene. 2015;565:246–51.
Szasz T, Webb RC. Rho-Mancing to Sensitize Calcium Signaling for Contraction in the Vasculature: Role of Rho Kinase.Adv Pharmacol. 2017;78:303–22.
Kim M, Park SW, Kim M, D Agati VD, Lee HT. Isoflurane activates intestinal sphingosine kinase to protect against renal ischemia-reperfusion-induced liver and intestine injury. Anesthesiology. 2011;114:363–73.
Lee HT, Ota-Setlik A, Fu Y, Nasr SH, Emala CW. Differential protective effects of volatile anesthetics against renal ischemiareperfusion injury in vivo. Anesthesiology. 2004;101:1313–24.
Cao L, Feng C, Li L, Zuo Z. Contribution of microRNA-203 to the isoflurane preconditioning-induced neuroprotection. Brain Res Bull. 2012;88:525–8.
Jia P, Teng J, Zou J, Fang Y, Zhang X, Bosnjak ZJ, Liang M, Ding X. MiR-21 contributes to xenon-conferred amelioration of renal ischemia-reperfusion injury in mice. Anesthesiology. 2013;119:621–30.
Olson JM, Yan Y, Bai X, Ge ZD, Liang M, Kriegel AJ, Twaroski DM, Bosnjak ZJ. Up-regulation of microRNA-21 mediates isoflurane-induced protection of cardiomyocytes. Anesthesiology. 2015;122:795–805.
Callis TE, Chen JF, Wang DZ. MicroRNAs in skeletal and cardiac muscle development. DNA Cell Biol. 2007;26:219–25.
Minami Y, Satoh M, Maesawa C, Takahashi Y, Tabuchi T, Itoh T, Nakamura M. Effect of atorvastatin on microRNA 221 / 222 expression in endothelial progenitor cells obtained from patients with coronary artery disease. Eur J Clin Investig. 2009;39:359–67.
Mann DL. MicroRNAs and the failing heart. N Engl J Med. 2007;356:2644–5.
Gimbrone MA Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardeña G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann N Y Acad Sci. 2000;902:230–9.
Lo Faro ML, Fox B, Whatmore JL, Winyard PG, Whiteman M. Hydrogen sulfide and nitric oxide interactions in inflammation. Nitric Oxide. 2014;41:38–47.
De Martin R, Hoeth M, Hofer-Warbinek R, Schmid JA. The transcription factor NF-kappa B and the regulation of vascular cell function. Arterioscler Thromb Vasc Biol. 2000;20:83–8.
Ge ZD, Pravdic D, Bienengraeber M, Pratt PF Jr, Auchampach JA, Gross GJ, Kersten JR, Warltier DC. Isoflurane postconditioning protects against reperfusion injury by preventing mitochondrial permeability transition by an endothelial nitric oxide synthase-dependent mechanism. Anesthesiology. 2010;112:73–85.
Smul TM, Lange M, Redel A, Burkhard N, Roewer N, Kehl F. Desflurane-induced preconditioning against myocardial infarction is mediated by nitricoxide. Anesthesiology. 2006;105:719–25.
Huang Z, Zhong X, Irwin MG, Ji S, Wong GT, Liu Y, Xia ZY, Finegan BA, Xia Z. Synergy of isoflurane preconditioning and propofol postconditioning reducesmyocardial reperfusion injury in patients. Clin Sci (Lond). 2011;121:57–69.
Durak I, Kavutcu M, Kaçmaz M, Avci A, Horasanli E, Dikmen B, Cimen MY, Oztürk HS. Effects of isoflurane on nitric oxide metabolism and oxidant status of Guinea pig myocardium. Acta Anaesthesiol Scand. 2001;45:119–22.
Dimopoulos GJ, Semba S, Kitazawa K, Eto M, Kitazawa T. Ca2+ − dependent rapid Ca2+ sensitization of contraction in arterial smooth muscle. Circ Res. 2007;100:121–9.
Yeon DS, Kim JS, Ahn DS, Kwon SC, Kang BS, Morgan KG. Role of protein kinase C- or RhoA-induced Ca2+ sensitization in stretch-induced myogenic tone. Cardiovasc Res. 2002;53:431–8.
Yang S, Wu Q, Huang S, Wang Z, Qi F. Sevoflurane and isoflurane inhibit KCl-induced class II phosphoinositide 3-kinase αsubunit mediated vasoconstriction in rat aorta. BMC Anesthesiol. 2016;16:e63.
Acknowledgements
Not applicable.
Funding
The work was supported by the National Nature Science Foundation of China (Grant Number 81471902) and Beijing Health System High-Level Health Technical Talents Cultivation Fund (Grant Number 2013–2-004).
Availability of data and materials
The datasets analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
LZ. and JM. designed the study and wrote the main manuscript text, BL. performed surgery, CW. assisted the surgeries and was in charge of Quantitative RT-PCR and Western blot for the LIMA, and DL. was in charge of the statistics and data interpretation. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
As described in the Methods section, the study was approved by Beijing Anzhen Hospital, Capital Medical University. All participants received detailed information about the study and provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhang, L., Wang, CB., Li, B. et al. RhoA/rho-kinase, nitric oxide and inflammatory response in LIMA during OPCABG with isoflurane preconditioning. J Cardiothorac Surg 14, 22 (2019). https://doi.org/10.1186/s13019-019-0835-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-019-0835-9