Abstract
Background
Purulent pericarditis is an uncommon entity, which is, in very rare cases, associated to infection of the aorta.
Case presentation
We present the case of a 42-year-old male patient, who was admitted to hospital complaining of tiredness, diarrhea and leg edema. Clinical examination revealed a hypotensive and obviously shocked patient. He was ultimately diagnosed with a rare combination of purulent pericarditis followed by false aneurysm of the ascending aorta. He was successfully treated by surgical pericardial drainage, replacement of the ascending aorta and antibiotics.
Conclusion
Mycotic aneurysms can rarely be associated with purulent pericarditis. Our literature review shows that there are two mechanisms explaining this association and that in most of the published cases infective endocarditis could not be demonstrated.
Similar content being viewed by others
Background
Purulent pericarditis has become an uncommon condition since the development of antibiotics. While it was a well-known complication of bacterial pneumonia, it is now much rarer and occurs mostly after thoracic trauma or surgery and in immunocompromised patients. S. aureus and Streptococci are the commonest identified pathogens.
Infected aneurysm can be caused by direct inoculation during trauma, hematogenous spread, contiguous infection and as vascular phenomenon during bacterial endocarditis. The later forms a subgroup called mycotic aneurysm. Risk factors for mycotic aneurysm are the same as for bacterial endocarditis, with the addition of preexisting aneurysm and atherosclerosis as factors promoting bacterial colonization of the arterial wall. Intracranial arteries are frequently involved, as are leg arteries, while infection of the aorta is rarer but often accompanied by a morphology of pseudo-aneurysm. Salmonella species and Staphylococcus (especially S. aureus) are the most frequently isolated pathogens.
To our knowledge, there are very few cases reporting the combination of these two conditions.
Case presentation
A 42-year-old man was admitted to a Swiss regional hospital with a complaint of tiredness associated with a 2 weeks’ history of diarrhea, jaundice and 3 days of legs edema. His medical history has shown intravenous drug use, untreated HIV, and advanced liver cirrhosis due to chronic hepatitis C infection. He had been treated the past month for a chronic ulcer of the right ankle.
On initial physical examination, the patient was obviously shocked with somnolence, dehydration, and marbling of the extremities. Cutaneous status was remarkable for presence of sequels of multiples intravenous injections, necrosis of 3 toes and persistence of the right ankle’s ulcer.
Vitals signs were the following: BP 116/79 mmHg, HR 152 bpm, temp 36.8 °C and oxygen saturation 93%, while breathing ambient air. Arterial blood gases were rapidly obtained and showed lactic acidosis with a pH of 7.28 and a concentration of lactates of 8 mmol/l. ECG showed sinus tachycardia with electrical alternans and the chest X- Ray revealed a massively enlarged cardiac silhouette (Fig. 1a). A point of care ultrasound (POCUS) was then performed and confirmed a large pericardial effusion (Fig. 1b).
Laboratory analyses indicated anemia, leukocytosis with severe lymphopenia, thrombopenia, hyponatremia, hyperkaliemia, a slightly elevated CRP and acute renal failure.
The diagnosis of obstructive shock caused by a large pericardial effusion was made and the patient was transferred to a reference hospital for treatment. On admission to the emergency service of this hospital a percutaneous pericardial drainage was rapidly employed to withdraw 1250 ml of purulent liquid with subsequent normalization of blood pressure. A computed tomography revealed multiple pulmonary, hepatic and splenic septic emboli, as well as lobar pulmonary embolism with bilateral thrombosis of the ilio-femoral veins. A transthoracic echocardiography showed a normal ventricular function and no valvular vegetations or other endocarditis echography signs. The pulmonary pressure was normal and there was no interatrial shunt at the color Doppler. The patient was then started on empirical Piperacillin-Tazobactam and Vancomycin and admitted to the ICU. Blood culture drawn at admission and culture of the pericardial effusion showed a Methicillin-sensitive Staphylococcus aureus and antibiotherapy spectrum was narrowed with administration of Flucloxacillin only.
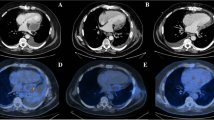
After 3 days, a follow-up transthoracic echocardiography showed a persistent circumferential pericardial effusion motivating a surgical pericardial drainage through a sub-xiphoidal midline approach. Approximately 1 week later (hospitalization day 10), a thoracic computed-tomography was repeated because of persistent fever and showed a false aneurysm of the ascending aorta measuring 2.5 × 3.7 cm, with signs of aortitis (Fig. 2a and b), as well as multiple new septic emboli. Given the risk of rupture associated with the development of the aneurysm in only 10 days, the patient was rapidly taken to the operating room (Fig. 2c) and a replacement of the ascending tubular aorta and anterior hemiarch using a 26 mm Dacron prosthesis was performed under cardiopulmonary bypass with systemic circulatory arrest in moderate hypothermia at 28 °C and antegrade cerebral perfusion through the bracchio-cephalic arterial trunk.
The patient survived the operation and recovered quite well. He accepted to take an antiretroviral therapy and was started on Truvada and Tivicay. Thrombosis phenomenon in the legs was treated with Rivaroxaban. After a few more days in our hospital the patient was discharged to a regional hospital near his home. The Flucloxacillin therapy was supposed to be pursued for 6 weeks after surgery, but as the patient decided to leave hospital without medical consent a few days later, he was discharged on oral Moxifloxacine once a day.
Eighteen months later the patient is doing well and has stopped all drug consumption.
Discussion
As said in the introduction, while purulent pericarditis and mycotic aneurysms are uncommon, the combination of the two is even rarer. A survey of Pubmed database has shown only 11 case reports of purulent pericarditis associated with mycotic aneurysm of the aorta [1,2,3,4,5,6,7,8,9,10,11]. Table 1 summarizes the major characteristics of these cases.
Almost two thirds of patients were female in their 60s and, Staphylococcus and Streptococcus species were found in eight of the 11 cases. More surprisingly, unlike our patient, more than half of the cases had no known predisposing condition for infective endocarditis. Mortality rate was 45% (5/11) and was essentially seen in patients treated conservatively. This is not surprising as infected aneurysms are best managed with combined treatment including antibiotics and surgery. Unlike in ours, where pericardial effusion appeared first, in a substantial part of the cases pericardial effusion occurred through a retrograde mechanism from the infected aneurysm.
Interestingly, despite repeated transthoracic and transoesophagal echocardiography, no evidence of endocardial involvement could be found as our patient continued to develop new septic emboli. This was also the case in most of the previously published cases. Nevertheless, according to Duke’s criteria, the most probable diagnostic remains infective endocarditis, although an alternative explanation could be paradoxical embolization of septic thrombosis of the legs through a patent foramen ovale.
Moreover, this case underlies the high potential for complications of bacterial infections around the heart. Indeed, in this case, the false aneurysm of the aorta is likely a complication of the purulent pericarditis and the probably underlying endocarditis. Thus, in case of bloodstream infections, especially with high risk pathogens (such as Salmonella and Staphylococcus species), clinicians must keep a high degree of suspicion regarding potential complications such as mycotic aneurysms, which can be rapidly deadly if not detected in time.
Conclusion
In summary, we report here an unusual case of purulent pericarditis caused by S. aureus infection, which was complicated by the development, in a very short period of time, of a false aneurysm of the ascending aorta, despite an appropriate antibiotic treatment. The condition was successfully managed by a combination of medical and surgical treatment.
Abbreviations
- ICU:
-
Intensive care unit
- IE:
-
Infective Endocarditis
- POCUS:
-
Point of care ultrasound
References
Fitzgerald JD, Mcnicol MW. Acute suppurative pericarditis with death from ruptured mycotic aneurysm of the aorta. Postgrad Med J. 1964;40:36–9.
Brahan RB, Kahler RC. Clostridium septicum as a cause of pericarditis and mycotic aneurysm. J Clin Microbiol. 1990;28(10):2377–8.
Shroyer KR, Svedlow GS, Adcock DM. Mycotic pseudoaneurysm of the thoracic aorta with purulent pericarditis. Am J Cardiovasc Pathol. 1990;3(4):347–52.
Aranda J, Tauth J, Henning RJ, O’Hara M. Pseudoaneurysm of the thoracic aorta presenting as purulent pericarditis and pericardial effusion. Catheter Cardiovasc Diagn. 1998;43(1):63–7.
Akashi Y, Ikehara Y, Yamamoto A, et al. Purulent pericarditis due to group B streptococcus and mycotic aneurysm of the ascending aorta: case report. Jpn Circ J. 2000;64(1):83–6.
Schneider S, Krülls-Münch J, Knörig J. A mycotic aneurysm of the ascending aorta and aortic arch induced by salmonella Enteritidis. Z Kardiol. 2004;93(12):964–7.
Patel S, Maves R, Barrozo CP, et al. Mycotic pseudoaneurysm and purulent pericarditis attributable to methicillin-resistant Staphylococcus Aureus. Mil Med. 2006;171(8):784–7.
Saito S, Matsuura A, Miyahara K, Takemura H, Sawaki S, Ito H. Infected aortic aneurysm, purulent pericarditis, and pulmonary trunk rupture caused by methicillin-resistant Staphylococcus Aureus. Gen Thorac Cardiovasc Surg. 2009;57(5):250–2.
Parikh SV, Memon N, Echols M, Shah J, DK MG, Keeley EC. Purulent pericarditis: report of 2 cases and review of the literature. Medicine (Baltimore). 2009;88(1):52–65.
Nagano N, Yamamoto T, Amano A, Kikuchi K. Infected aneurysm of the aortic arch with purulent pericarditis caused by Streptococcus Pneumoniae. Interact Cardiovasc Thorac Surg. 2010;10(3):459–61.
Sayah DM, Schwartz BS, Kukreja J, Singer JP, Golden JA, Leard LE. Scedosporium prolificans pericarditis and mycotic aortic aneurysm in a lung transplant recipient receiving voriconazole prophylaxis. Transpl Infect Dis Off J Transplant Soc. 2013;15(2):E70–4.
Acknowledgements
Not applicable
Funding
None declared.
Availability of data and materials
Please contact author for data requests.
Author information
Authors and Affiliations
Contributions
DM prepared the first draft of the manuscript and made the literature review. CT made substantial changes in the manuscript and supervised the editing process. MK contributed to the surgical aspects of the case. SDQ and DF provided radiology pictures and contributed to manuscript revision. OM contributed to manuscript revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written consent obtained.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Meier, D., Kirsch, M., Qanadli, S.D. et al. Case report of an unusual combination of purulent pericarditis and false aneurysm of the ascending aorta. J Cardiothorac Surg 13, 15 (2018). https://doi.org/10.1186/s13019-018-0699-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-018-0699-4