Abstract
Background
Recently, many studies have shown the role of hypoxia-inducible factor-1α (HIF-1α) expression in the outcome of bone tumor. However, the results remain inconclusive. It is necessary to carry out a meta-analysis of all the current available data to clarify the relationship between HIF-1α and survival or clinicopathological features of bone tumor.
Methods
PubMed, Cochrane Library, Web of Science, China National Knowledge Internet, and Wanfang databases were used to search the relationship between HIF-1α and bone tumor. Articles investigating clinicopathological and prognostic value of HIF-1α in bone tumor patients were enrolled in this meta-analysis. Overlapping articles, duplicate data, reviews, case reports, and letters without original data were excluded. The pooled risk ratios (RRs) and hazard ratios (HRs) were used to evaluate the clinicopathological and prognostic value of HIF-1α on bone tumor patients, respectively.
Results
A total of 28 studies including 1443 patients were included in this meta-analysis, which were involved in three different types of bone tumor including 3 chondrosarcomas, 2 giant cell tumors of bone, and 23 osteosarcomas. Our results showed that high expression levels of HIF-1α were associated with poorer OS (overall survival) (HR = 2.61, 95% CI 2.11–3.23, P < 0.001) and shorter DFS (disease-free survival) (HR = 2.02, 95% CI 1.41–2.89, P < 0.001) in bone tumor. In addition, this study also analyzed the role of HIF-1α expression in clinicopathological features, which were closely related with the severity of bone tumor, including differentiation, clinical stage, metastasis, and microvessel density. Our results indicated that HIF-1α overexpression was significantly associated with differentiation (RR = 1.56, 95% CI 1.00–2.43, P = 0.049), clinical stage (RR = 1.75, 95% CI 1.25–2.45, P = 0.001), metastasis (RR = 1.78, 95% CI 1.58–2.00, P < 0.001), and microvessel density (SMD = 2.34, 95% CI 1.35–3.34, P < 0.001) of bone tumor.
Conclusions
HIF-1α overexpression indicated an unfavorable factor for OS and DFS in bone tumor, suggesting that HIF-1α may serve as a potential prognostic marker for bone tumor.
Similar content being viewed by others
Background
Bone tumors are the fourth leading cause of cancer death in patients under 20 years, mainly presented as osteosarcoma, chondrosarcoma, Ewing sarcoma, giant cell tumor of bone, and fibrosarcoma [1]. Among them, osteosarcoma is the most common type of bone tumors, which often occurs in the distal femur and proximal tibia and commonly metastasizes to the lung. Although the 5-year survival rate of osteosarcoma patients has increased to 65–75% due to the improvement of surgical technology, chemotherapy, and radiotherapy [2], the survival rate of osteosarcoma patients with lung metastases remains just 28% [3]. Chondrosarcoma is a type of malignant bone tumors arising from tumor cells which produce cartilage matrix [4]. At present, surgery is the only curative treatment for patients with chondrosarcoma, because chondrosarcoma cells hardly respond to chemotherapy and radiation [5]. Giant cell tumor of bone is a benign bone tumor or sometimes semi-malignant neoplasm commonly affecting the knee [6]. Due to bone tumors appear mostly in children and adolescents, they will have a long-term impact on the life quality of patients [7]. Hence, it is necessary to explore the mechanism of the development and progression in bone tumor.
Hypoxia is an essential feature of many solid cancers and related to malignant transformation, metastases, and chemotherapy resistance [8, 9]. Hypoxia-inducible factors (HIFs) have severed as the critical molecular mediators in response to hypoxia. The HIF-1 is a heterodimer composed of HIF-1α and HIF-1β subunits. HIF-1β is constitutively expressed while HIF-1α is regulated according to oxygen concentration [10]. Under normoxic condition, HIF-1α is rapidly degraded by Von Hippel-Lindau (VHL) through the ubiquitin-proteasomal pathway. Under hypoxia condition, the degradation process is inhibited and HIF-1α transfers from the cell plasma to the nucleus, where it can bind to hypoxia-response elements (HREs) regulating the transcription of many genes relevant to oxygen transport, glucose metabolism, cell proliferation, and apoptosis [11, 12]. A large amount of studies have paid close attention to the expression of HIF-1α in the prognosis of various cancers including breast cancer, esophageal squamous cell carcinoma, hepatocellular carcinoma, gastric cancer, and lung cancer [13,14,15,16]. Furthermore, HIF-1α plays an important role in the bone tumor involved in pivotal aspects of tumor biology [17]. However, the clinicopathological and prognostic value of HIF-1α has been controversial in patients with bone tumor [18, 19].
In order to clarify the effect of HIF-1α on the clinicopathological and prognostic value in the bone tumor, a meta-analysis was performed to systematically evaluate the relationship between HIF-1α expression and bone tumor.
Materials and methods
This study was performed totally following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [20].
Search strategy
The PubMed (MEDLINE), Cochrane Library, Web of Science together with two Chinese databases, China National Knowledge Internet (CNKI) and Wanfang databases, were used to search for articles that evaluated the role of HIF-1α in the prognosis of bone tumor. Studies eligible for this analysis were updated on May 5, 2018, using the search terms “hypoxia-inducible factor-1”, “HIF-1”, and “bone tumor”, “bone sarcoma”, “osteosarcoma”, “chondrosarcoma”, “Ewing sarcoma”, “giant cell tumor”.
Criteria for inclusion and exclusion
The inclusion criteria are as follows: (1) articles investigating the relation between HIF-1α and bone tumor patients, (2) the HIF-1α expression in bone tumor tissues were detected, (3) patients were grouped according to the expression levels of HIF-1α, and (4) related clinicopathological features were described. The exclusion criteria are as follows: (1) literatures not pertinent to the HIF-1α, (2) studies not relevant to HIF-1α expression or lack of survival outcome and clinicopathological features, (3) overlapping articles or duplicate data, and (4) reviews, case reports, and letters without original data.
Data collection
Deqing Luo and Hongyue Ren screened the full text of selected studies to confirm eligibility and then extracted data independently. Disagreement was dissolved by consulting with a third author (Hui Liu). For each study, the following information was recorded: first author, publication year, country, histological type, the patient’s cases, the number of HIF-1α positive, the percentage of HIF-1α positive, survival, and follow-up time. The Newcastle-Ottawa Scale (NOS) score was used for assessing the quality, since all the included studies were non-randomized and retrospective studies. Studies with scores of 5 to 9 were regarded as high quality; otherwise, those with scores of zero to four were considered as low quality.
Statistical analysis
All analyses were performed using STATA 12 software (STATA Corp., College Station, TX). The effects of HIF-1α expression on outcome of the bone tumor were described as hazard ratios (HRs) with an estimate of 95% confidence intervals (CIs), which directly obtain from the publication or retrieve from Kaplan-Meier Curves by extracting several survival rates at specified times from the curves. The intensity of relationship between HIF-1α expression and clinicopathological features was assessed by risk ratios (RRs) and corresponding 95% CIs. Continuous data were expressed as standard mean difference (SMD) with 95% CIs. The chi-square-based Cochrane’s Q test and I2 index were conducted to evaluate the study of heterogeneity. If there was mild heterogeneity among studies (P > 0.10, I2 < 50%), the fixed effects model was applied to pooled data; otherwise, the random effects model was used (P < 0.10, I2 > 50%). Publication bias was calculated by Begg’s funnel plot and Egger’s test. Sensitivity analysis was used to evaluate the stability of the results by omitting individual study sequentially. A P value less than 0.05 was defined as statistical significance.
Results
Characteristics of studies
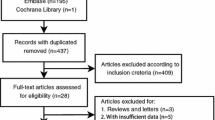
As shown in Fig. 1, a total of 888 studies were retrieved on initial literature search that related to the clinicopathological and prognostic value of HIF-1α in patients with bone tumor. Finally, after scanning the titles, abstracts, and full texts, 28 articles were included in the current meta-analysis [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]. The main and clinicopathological characteristics of the included studies were summarized in Table 1 and Additional file 1: Table S1, respectively. All studies were published between 2008 and 2017 with a total of 1443 patients from Germany, Japan, Canada, and China. Out of the 28 studies, 22 studies evaluated the relationship between HIF-1α expression and the clinicopathological features of bone tumor, and 14 studies reported survival data.
Three different types of bone tumor were involved in this meta-analysis, with 3 chondrosarcomas, 2 giant cell tumors of bone and 23 osteosarcomas. Sample sizes ranged from 20 to 108 cases (mean sample size, 52). Overall, the overall survival (OS) or disease-free survival (DFS) of these studies were ranged from 50 to 120 months, and 8 studies were evaluated to be of high quality.
Correlation between HIF-1α and the clinicopathological features
To explore the relationship between HIF-1α expression and the clinicopathological factors of patients with bone tumor, the analyses were conducted to stratify by gender, age, tumor size, differentiation, clinical stage, metastasis, and microvessel density (MVD). Among them, clinicopathologic factors, including tumor differentiation, clinical stage, metastasis, and MVD of bone tumor, are closely related with the malignant level of bone tumor and also used to estimate the prognosis of patients with bone tumor. As shown in Table 2 and Additional file 2: Figure S1, HIF-1α expression did not show any significant association with gender (RR = 0.93, 95% CI 0.83–1.04, P = 0.179; fixed effects model: χ2 = 12.97, I2 = 7.5, P = 0.371), age (RR = 1.19, 95% CI 1.00–1.41, P = 0.055; fixed effects model: χ2 = 8.72, I2 = 42.7, P = 0.121), and tumor size (RR = 1.19, 95% CI 0.99–1.44, P = 0.069; fixed effects model: χ2 = 6.86, I2 = 12.6, P = 0.334). However, high HIF-1α expression was related with tumor differentiation (RR = 1.56, 95% CI 1.00–2.43, P = 0.049; random effects model: χ2 = 28.33, I2 = 75.3, P < 0.001), clinical stage (RR = 1.75, 95% CI 1.25–2.45, P = 0.001; fixed effects model: χ2 = 8.89, I2 = 43.8, P = 0.113), and metastasis (RR = 1.78, 95% CI 1.58–2.00, P < 0.001; fixed effects model: χ2 = 24.91, I2 = 47.8, P = 0.024). In addition, as shown in Fig. 2, high HIF-1α expression was strongly associated with MVD of bone tumor (SMD = 2.34, 95% CI 1.35–3.34, P < 0.001; random effects model: χ2 = 69.97, I2 = 91.4, P < 0.001).
Association between HIF-1α and prognosis in patients with bone tumor
A total of ten studies have assessed the association of HIF-1α expression with OS. As shown in Fig. 3a, high expression level of HIF-1α significantly predicted unfavorable OS in bone tumor (HR = 2.61, 95% CI 2.11–3.23, P < 0.001), without any heterogeneity in the data (fixed effects model: χ2 = 5.70, I2 = 0, P = 0.770). Correspondingly, Galbraith graph also showed no heterogeneity in this meta-analysis (Fig. 4a). Furthermore, we investigated the relationship between HIF-1α expression and DFS of bone tumor. As shown in Fig. 3b, the combined data of five studies provided information on DFS demonstrated that patients with HIF-1α overexpression had shorter DFS (HR = 2.02, 95% CI 1.41–2.89, P < 0.001; fixed effects model: χ2 = 3.21, I2 = 0, P = 0.524). Correspondingly, it also did not show heterogeneity in the Galbraith graph (Fig. 4b).
Subgroup analyses
To further explore the relationship between HIF-1α expression and the prognosis of patients with bone tumor, subgroup analyses of OS (Table 3 and Additional file 3: Figure S2) and DFS (Table 4 and Additional file 4: Figure S3) were performed to stratify by region, histological type, the number of cases, follow-up time, or the quality of included articles. For OS, in the subgroup analysis based on histological type, the results showed that HIF-1α overexpression existed poor OS in osteosarcoma (HR = 2.60, 95% CI 2.09–3.24, P < 0.001) and chondrosarcoma (HR = 2.83, 95% CI 1.11–7.22, P = 0.030). The relation between HIF-1α overexpression and the OS of patients with bone tumor was also present in studies with less than 100 months (HR = 2.55, 95% CI 1.97–3.30, P < 0.001) as well as more than or equal 100 months follow-up time (HR = 2.76, 95% CI 1.88–4.04, P < 0.001). In addition, HIF-1α overexpression showed poor OS in the studies with smaller cases (n < 50) (HR = 2.40, 95% CI 1.84–3.12, P < 0.001), larger cases (n ≥ 50) (HR = 3.07, 95% CI 2.14–4.40, P < 0.001), high quality (HR = 2.70, 95% CI 2.13–3.43, P < 0.001), and low quality (HR = 2.30, 95% CI 1.44–3.68, P < 0.001).
In the subgroup for HIF-1α expression and DFS, HIF-1α overexpression showed poor DFS in the non-Asian (HR = 1.87, 95% CI 1.15–3.04, P = 0.011), the Asian regions (HR = 2.21, 95% CI 1.30–3.78, P = 0.004), osteosarcoma (HR = 2.21, 95% CI 1.30–3.78, P = 0.004), and chondrosarcoma (HR = 1.87, 95% CI 1.15–3.04, P = 0.011). Furthermore, HIF-1α overexpression showed shorter DFS in the studies with smaller cases (n < 40) (HR = 1.93, 95% CI 1.23–3.02, P = 0.004), larger cases (n ≥ 40) (HR = 2.19, 95% CI 1.21–3.97, P = 0.010), long follow-up time (≥ 100) (HR = 2.08, 95% CI 1.34–3.21, P = 0.001), and short follow-up time (< 100) (HR = 1.90, 95% CI 1.01–3.58, P = 0.047), high quality (HR = 3.22, 95% CI 1.19–8.68, P = 0.021), and low quality (HR = 1.88, 95% CI 1.28–2.77, P = 0.001).
Sensitivity analyses and publication bias
Sensitivity analysis was conducted to evaluate the effect of any single study on the prognosis. No significant difference was found after a sequential omission of one study at a time, suggesting that the conclusions of OS (Fig. 5a) and DFS (Fig. 5b) were stable. In addition, publication bias of the included literatures was performed to assess by Begg’s plot and Egger’s tests. As shown in Fig. 6a, the tests revealed that no evidence of publication bias in the analysis of OS (Begg’s P = 0.283 and Egger’s P = 0.150). In addition, there was no obvious evidence of publication bias on DFS assessed by Begg’s tests; however, Egger’s tests indicated a significant publication bias on DFS (Begg’s P = 0.086 and Egger’s P = 0.037) (Fig. 6b).
Discussion
HIF-1α is an important regulator of cellular response to hypoxia. Increased expression of HIF-1α, a marker of tumor hypoxia, is well associated with carcinogenesis and tumor progression in various kinds of cancer [49]. Recent studies have shown that overexpression of HIF-1α was linked with unfavorable prognosis in some malignancies [50]. A meta-analysis indicated no significant relationship between HIF-1α and the DFS of osteosarcoma [18]. However, a recent study found that HIF-1α might play an important role in the evolution of osteosarcoma [19]. Based on the aforementioned controversy, we conducted this meta-analysis to evaluate the role of HIF-1α in the prognosis and clinicopathological features of patients with bone tumor.
In this meta-analysis, a total of 888 studies with 1443 patients were obtained. The pooled results showed that HIF-1α overexpression was significantly associated with poorer OS and shorter DFS in patients with bone tumor. Moreover, significant results were also found in the subgroup analyses of OS and DFS by region, histological type, the number of cases, follow-up time, and the quality. What was more, HIF-1α overexpression was also significantly associated with the differentiation, clinical stage, metastasis, and MVD of bone tumor. Among them, tumor differentiation represents the severity of the bone tumor. Clinical stage of bone tumor is related to the prognosis and outcome of patients. The main reason for failure treatment of bone tumors is metastasis, which often depends on tumor angiogenesis. MVD is an important indicator of bone tumor angiogenesis and is associated with the prognosis of bone tumor. Hence, our results may provide some implications for doctors in practice. In addition, the results of OS and DFS were stable. There was no obvious evidence of publication bias on OS. However, Egger’s tests indicated a significant publication bias on DFS, because there are only five studies included in this meta-analysis. Besides, Ewing sarcoma was also one of the frequent bone tumors, but it was not enrolled in this meta-analysis. In fact, we have searched several original articles, which investigated the relationship between HIF-1α and Ewing sarcoma [51, 52]. Unfortunately, these articles were excluded due to lack of prognosis (OS and DFS) and clinicopathological features of Ewing sarcoma. Further large studies with high quality are required.
As a key transcriptional regulator, HIF-1α has critical effect on the development and progression of tumor cells by activating the targeting genes, which can regulate several biological processes including cell proliferation, survival, migration, angiogenesis, and glucose metabolism. In addition, HIF-1α can also play a significant role in bone tumor. Increasing evidences have indicated that HIF-1α was not only expressed under normoxia in the osteosarcoma cell line [53], but also overexpressed in metastatic osteosarcoma tumors [38]. More importantly, HIF-1α can contribute to the proliferation, migration, and chemoresistance in osteosarcoma, chondrosarcoma, and giant cell tumor. There was evidence that hypoxia promoted migration of human osteosarcoma cells by activating the HIF-1α/CXCR4 pathway [54]. In chondrosarcoma, HIF-1α could promote the expression of vascular endothelial growth factor (VEGF), which was the primary cytokine related to angiogenesis [55]. Furthermore, increased expression levels of HIF-1α played a prominent role in evasion of apoptosis and chondrosarcoma progression through upregulation of Bcl-xl [56].
Although we evaluated comprehensively the association between HIF-1α and bone tumor outcome, there were several limitations in this meta-analysis. Firstly, Egger’s tests indicated a significant publication bias on DFS. It was possibly because positive results were more likely to be published than negative ones, and further large studies are required. Secondly, HRs were extracted from Kaplan-Meier curves in a few studies, which may not have been entirely accurate. Thirdly, most of the included patients with bone tumor were from China, researches from other countries might obtain different outcomes. Finally, all of the included articles were retrospective studies and most of them had small sample size.
In conclusion, this meta-analysis provides a strong evidence of the correlation of HIF-1α overexpression with both clinicopathological features and survival in patients with osteosarcomas, chondrosarcomas, and giant cell tumors of the bone, suggesting HIF-1α could be used as a useful biomarker for predicting the prognosis of bone tumor patients.
Abbreviations
- CI:
-
Confidence intervals
- CNKI:
-
China National Knowledge Internet
- DFS:
-
Disease-free survival
- HIF-1α:
-
Hypoxia-inducible factor-1α
- HREs:
-
Hypoxia-response elements
- HRs:
-
Hazard ratios
- MVD:
-
Microvessel density
- NOS:
-
Newcastle-Ottawa Scale
- OS:
-
Overall survival
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RRs:
-
Risk ratios
- SMD:
-
Standard mean difference
- VHL:
-
Von Hippel-Lindau
References
Eyre R, Feltbower RG, Mubwandarikwa E, Eden TO, McNally RJ. Epidemiology of bone tumours in children and young adults. Pediatr Blood Cancer. 2009;53(6):941–52.
Mankin HJ, Hornicek FJ, Rosenberg AE, Harmon DC, Gebhardt MC. Survival data for 648 patients with osteosarcoma treated at one institution. Clin Orthop Relat Res. 2004;429:286–91.
Kempf-Bielack B, Bielack SS, Jurgens H, et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS). J Clin Oncol. 2005;23(3):559–68.
Gelderblom H, Hogendoorn PC, Dijkstra SD, et al. The clinical approach towards chondrosarcoma. Oncologist. 2008;13(3):320–9.
Bovee JV, Hogendoorn PC, Wunder JS, Alman BA. Cartilage tumours and bone development: molecular pathology and possible therapeutic targets. Nat Rev Cancer. 2010;10(7):481–8.
Hakim DN, Pelly T, Kulendran M, Caris JA. Benign tumours of the bone: a review. J Bone Oncol. 2015;4(2):37–41.
van den Berg H, Slaar A, Kroon HM, Taminiau AH, Hogendoorn P. Results of diagnostic review in pediatric bone tumors and tumorlike lesions. J Pediatr Orthop. 2008;28(5):561–4.
Cohen NA, Lai SY, Ziober AF, Ziober BL. Dysregulation of hypoxia inducible factor-1alpha in head and neck squamous cell carcinoma cell lines correlates with invasive potential. Laryngoscope. 2004;114(3):418–23.
Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270(3):1230–7.
Dachs GU, Patterson AV, Firth JD, et al. Targeting gene expression to hypoxic tumor cells. Nat Med. 1997;3(5):515–20.
Semenza GL. Hypoxia-inducible factor 1 and cancer pathogenesis. IUBMB Life. 2008;60(9):591–7.
Masoud GN, Li W. HIF-1alpha pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5(5):378–89.
Zhou J, Huang S, Wang L, et al. Clinical and prognostic significance of HIF-1alpha overexpression in oral squamous cell carcinoma: a meta-analysis. World J Surg Oncol. 2017;15(1):104.
Sun G, Wang Y, Hu W. Correlation between HIF-1alpha expression and breast cancer risk: a meta-analysis. Breast J. 2014;20(2):213–5.
Zhang ZG, Zhang QN, Wang XH, Tian JH. Hypoxia-inducible factor 1 alpha (HIF-1alpha) as a prognostic indicator in patients with gastric tumors: a meta-analysis. Asian Pac J Cancer Prev. 2013;14(7):4195–8.
Zheng SS, Chen XH, Yin X, Zhang BH. Prognostic significance of HIF-1alpha expression in hepatocellular carcinoma: a meta-analysis. PLoS One. 2013;8(6):e65753.
Zeng W, Wan R, Zheng Y, Singh SR, Wei Y. Hypoxia, stem cells and bone tumor. Cancer Lett. 2011;313(2):129–36.
Ouyang Y, Li H, Bu J, et al. Hypoxia-inducible factor-1 expression predicts osteosarcoma patients' survival: a meta-analysis. Int J Biol Markers. 2016;31(3):e229–34.
Ren HY, Zhang YH, Li HY, et al. Prognostic role of hypoxia-inducible factor-1 alpha expression in osteosarcoma: a meta-analysis. Onco Targets Ther. 2016;9:1477–87.
Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg. 2011;39(2):91–2.
Bao ZQ, Chai DM, Xiao YZ, Zhang CC, Wu M, Zhou JS. Clinical significance of vasculogenic mimicry and its relation with hypoxia inducible factor-1α in osteosarcoma. Chin J Histochem Cytochem. 2013;22(3):241–8.
Boeuf S, Bovee JV, Lehner B, Hogendoorn PC, Richter W. Correlation of hypoxic signalling to histological grade and outcome in cartilage tumours. Histopathology. 2010;56(5):641–51.
Chen WL, Feng HJ, Li HG. Expression and significance of hypoxemia-inducible factor-1alpha in osteosarcoma of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(2):254–7.
Chen C, Zhou H, Wei F, et al. Increased levels of hypoxia-inducible factor-1alpha are associated with Bcl-xL expression, tumor apoptosis, and clinical outcome in chondrosarcoma. J Orthop Res. 2011;29(1):143–51.
Chen Y, Yang Y, Yuan Z, Wang C, Shi Y. Predicting chemosensitivity in osteosarcoma prior to chemotherapy: an investigational study of biomarkers with immunohistochemistry. Oncol Lett. 2012;3(5):1011–6.
Geng YH, Wang CX, Chen PH. Expressions of Cox-2 and HIF-1α and their relationship with clinicopathologic characteristics of osteosarcoma. Chin J Tumor. 2008;28(5):427–30.
Guan GF, Lu Y, Ma Q, Wen YH, Yang LJ, Qiu XC. Expressions of HIF - 1α and CXCR4 in osteosarcoma and the significance. Chin J Modern Oncol. 2014;05:1162–5.
Guo M, Cai C, Zhao G, et al. Hypoxia promotes migration and induces CXCR4 expression via HIF-1alpha activation in human osteosarcoma. PLoS One. 2014;9(3):e90518.
Hu HJ. Expressions of HIF - 1α and MMP9 in osteosarcoma and the significance: Chinese master’s dissertation of central south university; 2009.
Hu T, He N, Yang Y, et al. DEC2 expression is positively correlated with HIF-1 activation and the invasiveness of human osteosarcomas. J Exp Clin Cancer Res. 2015;34(1):22–33.
Kubo T, Sugita T, Shimose S, et al. Expression of hypoxia-inducible factor-1alpha and its relationship to tumour angiogenesis and cell proliferation in cartilage tumours. J Bone Joint Surg Br. 2008;90(3):364–70.
Li GW. Expressions and correlation of HIF - 1α and VEGF in osteosarcoma. Chin J Henan Surg. 2012;18(1):23–5.
Li TF. Expressions of HIF - 1α and PTEN in osteosarcoma and the significance: Chinese master’s dissertation of university of south China; 2015.
Lian HX, Li YQ, Zhao M, Liu RJ. Relationship between HIF-1α,VEGF expression and angiogenesis in osteosarcoma. Hebei J Tradit Chin Med. 2013;09:1422–4.
Luo B. Expressions of HIF-1α and VEGF in giant cell tumor of bone and their relationship with tumor angiogenesis. Modern Med Health. 2009;25(1):14–6.
Ma Q, Guo JG. Expressions of c-myc and HIF-1α in osteosarcoma and their relationship with tumor angiogenesis. Shandong Med J. 2014;54(5):76–8.
Mao WB, Shao ZW, Pei H, Qiong Q. Relationship between the expression of HIF-1α, iNOS and GLUT-1 and angiogenesis in osteosarcoma. China Oncol. 2007;17(1):46–49.
Mizobuchi H, Garcia-Castellano JM, Philip S, Healey JH, Gorlick R. Hypoxia markers in human osteosarcoma: an exploratory study. Clin Orthop Relat Res. 2008;466(9):2052–9.
El Naggar A, Clarkson P, Zhang F, et al. Expression and stability of hypoxia inducible factor 1alpha in osteosarcoma. Pediatr Blood Cancer. 2012;59(7):1215–22.
Qian JR. Expressions of HIF - 1α in osteosarcoma and the significance: Chinese master’s dissertation of Shandong university; 2007.
Wang Y, Qiu JS, Qiao H, Liang HZ, Luo CQ, Wang R. Expression of hypoxia-inducible factor 1a, VEGF and p53 and their association with angiogenesis and prognosis in osteosarcoma. Chin J Clin Exp Pathol. 2004;02:182–6.
Wang S, Ren T, Huang Y, et al. BMPR2 and HIF1-alpha overexpression in resected osteosarcoma correlates with distant metastasis and patient survival. Chin J Cancer Res. 2017;29(5):447–54.
Wu SZ. Expression of hypoxia-inducible factor 1a, VEGF and CD105 and their association with angiogenesis in osteosarcoma: Chinese master’s dissertation of central south university; 2010.
Yang QC, Zeng BF, Dong Y, et al. Overexpression of hypoxia-inducible factor-1alpha in human osteosarcoma: correlation with clinicopathological parameters and survival outcome. Jpn J Clin Oncol. 2007;37(2):127–34.
Yin L, Hu YH, Ru JY, Jiang JH, Wang YF. Expressions of hypoxia-inducible factor-1α and CD105 in osteosarcoma and their clinical significance. J Modern Oncol. 2010;05:975–8.
Zeng CZ, Luo C, Yang Z, Wang L. Heparanase participates in the growth and invasion of human U-2OS osteosarcoma cells and its close relationship with hypoxia-inducible factor-1alpha in osteosarcoma. Neoplasma. 2010;57(6):562–71.
Zhao H, Wu Y, Chen Y, Liu H. Clinical significance of hypoxia-inducible factor 1 and VEGF-A in osteosarcoma. Int J Clin Oncol. 2015;20(6):1233–43.
Zheng XG, Tian K. Expressions of HIF - 1α and PTEN in osteosarcoma and the significance. Contemp Med. 2009;15(10):73–4.
Nepal M, Choi HJ, Choi BY, et al. Anti-angiogenic and anti-tumor activity of Bavachinin by targeting hypoxia-inducible factor-1alpha. Eur J Pharmacol. 2012;691(1–3):28–37.
Zhong H, De Marzo AM, Laughner E, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59(22):5830–5.
Hameiri-Grossman M, Porat-Klein A, Yaniv I, et al. The association between let-7, RAS and HIF-1alpha in Ewing sarcoma tumor growth. Oncotarget. 2015;6(32):33834–48.
Aryee DN, Niedan S, Kauer M, et al. Hypoxia modulates EWS-FLI1 transcriptional signature and enhances the malignant properties of Ewing's sarcoma cells in vitro. Cancer Res. 2010;70(10):4015–23.
van Uden P, Kenneth NS, Rocha S. Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem J. 2008;412(3):477–84.
Guan G, Zhang Y, Lu Y, et al. The HIF-1alpha/CXCR4 pathway supports hypoxia-induced metastasis of human osteosarcoma cells. Cancer Lett. 2015;357(1):254–64.
Lin C, McGough R, Aswad B, Block JA, Terek R. Hypoxia induces HIF-1alpha and VEGF expression in chondrosarcoma cells and chondrocytes. J Orthop Res. 2004;22(6):1175–81.
Chang Z, Huo L, Wu Y, Zhang P. HIF-1 alpha had pivotal effects on downregulation of miR-210 decreasing viability and inducing apoptosis in hypoxic chondrocytes. ScientificWorldJournal. 2014;2014:876363.
Acknowledgements
Not applicable.
Funding
This work was supported by the Youth Nursery Foundation of the Affiliated Southeast Hospital of Xiamen University, Zhangzhou, Fujian, China (grant Nos. 16Y012 and 16Y019), the military medical project of science and technology innovation (grant No. 14ZD35), and the Natural Science Foundation of Zhangzhou, Fujian, China (grant No. ZZ2017J36),.
Availability of data and materials
I state that data will not be shared since all the raw data can be seen in the figures in this article.
Author information
Authors and Affiliations
Contributions
DL and HL designed the study. HR, WZ, HX, and KL performed the data. DL and HL prepared and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Clinicopathological characteristics of included studies in the meta-analysis. (DOCX 32 kb)
Additional file 2:
Figure S1. Forest plots of the association between HIF-1α expression and the clinicopathological factors of patients with bone tumor including gender (A), age (B), tumor size (C), differentiation (D), clinical stage (E) and metastasis (F). (TIF 1328 kb)
Additional file 3:
Figure S2. Forest plots of subgroup analyses on the association between HIF-1α expression and OS including histological type (A), the number of case (B), follow-up time (C) and the quality of included articles (D). (TIF 1145 kb)
Additional file 4:
Figure S3. Forest plots of subgroup analyses on the association between HIF-1α expression and DFS including region (A), histological type (B), the number of case (C), follow-up time (D) and the quality of included articles (E). (TIF 1333 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Luo, D., Ren, H., Zhang, W. et al. Clinicopathological and prognostic value of hypoxia-inducible factor-1α in patients with bone tumor: a systematic review and meta-analysis. J Orthop Surg Res 14, 56 (2019). https://doi.org/10.1186/s13018-019-1101-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-019-1101-5