Abstract
Background
Trinity Evolution® (TE), a viable cellular bone allograft, previously demonstrated high fusion rates and no safety-related concerns after single-level anterior cervical discectomy and fusion (ACDF) procedures. This prospective multicenter clinical study was performed to assess the radiographic and clinical outcomes of TE in subjects undergoing two-level ACDF procedures.
Methods
In a prospective, multicenter study, 40 subjects that presented with symptomatic cervical degeneration at two adjacent vertebral levels underwent instrumented ACDF using TE autograft substitute in a polyetherethereketone (PEEK) cage. At 12 months, radiographic fusion status was evaluated by dynamic motion plain radiographs and thin cut CT with multiplanar reconstruction by a panel that was blinded to clinical outcome. Fusion success was defined by angular motion (≤4°) and the presence of bridging bone across the adjacent vertebral endplates. Clinical pain and function assessments included the Neck Disability Index (NDI), neck and arm pain as evaluated by visual analog scales (VAS), and SF-36 at both 6 and 12 months.
Results
At both 6 and 12 months, all clinical outcome scores (SF-36, NDI, and VAS pain) improved significantly (p < 0.05) compared to baseline values. There were no adverse events or infections that were attributed to the graft material, no subjects that required revisions, and no significant decreases to mean neurological evaluations at any time as compared to baseline. At 12 months, the per subject and per level fusion rate was 89.4 and 93.4%, respectively. Subgroup analysis of subjects with risk factors for pseudoarthrosis (current or former smokers, diabetic, or obese/extremely obese) compared to those without risk factors demonstrated no significant differences in fusion rates.
Conclusions
Patients undergoing two-level ACDF with TE in combination with a PEEK interbody spacer and supplemental anterior fixation had a high rate of fusion success without any serious adverse events related to the graft material.
Trial registration
Trinity Evolution in Anterior Cervical Disectomy and Fusion (ACDF) NCT00951938
Similar content being viewed by others
Background
Symptomatic cervical disc degeneration includes a multitude of pathologic processes including decreased disc height, disc herniation, and spondylosis resulting in radiculopathy and/or myelopathy. Anterior cervical discectomy and fusion (ACDF) is an established surgical treatment that achieves good to excellent clinical results in patients with symptomatic cervical degenerative disc disease [1]. Although multilevel ACDF is a safe and reliable procedure, multilevel procedures are associated with an increased rate of reoperation, higher non-union rates and longer time to fusion as compared to single-level procedures [2–6]. Additionally, patients who use tobacco [7] and particularly smokers who had a 2-level ACDF [8] have been associated with increased rates of pseudoarthrosis.
To minimize this risk of pseudoarthrosis, surgeons may select from a variety of bone graft materials with various qualities. Few bone graft substitutes contain all three essential bone-forming elements of autograft (osteogenicity, osteoconductivity, and osteoinductivity) [9] in a single, off-the-shelf product. Trinity Evolution® (TE) is a cellular bone allograft that consists of viable cellular cancellous bone matrix and demineralized cortical bone. TE possesses all three essential elements that are required for successful bone grafting, physiologic numbers of osteogenic cells (including mesenchymal stem cells and osteoprogenitor cells), osteoinductive proteins, and an osteoconductive matrix to which the cells are attached [10]. In a prospective study that evaluated the safety and effectiveness of TE in single-level ACDF, the fusion rate was 93.5% at 12 months, no serious allograft-related events occurred and comparisons to the literature revealed that TE may help negate any comorbid physiological barriers to fusion associated with risk factors such as smoking and diabetes [11].
The primary aim of this multicenter clinical study was to prospectively assess the safety and effectiveness of the TE viable cellular bone allograft in combination with a polyetherethereketone (PEEK) interbody spacer in two-level ACDF using patient reported and radiological outcome measures. To better assess effectiveness, the fusion rates were compared with the international literature that described a comparable surgical approach using other graft materials. A secondary aim of the study was to compare fusion rates between patients with and without risk factors for pseudoarthrosis.
Methods
Study design
From October 2009 to June 2012, a prospective, multicenter study was conducted at five investigational sites to evaluate the safety and effectiveness of a cellular bone allograft (Trinity Evolution® (TE)) in combination with a PEEK interbody spacer for ACDF surgery. All patients 18 years of age or older with symptomatic cervical degeneration at two adjacent vertebral levels between C3 and T1 were eligible for the study and those enrolled underwent ACDF with supplemental fixation and a PEEK interbody spacer (Orthofix, Inc., Lewisville, TX). TE was packed within and around the spacer. Exclusion criteria included the use of any other bone graft or bone graft substitute in addition to or in place of TE in and around the interbody spacer or arthrodesis at a single level only or at more than two levels. IRB approval was obtained for each site prior to the initiation of enrollment.
Surgical procedures
All operations were performed by five surgeons using comparable surgical techniques. A standard Smith-Robinson approach to the cervical spine was carried out through a transverse incision. After removal of disc material and endplate cartilage, subchondral bone was perforated and the neural structures were decompressed. During distraction, a PEEK cage (Orthofix Inc. Lewisville, TX) packed with TE was inserted into the intervertebral space. Additionally, TE was packed around the cage if space permitted. Rigid anterior plate-screw fixation was performed in all patients.
Postoperative management and data collection
Subjects were discharged from the hospital on the day of surgery or the day after surgery and were treated with comparable postoperative protocols. All subjects were allowed to ambulate on the first day after surgery. Post-operative immobilization in a cervical collar or brace was prescribed at the surgeon’s discretion.
Information regarding subject age, gender, body mass index (BMI), smoking status and the presence or absence of diabetes was collected. Subjects were evaluated clinically and radiographically at 6 (+/−1) weeks, 6 months (+/−1) and 12 (+/−1) months. At all timepoints, plain radiographs (flexion/extension, AP and lateral) and neurological evaluations (motor, sensory, or reflex) were collected. Neurologic evaluations included motor assessments of elbow flexors, wrist extensors, elbow extensors and finger extensions using a 0–5 scoring system. For sensory function, each cervical segment was assessed for absence, impaired, or normal function. For reflex assessment, biceps, brachioradialis, and triceps were evaluated using a four-point scale. Thin cut (≤1 mm) computed tomography with multiplanar reformatting (CT) was also performed for every subject at 12 months according to the study protocol.
Clinical endpoints included three health measurement instruments: the Neck Disability Index (NDI), visual analogue scale (VAS) (neck and arm), and the SF-36v2, which evaluated pain, function and quality of life (QOL). The NDI ranged from 0–50 points with higher scores representing greater functional improvement. The VAS scale ranged from 0 to 100 mm with 0 representing no pain and 100 representing severe pain on activity. The SF-36, an eight-scale profile of functional health and well-being scores, was summarized to obtain the physical composite score (PCS) and the mental composite score (MCS). In contrast to NDI and VAS, higher scores for SF-36 represent less disability.
Radiographic evaluation
At 12 months, the criteria for fusion required the presence of bridging bone across the adjacent endplates on thin cut CT scans with multiplanar reformatting and ≤4° angular motion on flexion/extension plain radiographs. Both levels were required to be fused in order for the subject to be judged as fused. Radiographic fusion status was determined via an independent review by three qualified reviewers who possessed substantial orthopedic experience and either an MD or a PhD. All three reviewers had to independently agree that bridging bone was present in order for the site to be judged as fused. All radiographic evaluations were performed by reviewers blinded to the patient’s clinical outcomes. At 6 months, fusion was assessed by bony bridging based on plain radiographs.
The quantitative assessments of intervertebral motion were produced by trained analysts using specialized motion analysis software, QMA™ (Quantitative Motion Analysis; Medical Metrics, Inc., Houston, TX). QMA™ has been validated to produce measurements of intervertebral rotation and translation and is accurate to within 1 degree and 1 mm [12, 13]. The reproducibility of the measurements has also been validated [12, 13].
International literature search
The literature search was conducted using PubMed with search terms for ACDF, PEEK, and two- or multilevel. Publications that were included must have reported a two-level ACDF procedure using a PEEK cage with supplemental fixation, the specific graft material, the follow-up times that fusion was assessed and the fusion incidence. Publications that were excluded were reports that described one-, three-, and four- level ACDF procedures or two-level ACDF reports that utilized autograft or allograft interbody spacers, a PEEK cage without graft material or a PEEK cage without rigid supplemental fixation (e.g., “stand-alone”).
Statistical methods
The data in the figures and the results are presented as the mean and standard error (SE) and mean and standard deviation (SD), respectively. A multiple paired t test with a subsequent Bonferroni correction was done for subject reported outcome measures. The Fisher’s exact test was used to compare fusion rates among subjects with risk factors for pseudoarthrosis. Significance was set at p ≤ 0.05. The statistical analyses were performed using SAS (version 9.3, Cary, NC).
Results
Forty subjects were enrolled in the study and arthrodesis was performed on 80 levels. Thirty-five and 38 subjects completed their 6 and 12 month study visits, respectively.
Baseline characteristics
The mean age and standard deviation was 48.5 +/−9 years and the age range was 26–65 years of age. Demographics are described in Table 1. Twenty-six (65.0%), thirteen (32.5%), and one (2.5%) subject received arthrodesis at C5-C7, C4-C6, and C3-C5, respectively.
Fusion
The per subject fusion rate increased over time and was determined to be 65.7% of subjects fused at 6 months and 89.4% at 12 months (Table 2; Fig. 1). The per level fusion rate mirrored the increase over time that was observed in the per subject fusion rate and was 54.3 and 93.4% at 6 and 12 months, respectively (Table 2; Fig. 1). The fusion rates at 12 months for subjects that were current or former smokers, diabetic, or obese were 94.1% (16/17), 100% (5/5), and 93.3% (14/15), respectively. Subgroup analysis of these high risk subjects compared to subjects without risk factors demonstrated no significant differences (p > 0.05) in fusion rates at 12 months (not shown).
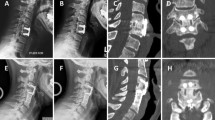
Two-level ACDF using Trinity Evolution that was performed on a 44-year-old obese female at C3-4 and C4-5. a Pre-operative flexion radiograph. b Pre-operative extension radiograph. c Twelve month flexion radiograph. d Twelve month extension radiograph. e Twelve month sagittal CT. f Twelve month coronal CT
Clinical findings
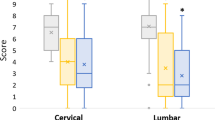
All patient reported outcomes (NDI, VAS neck and arm pain, SF-36 MCS and PCS) demonstrated significant improvements in pain and function at 6 and 12 months as compared to baseline (Figs. 2, 3, and 4).
Safety
There were no adverse events or infections that were related to TE and no pseudoarthroses that required revisions. There was no neurological deterioration encountered (motor, sensory, or reflex) at any time as compared to baseline.
Discussion
The primary aim of this multicenter, open-label clinical study was to prospectively assess the safety and effectiveness of Trinity Evolution cellular bone allograft (TE) in two-level ACDF procedures using a PEEK interbody cage and supplemental fixation, which is the standard of care for each of our five practices. The use of TE did not raise any safety concerns, since there were no adverse events, infections, or reoperations. All measures of subject pain and function (NDI, VAS neck and arm, SF-36 overall and MCS and PCS subscales) significantly improved at both 6 and 12 months as compared to baseline.
One secondary aim of the study was to compare the fusion rates of groups at risk of pseudoarthrosis with normal controls. Smokers [7] particularly smokers who had a two-level ACDF [8] have been associated with increased rates of pseudoarthrosis. Although the sample size was small, there were no significant differences observed between normal and at risk subjects. TE may help overcome the biological factors that impede healing in these groups, but this evaluation was underpowered and a clinically applicable conclusion cannot be drawn.
Because surgeons have several bone graft materials available, a literature review was performed to compare these fusion results to studies that used a comparable approach and instrumentation (Table 3). Evaluation of both safety and effectiveness can help surgeons select a preferred bone graft among the several types including cellular bone allograft, non-cellular allograft such as demineralized bone matrix (DBM), recombinant BMP containing grafts such as INFUSE®, and autograft. Since TE is a cellular bone allograft that contains DBM, one way to assess the potential benefit of TE is to compare the fusion incidence to studies that used DBM. The Topuz et al. study [14] demonstrated a 69.6% fusion rate using DBM, which is twenty percentage points lower than the 89.4% fusion rate for TE. Another study used DBM in conjunction with a synthetic graft material [15], which is a potential confounding factor for accurate data comparison. The use INFUSE® was described in ACDF procedures [16–18]. Although the fusion outcomes using INFUSE are high, there is a substantial safety issue when using INFUSE for ACDF procedures. FDA issued a public health notification of life-threatening cervical swelling (https://wayback.archive-it.org/7993/20170111190511/ http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm062000.htm) when INFUSE is used in the cervical spine. Table 3 also shows high fusion rate when autograft is used [15]. However, harvesting of autograft requires a second operative site which is associated with pain and morbidity that includes chronic harvest site pain, infection, increased operative time, and blood loss [19–23]. Thus, the results described herein appear promising because TE has the potential of increased arthrodesis rates as compared to allograft and TE lacks the safety concerns associated with INFUSE and autograft harvest.
Limitations to this study include a lack of a control group and thus TE treatment was not directly compared to autograft or non-cellular allograft treatments. Additionally, since the surgeons were not restricted with their use of operative approaches or fixation, either or both may have impacted outcomes. The impact of these factors on the outcome was not evaluated. Lastly, there was no sample size estimation in the protocol because there were no formal statistical hypotheses.
Conclusions
In conclusion, subjects who received Trinity Evolution in combination with a PEEK interbody device during a two-level ACDF procedure had a high rate of fusion success both overall and when stratified into high-risk groups, while having no serious adverse events related to the graft material.
Abbreviations
- BMI:
-
Body mass index
- CaS:
-
Calcium Sulfate
- CT:
-
Computed tomography
- DBM:
-
Demineralized bone matrix
- IRB:
-
Internal review board
- MCS:
-
Mental component score
- PCS:
-
Physical component score
- PEEK:
-
Polyetherethereketone
- QMA:
-
Quantitative motion assessment
- SD:
-
Standard deviation
- SE:
-
Standard error
- SF-36:
-
Short form 36
- TE:
-
Trinity Evolution
- VAS:
-
Visual analogue scale
References
Lee S-B, Cho K-S, Kim J-Y, Yoo D-S, Lee T-G, Huh P-W. Hybrid surgery of multilevel cervical degenerative disc disease: review of literature and clinical results. J Korean Neurosurg Soc. 2012;52(5):452–8.
Martin GJ, Haid RW, MacMillan M, Rodts GE, Berkman R. Anterior cervical discectomy with freeze dried fibula allograft. Overview of 317 cases and literature review. Spine. 1999;24(9):852–8.
Nirala AP, Husain M, Vatsal DK. A retrospective study of multiple interbody grafting and long segment strut grafting following multilevel anterior cervical decompression. British J Neurosurg. 2004;18(3):227–32.
Suchomel P, Barsa P, Buchvald P, Svobodnik A, Vanickova E. Autologous versus allogenic bone grafts in instrumented anterior cervical discectomy and fusion: a prospective study with respect to bone union pattern. Eur Spine J. 2004;13(6):510–5.
Veeravagu A, Cole T, Jiang B, Ratliff JK. Revision rates and complication incidence in single- and multilevel anterior cervical discectomy and fusion procedures: an administrative database study. Spine J. 2014;14(7):1125–31.
Wang JC, McDonough PW, Endow KK, Delamarter RB. Increased fusion rates with cervical plating for two-level anterior cervical discectomy and fusion. Spine. 2000;25(1):41–5.
Glassman SD, Anagnost SC, Parker A, Burke D, Johnson JR, Dimar JR. The effect of cigarette smoking and smoking cessation on spinal fusion. Spine. 2000;25(20):2608–15.
Hilibrand AS, Fye MA, Emery SE, Palumbo MA, Bohlman HH. Impact of smoking on the outcome of anterior cervical arthrodesis with interbody or strut-grafting. JBJS. 2001;83-A(5):668–73.
Vaccaro AR. The role of the osteoconductive scaffold in synthetic bone graft. Orthopedics. 2002;25:s571–578.
Rush SM. Trinity Evolution: mesenchymal stem cell allografting in foot and ankle surgery. Foot Ankle Specialist. 2010;3:140–3.
Vanichkachorn J, Peppers T, Bullard D, Stanley SK, Linovitz RJ, Ryaby JT. A prospective clinical and radiographic 12-month outcome study of patients undergoing single-level anterior cervical discectomy and fusion for symptomatic cervical degenerative disc disease utilizing a novel viable allogeneic, cancellous, bone matrix (Trinity Evolution®) with a comparison to historical controls. Eur Spine J. 2016;25(7):2233–8.
Reitman CA, Hipp JA, Nguyen L, Esses SI. Changes in segmental intervertebral motion adjacent to cervical arthrodesis. A prospective study. Spine. 2004;29:E221–6.
Reitman CA, Mauro KM, Nguyen L, Ziegler JM, Hipp JA. Intervertebral motion between flexion and extension in asymptomatic individuals. Spine. 2004;29:2832–43.
Topuz K, Colak A, Kaya S, et al. Two-level contiguous cervical disc disease treated with peek cages packed with demineralized bone matrix: results of 3-year follow-up. Eur Spine J. 2009;18:238–43.
Xie Y, Li H, Yuan J, et al. A prospective randomized comparison of PEEK cage containing calcium sulphate or demineralized bone matrix with autograft in anterior cervical interbody fusion. Int Orthop. 2015;39:1129–36.
Tumialan LM, Pan J, Rodts GE, et al. The safety and efficacy of anterior cervical discectomy and fusion with polyetheretherketone spacer and recombinant human bone morphogenetic protein-2: a review of 200 patients. J Neurosurg Spine. 2008;8(6):529–35.
Boakye M, Mummaneni PV, Garrett M, et al. Anterior cervical discectomy and fusion involving polyetheretherketone spacer and bone morphogenetic protein. J Neurosurg Spine. 2005;2(5):521–5.
Lovasik BP, Holland CM, Howard BM et al. Anterior cervical discectomy and fusion: comparison of fusion, dysphagia, and complication rates between recombinant human bone morphogenetic protein-2 and beta-tricalcium phosphate. World Neurosurg. Epub ahead of print.
Dimitriou R, Mataliotakis GI, Angoules AG, et al. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42 Suppl 2:S3–15.
Kurtz LT, Garfin SR, Booth RE. Harvesting autogenous iliac bone grafts. A review of complications and techniques. Spine. 1989;14:1324–31.
Gupta AR, Shah NR, Patel TC, et al. Perioperative and long-term complications of iliac crest bone graft harvesting for spinal surgery: a quantitative review of the literature. Int Med J. 2001;8:163–6.
Sawin PD, Traynelis VC, Menezes AH. A comparative analysis of fusion rates and donor site morbidity for autogeneic rib and iliac crest bone grafts in posterior cervical fusions. J Neurosurg. 1998;88(2):255–65.
Summers BN, Eisenstein SM. Donor site pain from the ilium: a complication of the lumbar spine fusion. J Bone Joint Surg. 1989;71B:667–80.
Acknowledgements
Not applicable.
Funding
Orthofix funded the clinical study. The funding body was responsible for the design of the study and interpretation of data.
Availability of data and materials
The datasets used and/or analyzed during the current study can be made available from the corresponding author on reasonable request.
Authors’ contributions
DEB, TAP, JSV, SKS, and PMA contributed to the surgical procedures and collection, analysis, and acquisition of data. EIW contributed to the data analysis and critical revisions. RH contributed to the data analysis. BLA contributed to the manuscript writing, literature search, and data interpretation. JTR contributed to the conception and design of the study and critical revisions. RJL contributed to the data analysis and critical revisions. All authors read and approved the final manuscript.
Competing interests
JTR, RH, and EIW are employed by and own stock in Orthofix, Inc., Lewisville, TX. In addition, JSV, TAP, DEB, SKS, RJL, and BLA are consultants of Orthofix, Inc., Lewisville, TX.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Institutional review boards approved the study and informed consent was obtained from all subjects. SKS: Western Institutional Review Board (WIRB); Study Number: 1111392; WIRB Protocol Number: 20090779; TAP: Scripps IRB (Memorial Hospital Encinitas, CORE); Protocol Number: IRB-09-5239; DEB: REX UNC Health Care IRB; JSV: Bon Secours Richmond Health System IRB; PMA: The University of Kansas Medical Center IRB; Project #: 12148.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Peppers, T.A., Bullard, D.E., Vanichkachorn, J.S. et al. Prospective clinical and radiographic evaluation of an allogeneic bone matrix containing stem cells (Trinity Evolution® Viable Cellular Bone Matrix) in patients undergoing two-level anterior cervical discectomy and fusion. J Orthop Surg Res 12, 67 (2017). https://doi.org/10.1186/s13018-017-0564-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-017-0564-5