Abstract
Background
Prognostication tools for early-stage non-small cell lung cancer (NSCLC) patients treated with stereotactic body radiotherapy (SBRT) are currently lacking. The purpose of this study was to develop and externally validate a nomogram to predict overall survival in individual patients with peripheral early-stage disease.
Methods
A total of 587 NSCLC patients treated with biologically effective dose > 100 Gy10 were eligible. A Cox proportional hazards model was used to build a nomogram to predict 6-month, 1-year, 3-year and 5-year overall survival. Internal validation was performed using bootstrap sampling. External validation was performed in a separate cohort of 124 NSCLC patients with central tumors treated with SBRT. Discriminatory ability was measured by the concordance index (C-index) while predictive accuracy was assessed with calibration slope and plots.
Results
The resulting nomogram was based on six prognostic factors: age, sex, Karnofsky Performance Status, operability, Charlson Comorbidity Index, and tumor diameter. The slope of the calibration curve for nomogram-predicted versus Kaplan-Meier-estimated overall survival was 0.77. The C-index of the nomogram (corrected for optimism) was moderate at 0.64. In the external validation cohort, the model yielded a C-index of 0.62.
Conclusions
We established and validated a nomogram which can provide individual survival predictions for patients with early stage lung cancer treated with SBRT. The nomogram may assist patients and clinicians with treatment decision-making.
Similar content being viewed by others
Background
Stereotactic body radiotherapy (SBRT) is the standard of care for medically inoperable early-stage non-small cell lung cancer (NSCLC) [1]. It is increasingly utilized also in the high risk operable patient population [2]. Survival outcomes, however, are variable, and predicting survival in this patient population has proven challenging [3, 4].
A major contributor to survival variability is the potentially high rate of competing non-cancer mortality. For example, severe chronic obstructive pulmonary disease (COPD), common in the SBRT lung population, is associated with a 70% mortality rate at 5 years in those with 3 or more acute exacerbations [5]. The proven safety of SBRT in elderly patients [6] and those with severe COPD [7, 8] has promoted an inclusive stance to patient eligibility. Consequently, despite high rates of local control and cancer-specific survival, overall survival (OS) remains poor and in the order of 40% at 5 years [2].
There is currently a paucity of accurate prognostic models for the early lung SBRT population. One study in the United Stated suggested the decision between curative-intent treatment and observation may be driven largely by institutional factors (academic vs non-academic) and patient financial or racial disparities rather than clinical factors or prognosis [9]. The ability to accurately predict survival on the individual patient level would be highly valuable. Not only would it assist patients with future planning and facilitate shared decision-making with clinicians, but it would also allow for judicious resource-allocation and potentially identify patients better served by a supportive care approach. Finally, it would allow for more accurate risk-stratification for clinical trials and comparative outcomes research.
Nomograms are a practical tool which incorporate prognostic factors for a given patient to calculate the expected probability of a clinical event such as 5-year overall survival. In resected early-stage NSCLC [10] as well as in a diverse lung cancer population undergoing a variety of treatments [11], nomograms have proven more accurate than TNM staging for survival prediction. The purpose of this study was to identify prognostic factors for survival in early lung cancer patients treated with SBRT and to build a nomogram to predict 6-month, 1-year, 3-year and 5-year overall survival.
Methods
Patients and treatment
Consecutive NSCLC patients treated between August 2005 and January 2017 with 4-dimensional SBRT at Erasmus MC were identified. Patients lacking histologic confirmation had findings on positron emission tomography (PET)-CT scan consistent with early-stage NSCLC, and had been recommended for SBRT by a multidisciplinary tumor board. Treatment planning protocols and follow-up schedule have been previously described [12, 13]. Inclusion criteria included peripheral early-stage disease (T1-T3 N0M0). Exclusion criteria included central location (within 2 cm of the proximal bronchial tree), synchronous intrapulmonary lesions, a diagnosis of small cell lung cancer, and delivered biologically effective dose (BED) < 100 Gy assuming an α/β ratio of 10. Tumor staging was originally performed according to AJCC 7th edition based on PET-CT scan (all patients received a staging PET scan) and patients were re-staged by AJCC 8th edition criteria for the present study. Mediastinal staging was performed by PET-CT, mediastinoscopy, and/or endoscopic ultrasound (EBUS and/or EUS).
Endpoints and covariates
The primary endpoint was OS at 5 years, calculated from first day of treatment until death, and patients still alive were censored at the date of last follow-up visit. Variables analyzed for association with survival included age, sex, Karnofsky Performance Status (KPS), operability, Charlson Comorbidity Index score (CCI), Cumulative Illness Rating Score (CIRS), smoking status (current/former vs never), Global Initiative for Chronic Obstructive Lung Disease (GOLD) score [14], previous malignancy, previous lung cancer, maximum axial tumor diameter, histology, and lower lobe location. GOLD scores were not reclassified to reflect 2017 criteria, which incorporate a comprehensive assessment of symptoms by validated questionnaires [15], as this data was not available retrospectively. Operability was determined by criteria as outlined in recently published clinical practice guidelines by the European Society for Medical Oncology (based primary on cardiac assessment and pulmonary function) [16].
Statistical analysis
Model building
The nomogram was based on a Cox proportional hazards model, using the following step-wise model building procedure. Variables with more than 1% missing values (histology 57% and smoking status 11%) were omitted from the initial model, and the decision regarding imputation (and then inclusion in the model) made subsequently based on assessment of their potential added predictive value with Cox univariate and multivariate analyses. Complete case analysis was used for variables with less than 1% missing values. The data provided evidence for interaction between the variables GOLD score, age and sex (p = 0.001) with the results difficult to interpret and depict in a nomogram (see further description within Results: Nomogram), and therefore GOLD score was not initially included. Thus, an initial model was built using the prognostic factors sex, age, KPS, operability, previous malignancy, previous lung cancer, lower lobe tumor location, and tumor diameter.
The model building steps were formulated as strict programmable decision rules aimed at arriving at the most parsimonious model with maximum predictive ability, so that the model building procedure could be internally validated. Initially, the prognostic factors were modeled flexibly, e.g. allowing highly non-linear relationships. Subsequently, following a predefined grid, less flexible functions were applied. The simplification was thus stopped once it began to come at the price of compromised model fit as compared to the most flexible model. Depending on the distribution of the prognostic factor, suitable measures of fit were used. Age and tumor diameter were modelled flexibly using restricted cubic splines (RCS) with 5 degrees of freedom (d.f.), and KPS was modelled as a nominal variable to allow maximum flexibility. To this model, in turn, RCS functions of CCI and CIRS of 4 d.f. each were added. Goodness-of-fit of each of these models was evaluated with respect to the initial model using a likelihood ratio test (LRT). The comorbidity index (CCI or CIRS) which resulted in a smaller p-value was selected (and the resulting model referred to as the full model henceforth). Subsequently, a gradient of RCS functions with d.f. ranging between 2 and 4 and a linear function of the comorbidity score were compared with a LRT to the full model. The functional form with the smallest p-value was selected. The effect of age and tumor diameter were modelled simultaneously and evaluated using Akaike’s Information Criteria (AIC) as the compared models are not nested, as suggested by Harrel [17]. The range of RCS of 5 d.f. to linear effect was evaluated. The model with the smallest AIC was selected. The variables previous malignancy and previous lung cancer were also assessed simultaneously using LRT with a p-value cut-off point of 0.1 for inclusion in the model. Sex, lower lobe tumor location, and operability were evaluated independently against a threshold for model inclusion of p = 0.1 from a LRT, a cut-off value chosen so that the model building procedure could be automated and then validated. Alternative functional forms of KPS score were also evaluated (linear and RCS with 2 and 3 d.f.) and compared to nominal variable modelling using AIC. The model with the smallest AIC is the final model.
Internal validation of the model building procedure
The model building procedure was validated by applying it to 1000 bootstrap samples and predicting the original sample based on the resulting model. Discriminative ability of the model was measured with the concordance index (C-index). Internal validation was also used to assess the degree of overfitting to the sample at hand (calibration slope), and the resulting optimism in C-index. The estimated optimism-corrected calibration slope was then used to shrink model predictions and thus increase their external validity [17]. Calibration plots in 1000 bootstrap samples were used to compare Kaplan-Meier-estimated and nomogram-predicted 6-month, 1-year, 3-year and 5-year OS.
External validation
An independent cohort of 124 NSCLC patients with centrally located tumors treated with SBRT at Erasmus MC between September 2004 – November 2016 was used for external validation.
The final model underlying the nomogram was used to predict 6-month, 1-year, 3-year, and 5-year OS of the patients in the external validation cohort. The model’s discriminative ability in this cohort was measured using the C-index. For the construction of the calibration plots, the predicted survival probabilities were grouped in four equally sized groups.
Statistical analyses were performed using IBM SPSS statistics version 22.0 software package (SPSS Inc., Chicago, IL, USA) and R software, version 3.4.1 (open source; www.r-project.org).
The study protocol was approved by the Medical Ethical Committee of the Erasmus Medical Center (MEC201679).
Results
Patients
A total of 587 patients met inclusion criteria. Baseline clinical and treatment characteristics are shown in Table 1. Median age was 75 years (range 44–91) with median CCI of 3 (range 0–10). Two-hundred and fifty-eight patients had biopsy confirmation of disease, while the remaining 329 had an FDG-avid lesion on PET deemed highly suspicious of NSCLC upon multidisciplinary tumor board review. Mediastinal staging was by PET for the majority of patients (n = 478) and invasive staging (mediastinoscopy, EBUS or EUS) was performed in 109 patients.
The external validation set consisted of 124 NSCLC patients with centrally located tumors treated with SBRT to a median dose of 55 Gy in 5 fractions. Baseline patient and tumor characteristics were similar to those of the primary patient cohort, however, median tumor diameter was larger and several patients had T4 tumors in the validation cohort (Table 1).
Survival
At the time of analysis, 252 patients (42.9%) were alive. Median follow-up time was 23.8 months (range 0.3–124.6) for all patients and 28.5 months (range 4.5–124.6) for surviving patients. Median OS was 38.4 months (95% confidence interval [CI] 34.2–42.6). Three-year and 5-year OS were 54.2 and 29.9%, respectively (Fig. 1). Median follow-up time in the validation cohort was 22.3 months (range 1.9–121.2) and median OS was 26.0 months (95% CI 19.5–32.5) (Fig. 1).
Nomogram
Six patients with unknown KPS score were omitted from the nomogram building procedure. Application of the model building procedure to the remaining 581 patients resulted in a final model based on the variables age, sex, operability, KPS, CCI and tumor diameter. The resulting nomogram is presented in Fig. 2. While age, CCI and tumor diameter were modelled as linear functions, KPS was best modelled as a quadratic function with restriction to linearity at both extremes of the scale, i.e. RCS function with 2 d.f. (Fig. 3). Univariate analysis demonstrated no additional predictive value from including histology, smoking status or GOLD score (p-values 0.38, 0.39, and 0.16, respectively). When added to the final model, histology and smoking remained insignificant and thus these variables were not included in the model. Conversely, GOLD score proved significant (p-value 0.004) when modeled as a nominal variable, however, survival effects were paradoxical: with respect to GOLD 0, GOLD 4 had a nearly identical effect on OS (HR 1.01 p = 0.76) while GOLD 1–3 showed favorable effects on survival with respect to GOLD 0 (HR 0.68, 0.57 and 0.63, and p-values 0.066, 0.002, and 0.022, respectively). When trying to understand these findings, we performed interaction tests with age and sex, which were significant (Chi2 43.7, 19 d.f., p = 0.001). In order to preserve greater parsimony and nomogram readability, and given the paradoxical effect of GOLD score severity on survival, GOLD score was not included in the model. The parameter estimates of the final model are shown in Table 2.
Validation
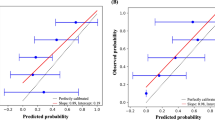
The frequencies of prognostic factor selection in 1000 bootstrap samples are presented in Supplementary Table 1. KPS, age and tumor diameter were selected in 100% of samples, while operability was selected in 96%. The results of validating the model building procedure are presented in Supplementary Table 2. The C-index in the original sample was 0.66, and corrected for optimism through bootstrap sampling to 0.64. The optimism-corrected calibration slope was estimated at 0.766. Nevertheless, calibration plots demonstrated high correlation between observed and predicted probability of 6-month, 1-year, 3-year and 5-year OS (Fig. 4).
Calibration plots of Kaplan Meier vs nomogram predicted survival for the original patient group (black solid line) and the validation cohort (dotted grey line) for a) 6-month b) 1-year c) 3-year and d) 5-year overall survival. The error bars indicate the 95% confidence intervals. A plot along the 45-degree line would indicate perfect agreement between predicted and actual survival
The model underlying the nomogram was used to predict OS of the patients from the external validation cohort. Its discriminative ability in this cohort as measured by C-index was assessed at 0.62, which is highly comparable with the results in the sample used to build the model. Fig. 4 presents calibration plots of the internal as well as the external validation.
Discussion
Survival prediction at the individual patient level can facilitate informed treatment decisions for patients and clinicians. Here, we have developed a nomogram to predict OS, with moderate discriminatory ability (C-index 0.64), and good predictive accuracy based on calibration plots. The model displayed good external validity, with a C-index only slightly lower than that of the original cohort (C-index 0.62). Survival outcomes and baseline characteristics of the studied population are similar to those reported elsewhere [2, 18, 19], suggesting applicability of our model to other early NSCLC SBRT populations.
The prognostic importance of the six variables included in the final nomogram is corroborated by previous investigations. Age [10, 20, 21], sex [10, 20,21,22,23], performance status [3, 19, 24], operability [3, 25], tumor diameter [17, 22, 26, 27] and Charlson Comorbidity Index [3, 18, 20] have previously been reported as significant predictors of survival in the early NSCLC population. Interestingly, in the present sample smoking status was not significantly associated with survival, a finding reported previously [3, 18] although conflicting reports exists [19, 24].
Matsuo et al. [22] investigated prognostic factors in 101 patients with early stage lung cancer treated with SBRT and identified only male sex (HR 3.40, p = 0.004 on multivariate analysis) and tumor diameter (HR 1.60 per 10 mm increase, p = 0.013 on multivariate analysis) as adverse prognostic features for 3-year OS. The population was of atypically high performance status (94% World Health Organization performance status [WHO PS] 0–1) and operability (37% of patients) which may have accounted for the lack of association of age, performance status, and operability with survival. Of note, Matsuo et al. did not evaluate comorbidity as a potential predictor. Kopek et al. [18] did include Charlson Comorbidity score as a prognostic variable and found it to be a powerful predictor of survival: those with a CCI score of 6 or more had a median survival of only 11 months compared to 41 months in patients scoring 3 or less. T stage was also significant on multivariate analysis, and contrary to our findings, sex and performance status were not prognostic. Other variables lacking significance included histology and GOLD classification, consistent with our results.
The nomogram of the present study is one of only a few published for the early stage lung cancer population. A multi-institutional Chinese study developed a nomogram for OS in early stage lung cancer patients, however this was in the setting of resected disease [10]. Nevertheless, it shares similarities with the present nomogram, including incorporation of age, sex, and tumor size as prognostic variables. Although the C-index indicated good discriminatory ability at 0.71, the nomogram is not a useful predictive tool for patients undergoing lung SBRT for several reasons. It relies on surgical variables such as volume of blood loss and pathologic N stage. Additionally, comorbidity was not found to be significantly associated with survival and thus was not incorporated into the nomogram, but because it was coded in the model only as present or absent, if lacked the sensitivity of more established metrics such as CCI.
In the early lung SBRT population, Louie et al. also developed a nomogram for predicting OS, with a C-index similar to the present nomogram (0.66), however, it showed a lower degree of external validity (C-index 0.55 and 0.52 in two external validation cohorts) [19]. Our nomogram differs from that of Louie et al. in several key features. Only the nomogram presented here incorporates operability as a prognostic variable. As SBRT is increasingly applied to the operable setting, incorporating this important variable confers particular utility to our nomogram. Indeed, operability has previously been reported as an important prognostic factor [2, 3, 25]. Onishi et al. [25] reported 5-year overall survival for medically operable patients as 64.8%, compared to 35.0% in inoperable patients (p < 0.001). An additional distinction of the present nomogram is incorporation of KPS rather than WHO PS as a performance status metric. Performance status is perhaps the variable which most consistently appears as a prognostic factor for OS in early lung cancer, and with one of the greatest magnitudes of effect [3, 19, 24]. By utilizing KPS, which has a greater number of categories than WHO PS, our nomogram has greater discriminative ability for small differences in performance status which may significantly affect overall survival. Finally, our nomogram may also be used to predict 1-year and 3-year OS, and these shorter-term survival estimates may be particularly useful for treatment decision-making. The 5 year survival estimates generated by the nomogram, however, should be interpreted with caution, as the median follow up of the study was 24 months.
The nomogram’s short-term survival estimates warrant particular consideration. Very poor short-term prognosis may tip the balance in favor of a supportive care approach, sparing a patient the unnecessary inconvenience and potential cost of curative treatment. Due to the aggressive natural history of NSCLC, cancer-related morbidity and mortality can reasonably be anticipated within an approximately 1-year timeframe [28]. Hence, survival longer than 6 months likely warrants active treatment. Conversely, a low probability of 6-month survival may support a palliative approach. The present nomogram, however, generates a minimum 6-month survival estimate of 80%; adverse prognostic factors including advanced age and high CCI score did not confer a very low probability of short-term survival. This suggests that age and comorbidity burden are not sufficient to justify withholding curative-intent SBRT. It also highlights the need to better identify patient and disease factors predictive of early mortality [29]. Klement et al. [3] aimed to develop a model to predict early mortality in early-stage NSCLC patients undergoing SBRT, and similarly found that patients at high risk of early mortality could not be reliably identified: 6-month mortality was only 8.8% for the group of patients at highest risk, compared to 4.1% for those with the lowest risk.
Weaknesses of the study include its retrospective nature. Additionally, the external validation cohort consisted of patients treated also at our institution, while validation in a cohort from a distinct centre would better demonstrate generalizability of our nomogram. Finally, the majority of patients lacked a histopathologic diagnosis of lung cancer, such that this could not be included as a potential prognostic factor in the nomogram. Previous studies have suggested inferior outcomes for squamous cell carcinoma lung tumors treated with SBRT [30]. It is also possible that some benign tumors were included. However, the incidence of benign disease following surgery for Dutch patients with a clinical diagnosis of NSCLC is generally less than 5% [31], and SBRT outcomes in one study were no different with versus without pathologic confirmation of malignancy [31]. Molecular tumor markers were also not available. Strengths of the study include the relatively large patient population, homogenous treatment, and completeness of data and long-term follow-up. Calibration plots showed good agreement between nomogram-predicted and Kaplan-Meier-estimated survival, with excellent agreement for 3-year OS, suggesting high reliability of the nomogram. The nomogram was externally validated in a distinct patient population with central tumors, and despite difference from the original study population, the nomogram performed well in the external validation cohort. Development of a distinct nomogram for central lung tumors could be an avenue of future investigation, and could assess additional prognostic factors unique to central lung tumors such as potential tumor under-dosing in order to respect normal tissue tolerance. Future investigations may incorporate novel biomarkers and metabolomics signatures which are emerging as prognostic in the early NSCLC population [32].
Conclusions
Here we present a validated a nomogram to predict OS in patients with early-stage NSCLC undergoing SBRT. The discriminatory ability is moderate and incorporation of emerging prognostic factors (for example molecular markers) may increase predictive ability for future models. Nevertheless, this prognostic tool may assist patients and clinicians in generating individual survival predictions.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AIC:
-
Akaike’s information criteria
- BED:
-
Biologically effective dose
- CCI:
-
Charlson comorbidity index score
- CI:
-
Confidence interval
- CIRS:
-
Cumulative illness rating score
- d.f :
-
Degrees of freedom
- HR:
-
Hazard ratio
- KPS:
-
Karnofsky performance status
- LRT:
-
Liklihood ratio test
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- RCS:
-
Restricted cubic splines
- SBRT:
-
Stereotactic body radiotherapy
References
Videtic GMM, Donington J, Giuliani M, Heinzerling J, Karas TZ, Kelsey CR, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: executive summary of an ASTRO evidence-based guideline. Pract Radiat Oncol. 2017;7:295–301.
Zheng X, Schipper M, Kidwell K, Lin J, Reddy R, Ren Y, et al. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys. 2014;90:603–11.
Klement RJ, Belderbos J, Grills I, Werner-Wasik M, Hope A, Giuliani M, et al. Prediction of early death in patients with early-stage NSCLC-can we select patients without a potential benefit of SBRT as a curative treatment approach? J Thorac Oncol. 2016;11:1132–9.
Young KA, Efiong E, Dove JT, Blansfield JA, Hunsinger MA, Wild JL, et al. External validation of a survival Nomogram for non-small cell lung Cancer using the National Cancer Database. Ann Surg Oncol. 2017;24:1459–64.
Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–31.
Cassidy RJ, Patel PR, Zhang X, Press RH, Switchenko JM, Pillai RN, et al. Stereotactic body radiotherapy for early-stage non-small-cell lung Cancer in patients 80 years and older: a multi-center analysis. Clin Lung Cancer. 2017;18:551–8.
Baumann P, Nyman J, Hoyer M, Gagliardi G, Lax I, Wennberg B, et al. Stereotactic body radiotherapy for medically inoperable patients with stage I non-small cell lung cancer - a first report of toxicity related to COPD/CVD in a non-randomized prospective phase II study. Radiother Oncol. 2008;88:359–67.
Takeda A, Enomoto T, Sanuki N, Handa H, Aoki Y, Oku Y, et al. Reassessment of declines in pulmonary function ≥1 year after stereotactic body radiotherapy. Chest. 2013;143:130–7.
Koshy M, Malik R, Spiotto M, Mahmood U, Weichselbaum R, Sher D. Disparities in treatment of patients with inoperable stage I non-small cell lung cancer: a population-based analysis. J Thorac Oncol. 2015;10:264–71.
Liang W, Zhang L, Jiang G, Wang Q, Liu L, Liu D, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. 2015;33:861–9.
Xiao HF, Zhang BH, Liao XZ, Yan SP, Zhu SL, Zhou F, et al. Development and validation of two prognostic nomograms for predicting survival in patients with non-small cell and small cell lung cancer. Oncotarget. 2017;8:64303–16.
van der Voort van Zyp NC, Prévost JB, Hoogeman MS, Praag J, van der Holt B, Levendag PC, et al. Stereotactic radiotherapy with real-time tumor tracking for non-small cell lung cancer: clinical outcome. Radiother Oncol. 2009;91:296–300.
Nuyttens JJ, van de Pol M. The cyber knife radiosurgery system for lung cancer. Expert Rev Med Devices. 2012;9:465–75.
Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–55.
Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–82.
Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv1–iv21.
Harrell FE Jr. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. 2nd ed. Switzerland: Springer; 2015.
Kopek N, Paludan M, Petersen J, Hansen AT, Grau C, Høyer M. Co-morbidity index predicts for mortality after stereotactic body radiotherapy for medically inoperable early-stage non-small cell lung cancer. Radiother Oncol. 2009;93:402–7.
Louie AV, Haasbeek CJ, Mokhles S, Rodrigues GB, Stephans KL, Lagerwaard FJ, et al. Predicting overall survival after stereotactic ablative radiation therapy in early-stage lung Cancer: development and external validation of the Amsterdam prognostic model. Int J Radiat Oncol Biol Phys. 2015;93:82–90.
Rosen JE, Keshava HB, Yao X, Kim AW, Detterbeck FC, Boffa DJ. The natural history of operable non-small cell lung Cancer in the National Cancer Database. Ann Thorac Surg. 2016;101:1850–5.
Ou SH, Zell JA, Ziogas A, Anton-Culver H. Prognostic factors for survival of stage I nonsmall cell lung cancer patients: a population-based analysis of 19,702 stage I patients in the California Cancer registry from 1989 to 2003. Cancer. 2007;110:1532–41.
Matsuo Y, Shibuya K, Nagata Y, Takayama K, Norihisa Y, Mizowaki T, et al. Prognostic factors in stereotactic body radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;79:1104–11.
McGovern SL, Liao Z, Bucci MK, et al. Is sex associated with the outcome of patients treated with radiation for nonsmall cell lung cancer? Cancer. 2009;115:3233–42.
Firat S, Bousamra M, Gore E, Byhardt RW. Comorbidity, and KPS are independent prognostic factors in stage I non-small cell lung cancer. Int J Radiat Biol Phys. 2002;52:1047–57.
Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2:S94–S100.
Allibhai Z, Taremi M, Bezjak A, Brade A, Hope AJ, Sun A, et al. The impact of tumor size on outcomes after stereotactic body radiation therapy for medically inoperable early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;87:1064–70.
Dunlap NE, Larner JM, Read PW, Kozower BD, Lau CL, Sheng K, et al. Size matters: a comparison of T1 and T2 peripheral non-small-cell lung cancers treated with stereotactic body radiation therapy (SBRT). J Thorac Cardiovasc Surg. 2010;140:583–9.
Wao H, Mhaskar R, Kumar A, Miladinovic B, Djulbegovic B. Survival of patients with non-small cell lung cancer without treatment: a systematic review and meta-analysis. Syst Rev. 2013;2:10.
Baker S, Sharma A, Peric R, Heemsbergen WD, Nuyttens JJ. Prediction of early mortality following stereotactic body radiotherapy for peripheral early-stage lung cancer. Acta Oncol. 2019;58:237–42.
Baine MJ, Verma V, Schonewolf CA, Lin C, Simone CB 2nd. Histology significantly affects recurrence and survival following SBRT for early stage non-small cell lung cancer. Lung Cancer. 2018;118:20–6.
Verstegen NE, Lagerwaard FJ, Haasbeek CJ, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy following a clinical diagnosis of stage I NSCLC: comparison with a contemporaneous cohort with pathologically proven disease. Radiother Oncol. 2011;101:250–4.
Burotto M, Thomas A, Subramaniam D, Giaccone G, Rajan A. Biomarkers in early-stage non-small-cell lung cancer: current concepts and future directions. J Thorac Oncol. 2014;9:1609–17.
Acknowledgements
Not applicable.
Funding
Research funding provided for SB by Accuray Incorporated. The funding body had no role in study design or collection, analysis or interpretation of data.
Author information
Authors and Affiliations
Contributions
SB and JJN conceived the study; SB and MD collected data; SB analyzed and interpreted data, and wrote manuscript; KB analyzed and interpreted data, provided statistical analyses; MD, MH, RC, IA, JP, WH, JJN provided critical review and revision of manuscript; All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Medical Ethical Committee of the Erasmus Medical Center (MEC201679).
Consent for publication
Not applicable.
Competing interests
The Department of Radiation Oncology, Erasmus MC Cancer Institute, has research contracts with Elekta and Accuray Incorporated.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
Frequency of variable selection in 1000 bootstrap samples.
Additional file 2: Table S2.
Results of internal validation of the model building procedure through 1000 bootstrap samples.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Baker, S., Bakunina, K., Duijm, M. et al. Development and external validation of a nomogram to predict overall survival following stereotactic body radiotherapy for early-stage lung cancer. Radiat Oncol 15, 89 (2020). https://doi.org/10.1186/s13014-020-01537-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-020-01537-z