Abstract
Background
Adjuvant (ART) and salvage radiotherapy (SRT) are two common concepts to enhance biochemical relapse free survival (BCRFS) in patients with prostate cancer (PC). We analyzed differences in outcome between ART and SRT in patients with steep decline of PSA-levels after surgery to compare outcome.
Methods
We evaluated 253 patients treated with postoperative RT with a median age of 66 years (range 42–85 years) treated between 2004 and 2014. Patients with additive radiotherapy due to PSA persistence and patients in the SRT group, who did not achieve a postoperative PSA level <0.1 ng/mL were excluded. Hence, data of 179 patients was evaluated. We used propensity score matching to build homogenous groups. A Cox regression model was used to determine differences between treatment options. Median follow-up was 32.5 months (range 1.4–128.0 months).
Results
Early SRT at PSA levels <0.3 ng/mL was associated with significant longer BCRFS than late SRT (HR: 0.32, 95%-CI: 0.14–0.75, p = 0.009). Multiple Cox regression showed pre-RT PSA level, tumor stage, and Gleason score as predictive factors for biochemical relapse. In the overall group, patients treated with either ART or early SRT showed no significant difference in BCRFS (HR: 0.17, 95%-CI: 0.02–1.44, p = 0.1). In patients with locally advanced PC (pT3/4) BCRFS was similar in both groups as well (HR: 0.21, 95%-CI:0.02–1.79, p = 0.15).
Conclusion
For patients with PSA-triggered follow-up, close observation is essential and early initiation of local treatment at low PSA levels (<0.3 ng/mL) is beneficial. Our data suggest, that SRT administered at early PSA rise might be equieffective to postoperative ART in patients with locally advanced PC. However, the individual treatment decision must be based on any adverse risk factors and the patients’ postoperative clinical condition.
Study registration
The present work is approved by the Ethics Commission of the Technical University of Munich (TUM) and is registered with the project number 320/14.
Similar content being viewed by others
Background
Although the recent ProtecT trial [1] showed no difference in outcome for patients treated either with surgery or radiotherapy (RT), radical prostatectomy (RP) is still the treatment option mostly chosen by patients with prostate cancer (PC) [2]. However, studies showed that approximately one-third to one-half [3] of the patients develop a biochemical relapse (BCR), which calls for treatment options e.g. postoperative local RT. Two postoperative approaches to reduce risk for relapse are commonly used: Adjuvant radiotherapy (ART), which should be performed within 4 months after surgery, triggered mainly by tumor size and surgical margins, and salvage radiotherapy (SRT), which is performed when prostate-specific antigen (PSA) levels increase during follow-up [4]. The term additive radiotherapy is used when RT is applied on basis of a persistence of PSA levels (most commonly PSA >0.1 ng/mL) after surgery.
Three large trials (EORTC 22911 [5, 6], SWOG 8794 [7, 8] and ARO [9,10,11]) with over 1700 patients in total showed a benefit for ART in biochemical relapse free survival (BCRFS) compared to observation. In all three trials, ART was compared to RP alone with a following wait-and-see policy.
Up to this point, SRT has only been examined in retrospective cohort studies or meta-analyses. Song et al. [12] and Stephenson et al. [13] investigated the oncological outcome of SRT. Song et al. showed a 5-year-BCRFS of 53.6%, while Stephenson et al. published a 6-year-BCRFS of 32.0%. Trock et al. [14] compared SRT with and without androgen-deprivation therapy (ADT) to patients treated with observation only. SRT was associated with a 3-times higher PC specific survival.
Since there is an obvious lack of data comparing ART to SRT directly, there is an ongoing debate on whether SRT is equal to ART. Budiharto et al. [15] evaluated patients with high-risk PC and showed a benefit for ART in this patient group. Briganti et al. [16] analyzed patients with pT3N0 R0-R1 tumors and found no differences in outcome.
Results of three randomized prospective trials on this topic are still on the way: the RAVES study [17] (ClincialTrials.gov Identifier: NCT00860652), the RADICALS trial (ClincialTrials.gov Identifier: NCT00541047) and the GETUG-17 trial (ClincialTrials.gov Identifier: NCT00667069). First results are expected in 2021. We previously reported data on toxicity in a patient cohort comparing immediate postoperative RT versus SRT [18]. In the present article we evaluate the oncological outcome after ART compared to SRT in the same cohort to answer the question whether SRT is equieffective to ART in terms of oncological outcome.
Methods
We retrospectively evaluated 253 patients with a median age of 66 years (range 42–85 years). Patients were treated at the Department of Radiation Oncology, Klinikum rechts der Isar, Technical University of Munich (TUM), Munich, Germany, between 2004 and 2014. ART was defined as RT within 6 months after surgery or in exceptional cases longer due to delayed start of RT because of postoperative side effects (e.g. urine incontinence). One patient in the ART group did not complete RT (total dose 52.0 Gy) due to severe pain caused by an anal fissure, which occurred pre-RT. SRT was defined as postoperative RT after 6 months and BCR with post-RT PSA level <0.1 ng/mL. Additive RT was defined as RT due to PSA persistence with PSA level ≥ 0.1 ng/mL after surgery.
Of all patients, 42 received ART (median time after RP: 4.4 months, range: 2.2–9.9 months), while SRT (median time after RP: 35.7 months, range: 5.7–200.1 months) was administered in 137 patients. Additive RT due to PSA persistence was given to 39 patients and were excluded from ART group. Thirty-five patients formally received salvage treatment but did not achieve a postoperative PSA level <0.1 ng/mL. Those patients were excluded, due to persistent PSA levels. The flow chart is shown in Fig. 1.
The primary endpoint was BCR after RT. BCR was defined as a post-RT PSA level >0.2 ng/mL after reaching the post-RT PSA nadir. Missing data and further follow-up were acquired by contacting patients via letter and/or phone. Before study initiation, ethical approval was obtained from the ethics committee of the Technical University of Munich (TUM), Germany (Medical Faculty, project number: 320/14).
ROC (Receiver Operating Characteristic) analysis was used to determine cut-off values for early salvage radiotherapy. We used adjusted Cox regression to compare BCRFS in both groups. Only patients without ADT (n = 111/137) were included in this sub-analysis. For further evaluation ART (n = 21) was solely compared to early SRT (n = 64) without ADT. We used propensity score matching (PSM) to build homogenous groups. Cox regression analysis was used to determine BCRFS. All other statistical analyses were performed descriptively with exploratory intention using proportions, means (range), and 95%-confidence intervals (95%-CI). A p-value <0.05 was considered as statistically significant. For all evaluations, we used SPSS version 21 (IBM, Armonk, USA).
Results
Based on the above-mentioned criteria, we included 179 patients in this evaluation. Patient characteristics are shown in Table 1.
Median pre-RT PSA level for ART was below detection limit with 0.04 ng/mL (range: 0.00–0.08 ng/mL) and for SRT 0.29 ng/mL (range: 0.02–10.0 ng/mL). A median total dose of 64.0 Gy (range: 52.0–70.2 Gy) was delivered with single doses of 1.8–2.14 Gy. Overall median follow-up was 32.5 months (range 1.4–128.0 months). In ART and SRT group 10 and 22 patients received additional irradiation to the pelvic lymph nodes. Table 2 shows rates of biochemical relapse and occurrence of metastases for patients with ART and SRT in overall group.
Early versus late salvage radiotherapy (SRT)
Data of 111 patients was used. ROC analysis determined a PSA of 0.3 ng/mL as a cut-off value, which resulted in 64 patients in early and 47 patients in late SRT group. We compared BCRFS of early SRT (PSA <0.3 ng/mL) and late SRT (PSA ≥0.3 ng/mL) with Cox regression adjusted for tumor stage (≤T2c vs. ≥T3a), nodal status (N0 vs. N1), Gleason score (≤7a vs ≥7b), and surgical margins (R0 vs. R1). BCRFS in both groups (<0.3 ng/mL versus ≥0.3 ng/mL) was significantly different (HR: 0.32, 95%-CI: 0.14–0.75, p = 0.009) (Fig. 2). Univariate Cox regression showed significance for pre-RT PSA level, tumor stage, and Gleason score. In multiple analysis all three variables remained independent predictive factors for early biochemical relapse (Table 3).
Overall group
For analysis of outcome, we only evaluated patients without ADT which resulted in 21 and 64 cases in ART and early SRT group, respectively. Before PSM, tumor stage and surgical margins showed significant differences in both groups. Therefore, we applied PSM for the two variables. Tumor characteristics before and after PSM are shown in Table 4. Sample size of patients with locally confined tumors was too small to report outcome analysis.
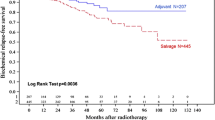
We built 21 pairs with patients of ART and early SRT group. BCRFS (see Fig. 3) was not significantly different between both groups (HR: 0.17, 95%-CI: 0.02–1.44, p = 0.1).
Locally advanced prostate Cancer (pT3/4)
For patients with locally advanced PC Cox regression showed no significant difference in BCRFS (see Fig. 4) of ART versus early SRT (HR: 0.21, 95%-CI:0.02–1.79, p = 0.15).
Discussion
Postoperative RT is a common approach with the goal to prolong BCRFS in patients who previously underwent RP. The question whether SRT versus ART is equieffective is still controversial. Our results suggest, that when early SRT at PSA levels <0.3 ng/mL is administered, patients show a significantly better BCRFS with a 68% reduced risk for BCR. Pre-RT PSA level, tumor stage, and Gleason score remained significant predictors in multiple Cox regression. However, no significant difference for patients receiving ART or early SRT in the overall group was seen. We couldn’t determine a difference in BCRFS in the subgroup analysis of patients with locally advanced (pT3/4) as well. For evaluation of locally confined tumors the sample size was too small.
In the past, three trials (EORTC 22911 [5, 6], ARO 96–02 [9,10,11], and SWOG 8794 [7, 8]) showed a significant benefit for ART compared to a wait-and-see strategy. Bolla et al. showed a BCRFS at 10 years of 60.6 and 41.1%, respectively. Wiegel et al. stated a BCRFS at 5 years of 77% for ART and 54% for a wait-and-see strategy. At 10 years, progression-free survival was 56% versus 35%, respectively. Thompson et al. showed a median BCRFS of 10.3 years for ART and 3.1 years for the wait-and-see group. Here, the primary endpoint was MFS, which accumulated to a median of 14.7 years for ART and 13.2 years for the wait-and-see group. However, Arcangeli et al. [21] performed a critical review of the three randomized trials and showed that in two of the three trials (SWOG 8794 and EORTC 22911) a proportion of patients had a detectable PSA and therefore received formally SRT rather than ART. Further, used doses are considered as inadequate, nowadays. Up to half of the patients in the observational arm received SRT at PSA above 1 ng/mL, which is considered inappropriate, nowadays. Moreover, only the SWOG 8794 trial showed an effect on overall survival.
SRT was only evaluated retrospectively, so far. Song et al. [12] determined a 6-year BCRFS of 32.0% for patients receiving SRT. Significant predictive factors for BCR were pre-RT PSA level ≥1.0 ng/mL, tumor stage ≥T3a, Gleason score ≥7, PSA doubling time <12 months and no visible lesion on pelvic MRI. In line with the described study, a high Gleason score was a predictive factor in our evaluation. Besides Gleason score ≥7b, ≥T3a-tumors were also significantly associated with BCR in the present study. Therefore, especially patients with high risk tumor features should be treated without delay with SRT in case of rising PSA levels. Trock et al. [14] compared observation only to SRT with and without ADT. A benefit for SRT was shown, while ADT had no influence on BCRFS. This remains surprising: ADT as gonadotropin-releasing hormone agonists/antagonists, and antiandrogens reduce the release or function of testosterone and therefore prevent the tumor cells from growth and release of PSA [22]. Consequentially, ADT prolongs BCRFS to the point of castration resistance. Recently, Shipley et al. [23] evaluated SRT with ADT versus placebo. Results showed that patients with additional administration of 24 months of ADT had a significantly better overall survival and a significantly lower rate of distant metastases and death from PC. However, the data of Shipley et al. suggest, that especially patients with pre-RT PSA levels >0.7 ng/mL benefit from addition of ADT. In an earlier study, Carrie et al. compared SRT alone versus SRT with addition of 6 months of ADT and found a significant benefit for the addition of ADT [24]. In patients with ART, ADT must be considered when a positive nodal status is present [25, 26].
To our knowledge all comparative studies of ART versus SRT to date are of retrospective nature. Selected studies are shown in Table 5. Five of the presented series (Budiharto et al. [15], Jereczek-Fossa et al. [27], Ost et al. [28], Mishra et al. [29] and Detti et al. [30]) showed a significant benefit regarding BCRFS in the ART group. However, after Mishra et al. [29] incorporated propensity score calculation in their data, there was only a trend towards significance in BCRFS. Jereczeck-Fossa et al. [27] stated no statistically significant difference in MFS in their cohort. Briganti et al. [16] and Fossati et al. [31] showed an equal effect on the oncological outcome. In comparison to the other series, Briganti et al. [16] only included pT3N0 tumors with positive and negative surgical margins. Further, Fossati et al. and Briganti et al. investigated early SRT with start of RT at PSA levels ≤0.5 ng/mL while all other series were not purely focused on RT at low PSA levels. Our data suggest as well, that patient with locally advanced tumors show similar outcome, when treated with early SRT compared to ART.
Since Stephenson et al. [13] showed a better outcome for patients receiving early SRT at PSA levels of 0.5 ng/mL or less the dictum of salvage treatment changed to “the earlier, the better” [32, 33]. The data of Bartkowiak et al. even advocates for a very early SRT at PSA levels of 0.2 ng/mL or less [34]. However, such low cut-off values are conflicting to the widely accepted definition of biochemical relapse after RP with two consecutive measurements of 0.2 ng/mL or higher [35]. Our data suggests an (very) early SRT at PSA levels less than 0.3 ng/mL. Therefore, close PSA monitoring remains an important follow-up strategy for patients after RP. The threshold of 0.3 ng/mL might be more beneficial in clinical routine, as well as in the discourse with patients. It must be kept in mind that our data derives from an era, where PSMA-PET imaging (Prostate-specific Membrane Antigen-Positron Emission Tomography) was not excessively used. In the last few years, PSMA-PET imaging has become an effective tool for staging and precise treatment of patients with BCR after RP [36, 37]. Whereas in the past, radiation oncologists had to administer empiric treatment to the prostate bed mostly without an imaging correlate, today the PSMA-PET accurately illustrates recurrent tumor sites in most cases. Nevertheless, negative PSMA-PET imaging shall not delay initiation of SRT [38], as discussed above early salvage treatment is crucial to good biochemical response. The perfect cut-off value of PSA indicating a high chance of visualization of tumor relapse in PSMA-PET imaging remains a topic of discussion. Perera et al. reported rates of 58% and 76% for PSA levels of 0.2–1.0 ng/mL and 1.0–2.0 ng/mL for PET scans with gallium-68 tracers [39]. However, the recently emerging use of fluorine-18 tracers might allow for better detection rates making the use of PSMA-PET imaging reasonable starting at PSA values as low as 0.2 ng/mL with a detection rate of 61.5% for patients with values between 0.2–0.5 ng/mL [40].
One point of criticism towards ART is the fact of possible overtreatment for patients who might never experience BCR. Previous series showed, that one-third to one-half [3] of the patients undergoing RP develop BCR. Patients receiving ART, that never might have relapsed, are exposed to possible toxicities and side effects caused by RT. In an earlier publication [18], we showed, that patients with immediate postoperative RT compared to SRT experience significantly higher rates of early gastrointestinal toxicities as proctitis, as well as early genitourinary side effects as urinary tract obstruction. Hence, the decision between ART or PSA-based follow-up and potential SRT should also be based on the patients’ postoperative clinical condition and any risk factors, as well as the patients’ preference. In terms of RT toxicity, patients may benefit from SRT with lower toxicity.
The European guidelines recommend discussion of ART in patients with pT3N0M0 tumors with high risk features such as positive surgical margins [4]. The German guideline recommends performing ART in patients with pT3N0M0 tumors with positive surgical margins (high grade of recommendation), pT3N0M0 tumors with negative surgical margins (moderate grade of recommendation) and pT2N0M0 tumors with positive surgical margins (low grade of recommendation) [38]. Positive surgical margins did not emerge as a predictive factor in our analysis. However, based on the previous results [15, 27,28,29,30] it remains discussable to use immediate postoperative RT depending on high risk tumor features such as tumor stage, positive surgical margins, high Gleason score, lymphovascluar invasion, perineural invasion, and high iPSA.
In comparison to all the mentioned series, we included patients with positive nodal status. ART in patients with intermediate to high risk tumors features and positive nodal status is reported to be beneficial [41]. However, no randomized data is published on this subject. Therefore, we suggest, that the decision on ART for patients with positive postoperative lymph node status should remain individual.
The median total doses of 60 Gy in the ART group and 64 Gy in the SRT group remain at the lower end of dosage given to the prostate bed, nowadays. In the last years, generally doses of 64–70 Gy are prescribed according to published data and guidelines [42]. The SAKK 09/10 trial currently compares dose-intensified SRT 64 Gy versus 70 Gy. The reported toxicity is low [43, 44], however data on outcome needs to be awaited.
The results of our study have limitations, as the data is of retrospective nature. We cannot account for the missing randomization: Patients receiving PSA-triggered SRT are negatively selected and might enter the study with a higher risk for BCR, while patients with no risk did not enter the analysis. Not all patients receiving RP are referred to the department of radiation oncology. Therefore, we cannot account for the referral practice. Moreover, patients who received ART might have never experienced a relapse. This being said, it is obvious that this flaw lies in the nature of the comparison and the only thing randomization would improve is the balance of the groups. The patient number and the limited follow-up time may be a further point of criticism. We cannot account for unknown covariates confounding the results. The tumor features (tumor stage, nodal status, surgical margins, Gleason score) differ in the ART and SRT group. Patients with high risk tumor features are more likely to be treated with ART as recommended in the guidelines. Therefore, we used PSM to deal with the imbalance. The heterogeneous definition for BCR after postoperative RT (see Table 5) remains a hurdle when comparing the data to other series. For primary RT, BCR is consistently defined by the Phoenix criteria [45]. The determination of BCR after postoperative RT remains difficult, hence, a consensual and consistent definition is desirable. Metastases were detected by imaging. However, no standardized follow-up imaging was performed with all patients as the data derives from the pre-PSMA-PET imaging era.
Up to date, three prospective trials are currently underway to determine whether ART and SRT are equieffective. The RAVES study [17] (ClincialTrials.gov Identifier: NCT00860652) is a randomized, multi-center phase 3 trial in Australia and New Zealand with 333 enrolled patients. The RADICALS trial (ClincialTrials.gov Identifier: NCT00541047) is a randomized, multicenter phase 3 study in the UK, Ireland, Denmark and Canada. Four thousand patients are expected to be included. Two studies are combined: In RADICALS RT patients with ART versus SRT are compared. In RADICALS HT, patients receiving RT with or without ADT are compared. The French GETUG-17 trial (ClincialTrials.gov Identifier: NCT00667069) is comparing ART versus SRT, both with concurrent ADT. Seven hundred eighteen patients shall be enrolled. The results of those prospective, randomized trials are eagerly awaited.
Conclusion
The debate on postoperative RT for patients with PC remains controversial. Our data strongly advocates for initiation of SRT at low pre-RT PSA levels <0.3 ng/mL. Especially patients with tumor stage ≥T3a and Gleason score ≥7b should be treated rapidly. Our data suggests ART and early SRT at PSA levels <0.3 ng/mL to be equieffective, espcially in patients with locally advanced PC. However, we recommend to base the treatment decsision individually on the patients’ postoperative clinical condition and the tumor features, foremost tumor stage, nodal status, Gleason score and surgical margins.
Availability of data and materials
The datasets generated and analysed during the current study are not publicly available due regional data protection law but are available from the corresponding author on reasonable request.
Abbreviations
- (p)T:
-
(Pathological) Tumor stage
- 3D-CRT:
-
Three-dimensional conventional Radiotherapy
- 95%-CI:
-
95%-Confidence interval
- ADRT:
-
Additive radiotherapy
- ADT:
-
Androgen deprivation therapy
- ARO:
-
Arbeitsgruppe Radiologische Onkologie
- ART:
-
Adjuvant radiotherapy
- BCR:
-
Biochemical relapse
- BCRFS:
-
Biochemical relapse-free survival time
- EORTC:
-
European Organisation for Research and Treatment of Cancer
- GETUG:
-
Groupe d’étude des tumeurs urogénitales
- Gy:
-
Gray
- HR:
-
Hazard ratio
- IMRT:
-
Intensity modulated radiotherapy
- ISUP:
-
International Society of Urological Pathology
- L:
-
Lymphovascular invasion
- M:
-
Metastasis
- N:
-
Nodal status
- ng/mL:
-
Nanogram per milliliter
- OP:
-
Surgery
- PC:
-
Prostate cancer
- PET:
-
Positron emission tomography
- PSA:
-
Prostate-specific antigen
- PSM:
-
Propensity score matching
- PSMA:
-
Prostate-specific membrane antigen
- R:
-
Surgical margin
- ROC:
-
Receiver operating characteristic
- RP:
-
Radical prostatectomy
- RT:
-
Radiotherapy
- SAKK:
-
Schweizerische Arbeitsgemeinschaft für klinische Krebsforschung
- SRT:
-
Salvage radiotherapay
- SWOG:
-
Swedish Oncology Group
- VMAT:
-
Volumetric intensity modulated arc therapy
References
Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate Cancer. N Engl J Med. 2016;375(15):1415–24.
van Tol-Geerdink JJ, Willem Leer J, Weijerman PC, van Oort IM, Vergunst H, van Lin EN, et al. Choice between prostatectomy and radiotherapy when men are eligible for both: a randomized controlled trial of usual care vs decision aid. BJU Int. 2013;111(4):564–73.
Fichtner J. The management of prostate cancer in patients with a rising prostate-specific antigen level. BJU Int. 2000;86(2):181–90.
Mottet N, Bellmunt J, Briers E, Bolla M, Bourke L, Cornford P, et al. EAU - ESTRO - ESUR - SIOG guidelines on prostate Cancer. Eur Urol. 2017;71(4):618–29.
Bolla M, van Poppel H, Collette L, van Cangh P, Vekemans K, Da Pozzo L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911). Lancet (London, England). 2005;366(9485):572–8.
Bolla M, van Poppel H, Tombal B, Vekemans K, Da Pozzo L, de Reijke TM, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet (London, England). 2012;380(9858):2018–27.
Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. Jama. 2006;296(19):2329–35.
Thompson IM, Tangen CM, Paradelo J, Lucia M, Miller G, Troyer D, et al. Adjuvant radiotherapy for pathologic T3N0M0 prostate Cancer significantly reduces risk of metastases and improves survival: long-term Followup of a randomized clinical trial. J Urol. 2009;181(3):956–62.
Wiegel T, Bottke D, Steiner U, Siegmann A, Golz R, Storkel S, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27(18):2924–30.
Wiegel T, Bartkowiak D, Bottke D, Thamm R, Hinke A, Stöckle M, et al. Prostate-specific antigen persistence after radical prostatectomy as a predictive factor of clinical relapse-free survival and overall survival: 10-year data of the ARO 96-02 trial. Int J Radiat Oncol Biol Phys. 2015;91(2):288–94.
Wiegel T, Bartkowiak D, Bottke D, Bronner C, Steiner U, Siegmann A, et al. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96-02/AUO AP 09/95 trial. Eur Urol. 2014;66(2):243–50.
Song W, Jeon HG, Sung HH, Jeong BC, Seo SI, Jeon SS, et al. Prognostic factors after salvage radiotherapy alone in patients with biochemical recurrence after radical prostatectomy. Int J Urol. 2016;23(1):56–61.
Stephenson AJ, Scardino PT, Kattan MW, Pisansky TM, Slawin KM, Klein EA, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25(15):2035–41.
Trock BJ, Han M, Freedland SJ, Humphreys EB, DeWeese TL, Partin AW, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. Jama. 2008;299(23):2760–9.
Budiharto T, Perneel C, Haustermans K, Junius S, Tombal B, Scalliet P, et al. A multi-institutional analysis comparing adjuvant and salvage radiation therapy for high-risk prostate cancer patients with undetectable PSA after prostatectomy. Radiother Oncol. 2010;97(3):474–9.
Briganti A, Wiegel T, Joniau S, Cozzarini C, Bianchi M, Sun M, et al. Early salvage radiation therapy does not compromise Cancer control in patients with pT3N0 prostate Cancer after radical prostatectomy: results of a match-controlled multi-institutional analysis. Eur Urol. 2012;62(3):472–87.
Pearse M, Fraser-Browne C, Davis ID, Duchesne GM, Fisher R, Frydenberg M, et al. A phase III trial to investigate the timing of radiotherapy for prostate cancer with high-risk features: background and rationale of the radiotherapy -- adjuvant versus early salvage (RAVES) trial. BJU Int. 2014;113(Suppl 2):7–12.
Vogel MME, Kessel KA, Gschwend JE, Weichert W, Wilkens JJ, Combs SE. Early and late toxicity profiles of patients receiving immediate postoperative radiotherapy versus salvage radiotherapy for prostate cancer after prostatectomy. Strahlenther Onkol. 2018;195(2):131–44.
Mohler JL, Armstrong AJ, Bahnson RR, D'Amico AV, Davis BJ, Eastham JA, et al. Prostate Cancer, version 1.2016. J Natl Compr Cancer Netw. 2016;14(1):19–30.
Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40(2):244–52.
Arcangeli S, Ramella S, De Bari B, Franco P, Alongi F, D'Angelillo RM. A cast of shadow on adjuvant radiotherapy for prostate cancer: a critical review based on a methodological perspective. Crit Rev Oncol Hematol. 2016;97:322–7.
Siddiqui ZA, Krauss DJ. Adjuvant androgen deprivation therapy for prostate cancer treated with radiation therapy. Transl Androl Urol. 2018;7(3):378–89.
Shipley WU, Seiferheld W, Lukka HR, Major PP, Heney NM, Grignon DJ, et al. Radiation with or without Antiandrogen therapy in recurrent prostate Cancer. N Engl J Med. 2017;376(5):417–28.
Carrie C, Hasbini A, de Laroche G, Richaud P, Guerif S, Latorzeff I, et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 2016;17(6):747–56.
Briganti A, Karnes RJ, Da Pozzo LF, Cozzarini C, Capitanio U, Gallina A, et al. Combination of adjuvant hormonal and radiation therapy significantly prolongs survival of patients with pT2-4 pN+ prostate cancer: results of a matched analysis. Eur Urol. 2011;59(5):832–40.
Gupta M, Patel HD, Schwen ZR, Tran PT, Partin AW. Adjuvant radiation with androgen-deprivation therapy for men with lymph node metastases after radical prostatectomy: identifying men who benefit. BJU Int. 2018;123(2):252–60.
Jereczek-Fossa BA, Zerini D, Vavassori A, Fodor C, Santoro L, Minissale A, et al. Sooner or later? Outcome analysis of 431 prostate cancer patients treated with postoperative or salvage radiotherapy. Int J Radiat Oncol Biol Phys. 2009;74(1):115–25.
Ost P, De Troyer B, Fonteyne V, Oosterlinck W, De Meerleer G. A matched control analysis of adjuvant and salvage high-dose postoperative intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2011;80(5):1316–22.
Mishra MV, Scher ED, Andrel J, Margules AC, Hegarty SE, Trabulsi EJ, et al. Adjuvant versus salvage radiation therapy for prostate cancer patients with adverse pathologic features: comparative analysis of long-term outcomes. Am J Clin Oncol. 2015;38(1):55–60.
Detti B, Scoccianti S, Cassani S, Cipressi S, Villari D, Lapini A, et al. Adjuvant and salvage radiotherapy after prostatectomy: outcome analysis of 307 patients with prostate cancer. J Cancer Res Clin Oncol. 2013;139(1):147–57.
Fossati N, Karnes RJ, Boorjian SA, Moschini M, Morlacco A, Bossi A, et al. Long-term impact of adjuvant versus early salvage radiation therapy in pT3N0 prostate Cancer patients treated with radical prostatectomy: results from a multi-institutional series. Eur Urol. 2017;71(6):886–93.
Pfister D, Bolla M, Briganti A, Carroll P, Cozzarini C, Joniau S, et al. Early salvage radiotherapy following radical prostatectomy. Eur Urol. 2014;65(6):1034–43.
Tendulkar RD, Agrawal S, Gao T, Efstathiou JA, Pisansky TM, Michalski JM, et al. Contemporary update of a multi-institutional predictive Nomogram for salvage radiotherapy after radical prostatectomy. J Clin Oncol. 2016;34(30):3648–54.
Bartkowiak D, Thamm R, Bottke D, Siegmann A, Bohmer D, Budach V, et al. Prostate-specific antigen after salvage radiotherapy for postprostatectomy biochemical recurrence predicts long-term outcome including overall survival. Acta Oncol. 2017;57(3):362–7.
Mottet N, Bellmunt J, Briers E, Bolla M, Cornford P, Henry MA, et al. EAU - ESTRO - SIOG Guidelines on Prostate Cancer 2016 [Available from: https://uroweb.org/wp-content/uploads/EAU-Guidelines-Prostate-Cancer-2016.pdf (Archived by WebCite® at http://www.webcitation.org/6nYz1to9J). Accessed 16 Jan 2017.
Habl G, Sauter K, Schiller K, Dewes S, Maurer T, Eiber M, et al. (68) Ga-PSMA-PET for radiation treatment planning in prostate cancer recurrences after surgery: individualized medicine or new standard in salvage treatment. Prostate. 2017;77(8):920–7.
Schiller K, Sauter K, Dewes S, Eiber M, Maurer T, Gschwend J, et al. Patterns of failure after radical prostatectomy in prostate cancer - implications for radiation therapy planning after (68)Ga-PSMA-PET imaging. Eur J Nucl Med Mol Imaging. 2017;44(10):1656–62.
Leitlinienprogramm Onkologie. Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): Interdisziplinäre Leitlinie der Qualität S3 zur Früherkennung, Diagnose und Therapie der verschiedenen Stadien des Prostatakarzinoms, Langversion 5.0, AWMF Registernummer: 043/022OL. 2018 [Available from: https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Prostata_5_0/LL_Prostata_Langversion_5.0.pdf (Archived by WebCite® at http://www.webcitation.org/701q4eCrl). Accessed 08 June 2018.
Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, et al. Sensitivity, specificity, and predictors of positive (68)Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate Cancer: a systematic review and meta-analysis. Eur Urol. 2016;70(6):926–37.
Giesel FL, Knorr K, Spohn F, Will L, Maurer T, Flechsig P, et al. Detection efficacy of [(18)F]PSMA-1007 PET/CT in 251 Patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2019;60(3):362–68.
Abdollah F, Karnes RJ, Suardi N, Cozzarini C, Gandaglia G, Fossati N, et al. Impact of adjuvant radiotherapy on survival of patients with node-positive prostate cancer. J Clin Oncol. 2014;32(35):3939–47.
Valicenti RK, Thompson IJ, Albertsen P, Davis BJ, Goldenberg SL, Wolf JS, et al. Adjuvant and salvage radiation therapy after prostatectomy: American Society for Radiation Oncology/American urological association guidelines. Int J Radiat Oncol Biol Phys. 2013;86(5):822–8.
Ghadjar P, Hayoz S, Bernhard J, Zwahlen DR, Hölscher T, Gut P, et al. Acute toxicity and quality of life after dose-intensified salvage radiation therapy for biochemically recurrent prostate Cancer after prostatectomy: first results of the randomized trial SAKK 09/10. J Clin Oncol. 2015;33(35):4158–66.
Ghadjar P, Hayoz S, Bernhard J, Zwahlen DR, Stein J, Holscher T, et al. Impact of dose intensified salvage radiation therapy on urinary continence recovery after radical prostatectomy: results of the randomized trial SAKK 09/10. Radiother Oncol. 2018;126(2):257–62.
Roach M 3rd, Hanks G, Thames H Jr, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix consensus conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965–74.
Acknowledgements
Not applicable.
Funding
No funding to declare.
Author information
Authors and Affiliations
Contributions
MV wrote the manuscript. SC advised and edited the manuscript and proposed the initial concept. KK, KS, MD, JG, WW advised and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present work is approved by the Ethics Commission of the Technical University of Munich (TUM) and is registered with the project number 320/14.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Vogel, M.M.E., Kessel, K.A., Schiller, K. et al. Adjuvant versus early salvage radiotherapy: outcome of patients with prostate cancer treated with postoperative radiotherapy after radical prostatectomy. Radiat Oncol 14, 198 (2019). https://doi.org/10.1186/s13014-019-1391-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-019-1391-0