Abstract
Background
The cranial border of the target volume (TV) in rectal cancer patients treated with neoadjuvant chemoradiation (nCRT) is mostly defined at the level of L5/S1. However, current studies have shown that relapse cranially of the target volume after neoadjuvant nCRT and surgery is very rare. A reduction of cranial TV margins could be reasonable to reduce toxicity to the organs at risk (OAR). In this study we compared the dose distribution to the OAR for different cranial longitudinal margins using a dose-volume histogram (DVH) analysis.
Methods
Ten patients with loco regional advanced rectal cancer were analysed retrospectively. All patients were planned for Volumetric Arc Therapy Radiation Therapy (VMAT). Next to the original PTV (PTV0), three new planning target volumes (PTV) were defined for each patient: The PTV0 reduced by 1 cm, 2 cm and 3 cm on cranial extension. For each PTV a treatment plan with a total dose of 50.4 Gy with daily doses of 1.8 Gy was calculated. Dose to the OAR were evaluated and compared.
Results
For the bone marrow, the small bowel and the peritoneal space all clinically relevant relative dose parameters (V10-V50) as well as the Dmedian could be significantly reduced with every cranial target volume reduction of 1 cm. For V10 of the peritoneal space the dose could be nearly halved with a 3 cm shortened TV. After TV reduction of 3 cm also for the urinary bladder a significant dose reduction of the Dmedian could be achieved.
Conclusions
Considering the very low recurrence rates in the TME and IMRT era, the distribution patterns of these relapses as well as the relevant side effects of nCRT, we would agree with existing recommendations of reduction of the cranial target volume in nCRT treated rectal cancer patients.
Similar content being viewed by others
Background
Neoadjuvant short term radiation therapy (RT, 5 × 5 Gy) or conventional chemoradiation (CRT) are the standard treatment protocols for patients with locally advanced rectal cancer (UICC-Stage II or III) for potential curative disease of the lower and middle third [1]. Several studies have demonstrated that with n(C)RT loco regional relapse rates can be significantly reduced and sphincter preservation rates in lower carcinoma can be improved [2,3,4].
In the last decade there have been significant changes in both, surgery and radiation techniques in the treatment of rectal cancer patients. The total mesorectal excision (TME) alone can provide very good local control rates. In some trials, long term local control, especially for T3 N0 tumors, could reach more than 90% compared with conventional operation techniques [4, 5]. But even though local control rates improved with TME alone, not all institutes can achieve such good results and even with excellent surgical technique, neoadjuvant radiotherapy can usually halve the number of loco regional relapse [4, 6]. Furthermore, intensity modulated radiation therapy (IMRT) of the pelvic is more conformal than conventional radiation therapy and achieves better dose sparing of OAR [7,8,9]. But though modern n(C)RT techniques can effectively improve loco regional control, it is not without side effects. Especially the hematologic and acute gastrointestinal side effects are still relevant and can lead to treatment breaks [10,11,12].
Radiation contouring guidelines mainly based on data before the TME era, although studies dealing with patterns of relapse have shown that loco regional relapse is mainly below the level of S1-S2 [13], without primary nodal involvement and a negative circumferential resection margin even below S2-S3 [14]. The RTOG Consensus Panel for elective Clinical Target Volumes (CTV) in Anorectal Cancer recommends an inclusion of the presacral space where common iliac vessels bifurcate into external/internal iliacs (approximate boney landmark: sacral promontory) [15]. In case of positive lymph nodes, the current International Consensus Guidelines on Clinical Target Volume Delineation in Rectal Cancer recommend an inclusion of the prescaral space at the level of the bifurcation of the aorta in common iliac arteries or 5 mm above the last positive lymph node into the CTV [16].
It was shown that lowering the cranial CTV margins reduces the small bowel exposure with 3D and IMRT radiotherapy for long course neo-adjuvant treatment [17]. With this study we want to verify to what extent a reduction of different cranial CTV margins in nCRT treated rectal cancer patients leads to positive effects on dose-volume distribution.
Methods
Patient characteristics
A total of 10 rectal cancer patients with UICC stadium III (T3N+) treated with a conventional nCRT concept (total dose 50.4 Gy, single dose 1.8 Gy) from 2014 to 2017 in our institution were included in this study. We selected patients with tumors 5–12 cm from the anal verge. All patients received at least one pre-therapeutic MRI, a proctorectoscopy and a planning CT scan. Only those patients were included, where the last macroscopic suspected visible tumor (on primary tumour site or suspected lymph nodes) in pre-treatment MRI could be identified 4 cm below the cranial boarder of the pelvic pre-sacral space (bifurcation of the common iliac arteries). All patients received concomitant chemotherapy. Either 5-Fluoruracil (5FU) on day 1–4 and 29–32 with 1000 mg/qm body surface area (BSA) or Capecitabine 825 mg/qm BSA two times a day, 5 days per week. Patients´ characteristics are shown in Table 1.
Contouring
Contouring and treatment planning was performed using Eclipse 13.0 planning system (Varian Medical Systems, Palo Alto, CA, USA). Contouring was performed on planning CTs with 3 mm slice thickness. All patients were immobilized in prone position. For the definition of the target volumes, we also used MRI-scans and the information of the proctorectoscopy as well as PET-CT/MRI information, if available. The clinical target volume (CTV) definition for standard PTV (PTV0) were based on the recommendations of the RTOG Consensus Panel Contouring Atlas [15].
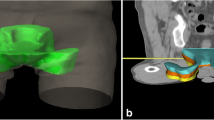
Three new clinical target volumes were defined and extended by 1 cm to create the PTVs. First, the original CTV was reduced by 1, 2 and 3 cm (longitudinal) and if necessary, adjusted to bones and vessels. By using 1 cm safety margin of these CTVs, PTVs were defined for each patient: The standard PTV minus 1 cm cranially (PTV-1), the standard PTV minus 2 cm cranially (PTV-2) and the standard PTV minus 3 cm cranially (PTV-3) (Fig. 1). The goal was to keep the shortest PTV (PTV-3) at least 2 cm above the last macroscopically visible primary tumor or lymph node metastases on MRI scan.
The small bowel, as well as the peritoneal space as a surrogate, the urinary bladder, the bone marrow, the femoral heads and the genitals were contoured as OARs on the planning CT-scans. We defined the pelvic bones as they could be identified on planning CT-scan. Cranial lineation was 2.1 cm above PTV0 and caudal lineation was 2.1 cm below the PTV0. The whole of sacral, iliac, ischial and pubic bone as well as the acetabulum and L5 and L4 were included. The small bowel loops were contoured as they could be identified on planning CT scan. The peritoneal space includes the small bowel, the large bowel, peritonealised large bowel and intraperitoneal vessels. The sexual organs as well as the urinary bladder were excluded. Contouring was done almost analogous to Robyn Banerjee et al. 2012 [18]: Superiorly we used 7 slices above the cranial end of the original PTV. Inferiorly it was 3 mm below the lowest identified small bowel loop or on the level of the peritoneal sigmoid colon. The anterior boarder was the abdominal muscles and posterior the vertebral bodies, vena cava and aorta as well as the psoas muscles. Laterally, the boundary was the pelvic side wall and the muscles.
Treatment planning and evaluation
For all ten patients we optimized four treatment plans for PTV0–3 using Volumetric-modulated arc therapy (VMAT) with two full arcs (358° rotation) and 15 MeV photons. Plans were optimized for treatment with a Varian Clinac DHX linear accelerator (Varian Medical Systems, Palo Alto, CA, USA).
The planning goal was to achieve a homogeneous dose distribution within the PTV and to reduce the dose to OARs, in particular the small bowel, bladder, bone marrow, femoral heads and the genitals.
Dose calculations were performed using the Anisotropic Analytical Algorithm (AAA) and heterogeneity correction. The prescribed dose for the neoadjuvant treatment plans was 50.4 Gy with single doses of 1.8 Gy. All plans were normalized to a median dose of the PTV corresponding to the prescription dose.
To compare dose distribution to the OAR we analysed absolute median dose (Dmedian) and maximum dose (Dmax) on all treatment plans (PTV, PTV-1, PTV-2, PTV-3). For the small bowel loops and the peritoneal space, as a surrogate for the small bowel, we also analysed relative dose parameters (volume receiving 10 Gy, 15 Gy, 20 Gy, 25 Gy, 30 Gy, 35 Gy, 40 Gy, 45 Gy, 50 Gy) for all treatment plans. The volumes receiving 10 Gy, 20 Gy, 30 Gy and 40 Gy (V10 - V40) of the bone marrow and the absolute dose of 65 cc, 100 cc, 180 cc and 830 cc to the peritoneal space were additionally evaluated for all treatment plans.
For all dose parameters of the OAR, a two-sided Wilcoxon test was performed with SPSS 25.0 (SPSS Inc., Chicago, IL, USA) to identify significant differences between the plans for PTV0 and PTV1–3. A p-value < 0.05 was considered to indicate statistical significance.
Results
Dosimetric analysis
The standard PTV (PTV0) (the original planned target volume) differed in the cranial boarder in the 10 patients. The highest extension was at the level of L4 (vertebral middle), the lowest was at the level of L5/S1 (superior/anterior). In mean, it was in the longitudinal middle of L5. The cranial margin of PTV minus 3 cm (PTV-3) varies between the level of L5/S1 (posterior) and the level of S2 (inferior/anterior). Patient characteristics are found in Table 1.
The mean volumes of the PTV0, PTV-1, PTV-2 and PTV-3 were 1524 cc, 1466 cc, 1366 cc and 1255 cc. This is a reduction of just 18% from PTV0 to PTV-3. The dose coverage of all PTVs was performed accurately, which resulted in identical mean Dmean, mean Dmedian and mean Dmax. For the bone marrow, the small bowel loops and the peritoneal space nearly all absolute and relative dose parameters were significantly reduced for the new PTVs (PTV-1, PTV-2, PTV-3) compared to PTV0 (Tables 2 and 3, Fig. 2).
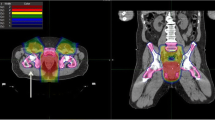
Change in dose distribution after a target volume reduction of 3 cm. Protection of OARs. Differences in dose distribution (colour-wash) of the original bPTV (left) and the cranial 3 cm reduced PTV (3 cm, right). Especially the low and middle dose range covering less volume of the peritoneal space (green shape) and the bladder (yellow shape)
The bone marrow had a mean volume of 1412 cc. Dmedian of the bone volume and all relative dose parameters (V10, V20, V30, V40) for all cranial shortened PTVs (PTV-1, PTV-2 and PTV-3) compared to the PTV0 were significant reduced (p ≤ 0.008). Relatively, the volume in low dose range didn’t decrease as much as in high dose range. The V10 of the PTV-3 (70%) was 19% less than of the PTV0 (87%) whereas the V40 was reduced by 29%. As expected, the Dmax did not change within the different plans (all 54.0 Gy).
The peritoneal space had nearly constant caudal extension to the level of S1/S2 in the different patients. The situation was similar for the small bowel. The mean volume of the peritoneal space was 1847 cc. For both, the small bowel loops and for the peritoneal space, a significant reduction of the relative dose parameters (V10, V15, V20, V25, V30, V35, V40, 45, Dmedian, Dmax) and for the peritoneal space also for the evaluated volumes (180 cc, 830 cc) was shown. The Dmedian for the peritoneal space for the PTV0, PTV-1, PTV-2 and PTV-3 was 20.9 Gy, 15.5 Gy, 9.9 Gy and 5.0 Gy (for all p = 0.005). The biggest relative differences in dose distribution of the peritoneal space are shown for medium-sized volumes (180 cc). Generally the dose to large volumes changed more than for very small volumes. The dose of 830 cc of the peritoneal space for the PTV-1, PTV-2 and PTV-3 was 14.9 Gy, 9.6 Gy and 7.5 Gy and all decreased significantly compared to 18 Gy of the PTV0 (p = 0.012). In contrast, for 65 cc, a significant dose change was just seen for PTV-3. Analogous, the largest differences in the volume of the peritoneal space were seen for low doses. The V10 of the PTV-3 was about halved compared to PTV0 while the V50 could just be reduced by about a quarter.
The mean volume of the small bowel was much higher (2977 cc) and differed much between the patients due to different cranial extensions of the planned CT imaging. This affected the Dmedian but not the relative dose parameters. Compared to the peritoneal space, a similar outcome was found for the relative dose parameters of the small bowel loops. The V10 (18%, 14% and 10%) of PTV-1, PTV-2 and PTV-3 was significant lower (for all p = 0.008) compared to PTV0 (21%) while there was not a big change in V50 (all 0%) compared to 1% (p = 0.028–0.612). The Dmax still remained high for both, small bowel loops and peritoneal space for all PTVs and did not show statistically significant changes.
A significant reduction of the Dmedian for the urinary bladder was just seen with 3 cm lowered PTV. The PTV-3 was 25.7 Gy compared to PTV0 with 28.0 Gy (p = 0.013) while there were no differences in different Dmax. For the sigmoid colon there was a slight decrease in dose distribution with reduced margins of the target volume (not significant) and the Dmax remained almost the same for all PTVs (about 52.0 Gy). Finally, as expected, the Dmedian and Dmax of the femoral head (left) for different PTVs did not differ significantly.
Discussion
Benefit of the reduction of the target volume
We could show that each cranial reduction of 1 cm from the standard PTV in rectal cancer patients of the lower and middle third can significantly reduce the dose to the bone marrow and small bowel (loops & peritoneal space) for almost all relevant dose parameters (Tables 2 and 3, Fig. 2). Also for urinary bladder the Dmedian can be reduced with a 3 cm lowered cranial margin. In similar study approaches, for example in esophageal carcinoma, it has already been shown that a reduction of longitudinal margins would probably lead to an expected lower rate of side effects [19]. So the main question is whether it is to be expected that such margin reductions can also be translated into better therapy tolerance in rectal cancer. In large prospective trials the rate of severe acute enteritis was about 15% [20, 21]. Chronical consequences of small bowel radiation can be obstruction, perforation, fistula, bleeding, persistent diarrhea and malabsorption [22, 23].
The relevance of dose-volume relationships and side effects in different types of pelvic cancer treated with chemoradiation was demonstrated. The importance of dose sparing of bone marrow was required for anal cancer [24]. For anal cancer patients treated with definitive CRT Cheng et al. could proof a highly significant correlation of ≥grade 3 hematologic toxicity with the mean dose and low-dose dose parameters (V5, V10, V15, V20) and recommended dose constraints to the lumbo-sacral-spine with V10 ≤ 80% [25]. In 50 patients treated with IMRT, a higher V20 of the pelvic bone marrow was associated with lower white blood cell nadir (p = 0.048) and patients with V40 ≥ 41% of the lumbo-sacral bone marrow had higher risk to develop ≥Grade 3 hematologic toxicity [26]. The most comparable study design was from Wan et al. [27]. Here the V40 of the lumbosacral spine was associated with clinically significant grade ≥ 2 hematologic toxicity in patients receiving conventional concurrent CRT (50 à 2 Gy, IMRT, Capecitabine) (grade ≥ 2hematologic toxicity with V40 ≥ 60% vs. V40 < 60% was 38.3% vs.13%, p = 0.005). Interestingly, no case of severe acute toxicity was registered in a radiation dose intensification study in rectal cancer [28].
In our study, V10, V20, V30 and V40 of the whole bone of the pelvis (including lumbosacral spine) would be decreased significantly with each cranial reduction of the PTV of 1 cm compared to the standard PTV (PTV0). The V10 for the bone in the pelvis (mainly lumbosacral spine) was 87% for PTV0, 84% for PTV-1, 78% for PTV-2 and 70% for PTV-3 (p ≤ 0.007). The effect was less but still significant in high dose range. The V40 was 29% for PTV0, 27% for PTV-1 (p = 0.017), 24% for PTV-2 (p = 0.007) and 20% for PTV-3 (p = 0.005). Even so the definition of the bone marrow and the type of chemotherapy could vary in those studies, with significant reduced relative dose parameters it is to be assumed that acute ≥2 and ≥ 3 hematologic toxicity would be significantly reduced as well.
The survey and evaluation of the dose distribution on the small bowel is complex. A major problem is the definition of small bowel loops on the planning CT scan because of inconsistent position of the small bowel throughout the treatment course. The evidence for relationships between dose distributions and clinical side effects is still limited, therefore clear recommendations concerning small bowel constraints are still difficult [29]. Some authors prefer the definition of the peritoneal space as a more reliable surrogate for the small bowel, whereas other studies have shown that both approaches, small bowel and peritoneal space, can be useful for dose comparison [18]. Therefore, in our study, we defined the small bowel loops and the peritoneal space. Furthermore all of our rectal cancer patients were irradiated in prone position because there is good evidence that prone position is superior to supine position in terms of dose distribution to the small bowel in patients who underwent pelvic irradiation with 3D conformal RT and most likely applies to the VMAT-IMRT as well [30, 31]. Our study design and peritoneal space definition orientated on Banerjee et al.. They have published data of 67 patients who were irradiated with nCRT and compared peritoneal space versus small bowel and evaluated dose parameters to predicting grade ≥ 3 acute toxicity. The mayor findings were, that peritoneal space V15 less than 830 cc and a small bowel V15 less than 275 cc correlated with < 10% risk of grade ≥ 3 acute toxicity [18]. In our study the volume receiving 15 Gy could be roughly halved with a PTV-reduction of 3 cm. The V15 of the peritoneal space decreased significantly with every cm. Furthermore, we had similar findings for V15 of the small bowel (551 cc, 444 cc, 339 cc and 240 cc; p = 0.008). Though, only the V15 of the peritoneal space for PTV-2 and -3 cm was below 830 cc and for the small bowel only the V15 of the PTV-3 was below 275 cc.
There are several mathematical models to estimate dose/volume-relationships and probability of acute side effects. Roeske et al. e. a. suggest that the volume of the peritoneal space receiving the prescription dose (45–50 Gy) should be < 195 cc [32]. As mentioned, in our study the relative changes in the dose distribution on high dose range wasn’t as high as in low dose range. In our treatment plans the V45 and V50 were 292 cc and 138 cc for the standard PTV whereas it decreased to 213 cc and 103 cc for the PTV-3 (both: p = 0.005). Those changes were all statistic significant. In summary of the results and the clinical relationships of the DVH and the toxicity, it should be noted that cranial target volume reduction can significantly reduce all parameters that have been clinically relevant in the literature. That might reduce acute and late intestinal toxicity but has to be proven in prospective trials which use cranially reduced CTV margins.
The opportunity of smaller cranial margins in n(C)RT treated rectal cancer patients
The opportunity of smaller cranial margins in n(C)RT treated rectal cancer patients, especially with IMRT, of the middle and lower third is currently discussed [33]. In a recently published editorial, Te Vuong, Aurelie Garant & Fleure Gallant summarised the situation very well [34]. The TME trial could show a further reduction of local recurrence with short term neoadjuvant RT. After a median follow up of 6.1 years, RT with 5 × 5 Gy local relapse was 5.6% compared to 10.9% with TME alone [4]. Moreover RT is most effective if the quality of TME is high [35]. Next to the fact that loco-regional relapse after RT and TME is very rare, studies dealing with patterns of local relapse have shown that locoregional relapse is mainly below the level of S1-S2. In an update of the TME-trial, patterns of local relapse were published. Local relapse after RT and TME was still very rare (1.1% of all patients) and most likely posterior or laterally of the primary tumor site [36]. A Swedish study analysed the site of recurrence of rectal cancer patients treated with abdominal resection (mostly with n(C)RT). All cases of relapse (155 of 2315) have been in the lower 75% of the pelvis, means below the S1/S2 interspace [13]. Additionally, in a three-dimensional analysis of recurrence patterns in rectal cancer patients with recurrence after TME, Nijkamp et al. could demonstrate that patient without primary node involvement had no recurrences cranially of the S2-S3 interspace [14]. With CTV reduction to the S2-S3 interspace, over 60% (three-field conventional RT) and 80% (IMRT) reduction in absolute small bowel exposure (dose levels from 15 to 35 Gy) could be achieved. A cranial PTV reduction should therefore be possible without an increased risk for locoregional recurrence rates. Ongoing randomized prospective trials on the timing of surgery after IMRT-based neoadjuvant chemoradiation treatment (ClinicalTrials.gov, number NCT03465982; number NCT02551237) could provide a valid contribution to the definition of the patterns of local relapse and acute toxicity, to consolidate the clinical rationale of the reduction of the cranial target volumes for patients with rectal cancer.
Conclusion
Reduction of the cranial target volume in nCRT for patients with rectal cancer of the lower and middle third can lead to a significant reduction of the dose parameters proven to be crucial for toxicity rates, especially acute gastrointestinal and hematologic side effects. Due to the anatomical conditions, meaningful dose restrictions to OAR that other studies have shown can best be achieved by cranial CTV reduction. Considering the very low recurrence rates in the TME and IMRT era, the distribution patterns of these relapses as well as the relevant side effects of the neoadjuvant irradiation, we would agree with existing recommendations of a reduction of the cranial target volumes at least up to the level of S1/S2 interspace.
Abbreviations
- 2D/3D:
-
2/3 dimensional
- BM:
-
Bone marrow
- BSA:
-
Body surface area
- CRT:
-
Chemoradiation
- CTV:
-
Clinical target volume
- E. a.:
-
For example
- Gy:
-
Gray
- IMRT:
-
Intensity modulated radiotherapy
- nCRT:
-
Neoadjuvant chemoradiation
- OAR:
-
Organs at risk
- PC:
-
Peritoneal Space
- PET:
-
Positron Emission Tomography
- PTV:
-
Planning target volume
- RC:
-
Rectal cancer
- RT:
-
Radiation therapy
- RT:
-
Radiotherapie
- SB:
-
Small bowel
- SIB:
-
Simultaneously integrated boost
- VMAT:
-
Volumetric modulated arc therapy
References
S3-Leitlinie kolorektales Karzinom. Langversion 2.0 - November 2017 - AWMF-Registernummer: 021/007OL. Stuttgart: Thieme; 2017.
Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–33.
Cammà C, Giunta M, Fiorica F, Pagliaro L, Craxì A, Cottone M. Preoperative radiotherapy for resectable rectal cancer: a meta-analysis. JAMA. 2000;284:1008–15.
Peeters KCMJ, Marijnen CAM, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693–701.
Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg. 1998;133:894–9.
Enríquez-Navascués JM, Borda N, Lizerazu A, et al. Patterns of local recurrence in rectal cancer after a multidisciplinary approach. World J Gastroenterol. 2011;17:1674–84.
Portelance L, Chao KS, Grigsby PW, Bennet H, Low D. Intensity-modulated radiation therapy (IMRT) reduces small bowel, rectum, and bladder doses in patients with cervical cancer receiving pelvic and Para-aortic irradiation. Int J Radiat Oncol Biol Phys. 2001;51:261–6.
Mell LK, Tiryaki H, Ahn K-H, Mundt AJ, Roeske JC, Aydogan B. Dosimetric comparison of bone marrow-sparing intensity-modulated radiotherapy versus conventional techniques for treatment of cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71:1504–10.
Wee CW, Kang H-C, Wu H-G, et al. Intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy in rectal cancer treated with neoadjuvant concurrent chemoradiation: a meta-analysis and pooled-analysis of acute toxicity. Jpn J Clin Oncol. 2018;48:458–66.
Mitra D, Hong TS, Horick N, et al. Long-term outcomes and toxicities of a large cohort of anal cancer patients treated with dose-painted IMRT per RTOG 0529. Adv Radiat Oncol. 2017;2:110–7.
Yang TJ, Oh JH, Apte A, Son CH, Deasy JO, Goodman KA. Clinical and dosimetric predictors of acute hematologic toxicity in rectal cancer patients undergoing chemoradiotherapy. Radiother Oncol. 2014;113:29–34.
Fernández-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol. 2010;28:859–65.
Syk E, Torkzad MR, Blomqvist L, Nilsson PJ, Glimelius B. Local recurrence in rectal cancer: anatomic localization and effect on radiation target. Int J Radiat Oncol Biol Phys. 2008;72:658–64.
Nijkamp J, Kusters M, Beets-Tan RGH, et al. Three-dimensional analysis of recurrence patterns in rectal cancer: the cranial border in hypofractionated preoperative radiotherapy can be lowered. Int J Radiat Oncol Biol Phys. 2011;80:103–10.
Myerson RJ, Garofalo MC, El Naqa I, et al. Elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys. 2009;74:824–30.
Valentini V, Gambacorta MA, Barbaro B, et al. International consensus guidelines on clinical target volume delineation in rectal cancer. Radiother Oncol. 2016;120:195–201.
Nijkamp J, Marijnen C, van Herk M, van Triest B, Sonke J-J. Adaptive radiotherapy for long course neo-adjuvant treatment of rectal cancer. Radiother Oncol. 2012;103:353–9.
Banerjee R, Chakraborty S, Nygren I, Sinha R. Small bowel dose parameters predicting grade ≥ 3 acute toxicity in rectal cancer patients treated with neoadjuvant chemoradiation: an independent validation study comparing peritoneal space versus small bowel loop contouring techniques. Int J Radiat Oncol Biol Phys. 2013;85:1225–31.
Münch S, Oechsner M, Combs SE, Habermehl D. DVH- and NTCP-based dosimetric comparison of different longitudinal margins for VMAT-IMRT of esophageal cancer. Radiat Oncol. 2017;12:128.
Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40.
Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773–80.
Vistad I, Kristensen GB, Fosså SD, Dahl AA, Mørkrid L. Intestinal malabsorption in long-term survivors of cervical cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2009;73:1141–7.
Ceelen W, Fierens K, van Nieuwenhove Y, Pattyn P. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer: a systematic review and meta-analysis. Int J Cancer. 2009;124:2966–72.
Franco P, Arcadipane F, Ragona R, et al. Hematologic toxicity in anal cancer patients during combined chemo-radiation: a radiation oncologist perspective. Expert Rev Anticancer Ther. 2017;17:335–45.
Cheng JC-H, Bazan JG, Wu J-K, Koong AC, Chang DT. Lumbosacral spine and marrow cavity modeling of acute hematologic toxicity in patients treated with intensity modulated radiation therapy for squamous cell carcinoma of the anal canal. Pract Radiat Oncol. 2014;4:198–206.
Franco P, Ragona R, Arcadipane F, et al. Dosimetric predictors of acute hematologic toxicity during concurrent intensity-modulated radiotherapy and chemotherapy for anal cancer. Clin Transl Oncol. 2017;19:67–75.
Wan J, Liu K, Li K, Li G, Zhang Z. Can dosimetric parameters predict acute hematologic toxicity in rectal cancer patients treated with intensity-modulated pelvic radiotherapy? Radiat Oncol. 2015;10:162.
Alongi F, Fersino S, Mazzola R, et al. Radiation dose intensification in pre-operative chemo-radiotherapy for locally advanced rectal cancer. Clin Transl Oncol. 2017;19:189–96.
Kavanagh BD, Pan CC, Dawson LA, et al. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010;76:S101–7.
Martin J, Fitzpatrick K, Horan G, et al. Treatment with a belly-board device significantly reduces the volume of small bowel irradiated and results in low acute toxicity in adjuvant radiotherapy for gynecologic cancer: results of a prospective study. Radiother Oncol. 2005;74:267–74.
White R, Foroudi F, Sia J, Marr MA, Lim Joon D. Reduced dose to small bowel with the prone position and a belly board versus the supine position in neoadjuvant 3D conformal radiotherapy for rectal adenocarcinoma. J Med Radiat Sci. 2017;64:120–4.
Roeske JC, Bonta D, Mell LK, Lujan AE, Mundt AJ. A dosimetric analysis of acute gastrointestinal toxicity in women receiving intensity-modulated whole-pelvic radiation therapy. Radiother Oncol. 2003;69:201–7.
Joye I, Haustermans K. Clinical target volume delineation for rectal cancer radiation therapy: time for updated guidelines? Int J Radiat Oncol Biol Phys. 2015;91:690–1.
Vuong, Te; Garant, Aurelie; Gallant, Fleure. Intensity-modulated radiation therapy for patients with rectal cancer: Editorial; 2014. https://www.futuremedicine.com/doi/pdf/10.2217/crc.14.20.
Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811–20.
Kusters M, Marijnen CAM, van de Velde CJH, et al. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur J Surg Oncol. 2010;36:470–6.
Funding
This research did not receive any specific grant from funding agencies in 448 the public, commercial, or not-for-profit sectors.
Availability of data and materials
The present data is summarized in this paper (METHODS). The complete dataset can be retrieved from the authors upon formal request from interested readers.
Author information
Authors and Affiliations
Contributions
DH and SC treated the patients and provided the data and study infrastructure. DH developed the study design, collected, and interpreted data, performed statistical analysis and drafted the manuscript. DH made substantial contributions to conception and design of the study, interpreted data and revised the manuscript. SM, KB, MM, JP and SC contributed significantly to the discussion and interpretation of the results. MO calculated the therapy plans. HD, MO and DH made the main contributions to conception and design of the study, analysed and interpreted data and drafted the manuscript. All co-authors read and revised the revised the manuscript. The final version of the manuscript was approved by all co-authors.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was performed in accordance with the ethics standards at the Technical University of Munich (TUM) (ethical vote: 06.04.2018s, 90/18 S).
Name of committee: Ethikkommission der Technischen Universität München.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Dapper, H., Oechsner, M., Münch, S. et al. Dosimetric analysis and comparison of reduced longitudinal cranial margins of VMAT-IMRT of rectal cancer. Radiat Oncol 13, 169 (2018). https://doi.org/10.1186/s13014-018-1120-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-018-1120-0