Abstract
Background
The importance of cerebrospinal fluid (CSF) diagnostics for psychiatry is growing. The CSF/blood albumin quotient (QAlb) is considered to be a measure of the blood–CSF barrier function. Recently, systematically higher QAlb in males than in females was described in neurological patients. The aim of this study was to investigate whether a sex difference could also be detected in a well-characterized psychiatric cohort.
Methods
The patient cohort comprised 989 patients, including 545 females and 444 males with schizophreniform and affective syndromes who underwent CSF diagnostics, including QAlb measurement. The basic CSF findings and antineuronal autoantibody data of this cohort have already been published. This re-analysis employed analysis of covariance with age correction for QAlb mean values and chi2-testing for the number of increased age-corrected QAlb levels to investigate sex differences in QAlb.
Results
The QAlb levels were elevated above reference levels by 18% across all patients, and a comparison between male and female patients revealed a statistically significant sex difference, with increased values in 26% of male patients and a corresponding rate of only 10% in female patients (chi2 = 42.625, p < 0.001). The mean QAlb values were also significantly higher in males (6.52 ± 3.69 × 10–3) than in females (5.23 ± 2.56 × 10–3; F = 52.837, p < 0.001).
Discussion
The main finding of this study was a significantly higher QAlb level in male compared to female patients with psychiatric disorders, complementing previously described sex differences in neurological patient cohorts. This result indicates bias from some general factors associated with sex and could be partly explained by sex differences in body height, which is associated with spine length and thus a longer distance for CSF flow within the subarachnoid space down the spine from the occipital area to the lumbar puncture site in males compared to females. Hormonal influences caused by different estrogen levels and other sex-specific factors could also play a relevant role. The significance of the study is limited by its retrospective design, absence of a healthy control group, and unavailability of exact measures of spine length.
Similar content being viewed by others
Background
Interest in the immunological pathways underlying the etiology and pathophysiology of a subgroup of patients with mental disorders has increased in recent years. The cerebrospinal fluid (CSF) is a dynamic, metabolically active secretion that can provide important information pertaining to inflammatory changes involving the brain [1]. Therefore, diagnostic examinations of CSF composition have been recommended increasingly in the study of severe mental disorders [2]. The blood–brain barrier (BBB) is the primary interface dividing peripheral circulation from the central nervous system (CNS) [3]. Its most notable component is the cerebral microvascular endothelium, which functions as a diffusion barrier with selective uptake and efflux transporters and specific vesicular transport [3,4,5,6]. Endothelial tight junctions restrict the paracellular pathway between the blood and brain for polar solutes [5, 7]. The CSF/blood albumin quotient (QAlb) has been considered the gold standard for assessment of the BBB function [7]. However, a complicated system of three or more brain barriers and interfaces is now recognized [8,9,10,11]. Therefore, QAlb levels are now considered a measure of the overall blood-CSF-barrier (BCSFB) [12, 13] in the framework of the flow-diffusion model of BCSFB (dys-)function originally proposed by Reiber [13,14,15]. Thus, the overall BCSFB is a construct created to explain the complicated interrelationship between CSF and surrounding tissues and the many dynamic influences of exchange involved, as most recently seen in analysis of CSF composition for clinical purposes. Albumin is synthesized predominantly in the liver, from which it rapidly enters the bloodstream and enters CSF circulation mainly during the process of CSF production by the choroid plexus in the brain ventricles, with albumin representing approximately 80% of normal CSF total protein [12]. However, recent research results also demonstrated that under certain circumstances albumin can also be produced in the brain by microglial cells [16, 17]. With increasing age, the permeability of the BBB/BCSFB increases, whereas CSF production and thus the CSF turnover rate declines [12, 13]. Both of these factors contribute to a distinct age-dependent increase of QAlb; thus, QAlb reference values are generally corrected for age [12, 13]. Moreover, QAlb could be influenced by other factors such as body weight, body mass index, a reduction in CSF production from reasons other than age, alcohol and nicotine consumption [1, 14]. Interestingly, an influence of sex on QAlb was recently also noticed and discussed in two papers that investigated patient cohorts with a spectrum of neurological disorders [18, 19].
The aim of this study was to investigate whether similar sex differences in QAlb can be detected in a cohort of psychiatric patients by reanalyzing the data of a recently published study of a well-characterized sample of patients with schizophreniform and affective psychosis [20].
Methods
The retrospective data analyses received approval from the local ethics committee of the University of Freiburg (EK Fr 396/18). Between January 2006 and November 2019, 992 patients were investigated [20], of which only those patients who had been tested for QAlb were analyzed in the current study (N = 989).
Female and male patient groups
All patients received a lumbar puncture (LP) for the organic clarification of their mental disorder. Schizophreniform syndromes (according to the International Statistical Classification of Diseases and Related Health Problems criteria, version 10 [ICD-10]: F20.X–F29.X, F06.0–2, F10.5–F19.5) and affective syndromes (unipolar depression following ICD-10: F32.X, F33.X, F06.3; bipolar disorder following ICD-10: F30.X, F31X, F06.3) were clinically diagnosed by experienced psychiatrists and classified according to their predominant initial psychiatric syndromes. Preexisting or newly occurring neurological comorbidities were itemized but not considered as exclusion criteria whereas a comorbid diagnosis of dementia (ICD-10: F00.X–F04.X) led to exclusion. The clinical characteristics were extracted from the patients’ discharge letters. The clinical findings and psychometric scores, including Clinical Global Impression (CGI), Global Assessment of Functioning (GAF), and psychopathological scores following the German Association for Methodology and Documentation in Psychiatry (AMDP), were extracted from the basic clinical documentation.
Laboratory methods
Paired CSF and serum samples were drawn at the same time following guideline recommendations [21]. The CSF and serum diagnostics were performed in the CSF laboratory of the Department of Neurology of the University Medical Center Freiburg (https://www.uniklinik-freiburg.de/neurologie/klinik/diagnostische-einrichtungen/liquor-labor.html), and the CSF and serum samples were analyzed immediately after centrifugation. The basic quantitative protein diagnostics via nephelometry included total CSF protein concentration, serum/CSF albumin, and serum/CSF IgG concentration (ProSpect System, Siemens, Erlangen, Germany). The CSF and serum albumin were used to calculate the QAlb using the formula QAlb = [Alb]CSF/[Alb]serum × 1000. The established age-dependent reference values for QAlb were used: < 40 years: < 6.5 × 10–3; 40–60 years: < 8 × 10–3; and > 60 years: < 9.3 × 10–3 [22]. In addition, the white blood cell (WBC) count and cytological staining were assessed by manual microscopy (Leica DMRB, Germany) using a Fuchs-Rosenthal counting chamber (Hecht-Assistant, Germany). All samples with elevated WBC counts were checked for blood contamination. In case of blood contamination the WBC counts were corrected according to the following formula: 1 cell/µl of WBC count reduction per 1000 red blood cells/µl. Isoelectric focusing followed by immunofixation (Hydragel Isofocusing, Sebia, France) were used to determine oligoclonal bands (OCBs).
Instrument-based diagnostics
Electroencephalography (EEG)
The admission procedures included an EEG examination in resting state and under hyperventilation (HV). The evaluation was performed as part of clinical routine [20].
Magnetic resonance imaging (MRI)
At the least, the MRI protocol included T1-weighted (MPRAGE with isotropic 1 mm3 voxels on 3 T or axial 5 mm thick fast spin-echo slices on a 1.5 T scanner), FLAIR (3D SPACE sequence with isotropic 1 mm3 voxels on 3 T or coronal 3 mm thick fast spin-echo slices on a 1.5 T scanner), and DWI sequences (axial 5 mm thick slices). The evaluation was performed by experienced senior physicians in neuroradiology, and the MRI findings were classified as in the main study [20].
Available data sets
The initial study cohort included 992 patients [20], of which the QAlb of 989 patients was available. Those 989 patients were analyzed in the current study. Due to the retrospective approach, some parameters are missing. The available data sets are presented in Table 1.
Statistical analyses
Statistical analysis was carried out using the Statistical Package for the Social Sciences (SPSS), version 24 (IBM Corp., Armonk, NY, USA). The results are mainly presented in a descriptive manner. Independent samples t-tests were conducted comparing age and dimensional variables between the subgroups of patients (without age difference). ANCOVA analyses were used to compare other dimensional variables (e.g., CSF QAlb between female and male patients) between the subgroups corrected for age where indicated. The age-corrected QAlb values were compared using chi2 tests. A binary logistic regression (“Wald statistics”) was performed for age-dependent categorical variables (e.g., the number of elevated CSF protein levels) between groups with age differences. The correlations between QAlb levels and IRDA/IRTA rates, clinical findings (number of suicide attempts and number of earlier inpatient stays), and psychometric scores (GAF, CGI, and AMDP) were analyzed using Spearman correlation. The correlation analyses were performed separately for female and male patients. A p-value of < 0.05 was defined as statistically significant for QAlb comparisons (the primary outcome parameter). This significance level was also used for further group comparisons and correlation analyses. Due to the exploratory approach of the additional statistical analyses, no correction for multiple testing was performed. Figure 1 was created using R [23].
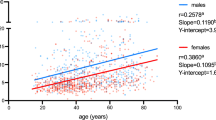
Scatter plot diagram with levels of albumin quotients (y-axis) and sex (x-axis). Single case data is shown. A box plot is added in red (quartiles and median of the distribution; the notches in the box sides give an approximation of the 95% confidence interval around the median). The data are presented on logarithmic scales
Results
Description of the study population
The CSF of 545 female and 444 male patients was analyzed. The two subgroups differed significantly in age (females: 43.77 ± 17.21 years, males: 41.33 ± 18.61 years; p = 0.033). Detailed characterizations are presented in Table 2, and medications at the time of LP or upon admission are listed in Table 3.
CSF-blood albumin quotients
In total, 18% of all patients exhibited elevated QAlb levels compared to age adjusted control levels [22]. Comparing male and female patients revealed a statistically significant difference (chi2 = 42.625, p < 0.001), with a higher number of elevated age-corrected QAlb in male patients (26%) than in female patients (10%). The mean values of the QAlb also differed significantly (F = 52.837, CI = [0.892; 1.676], p < 0.001) between female (M ± SD × 10–3 = 5.23 ± 2.56) and male patients (M ± SD × 10–3 = 6.52 ± 3.69), as illustrated in Fig. 1. This significant sex difference was found irrespective of clinical syndromes (Table 4).
The effect of neurological comorbidity as a confounding factor on sex differences
In order to rule out an effect of neurological comorbidities, a post hoc analysis was performed in the subgroup of patients without neurological comorbidities only. Female (age: M = 42.39 years, SD = 16.42; N = 434) and male (age: M = 38.24 years, SD = 17.26; N = 354) patients without neurological comorbidities (N = 788, 80%) differed significantly in age (F = 1.799, p = 0.001). Significant differences (F = 36.029, p < 0.001) in levels of QAlb were discerned, with higher rates in male (M ± SD × 10–3 = 6.21 ± 3.41) than in female patients (M ± SD × 10–3 = 5.18 ± 2.57) in the age-corrected ANCOVA analysis.
Cerebrospinal fluid basic parameters and instrument-based diagnostics
The CSF protein levels were elevated in male as compared to female patients (β = 0.859, Wald = 39.542, p < 0.001). Overall, male patients exhibited some degree of CSF alteration significantly more frequently than female patients (β = 0.747, Wald = 30.608, p < 0.001). There were no significant sex differences in white blood cell counts, the IgG index, or oligoclonal bands (Table 5). EEG analyses revealed no significant sex differences. Female patients more frequently had inflammatory changes in the MRI (Wald = 4.360, p = 0.037, Table 6).
Correlation analyses
Female patients
The QAlb correlated significantly with the AMDP scores for fears and compulsions (r = − 0.161, p = 0.001; N = 422), and the AMDP scores for delusions (r = − 0.114, p = 0.019; N = 421).
Male patients
The QAlb were significantly correlated with CGI score on admission (r = 0.168, p = 0.001; N = 388), number of earlier suicide attempts (r = 0.213, p = 0.003; N = 197), and the AMDP scores for fears and compulsions (r = -0.161, p = 0.003; N = 352).
Discussion
The main result of this study is a significantly elevated QAlb in men compared to women, which at first glance and in accordance with traditional interpretations suggests that the integrity of the BBB is more often impaired in men than in women with schizophrenic and affective psychosis. However, due to current recognition that there are three or more brain barriers and interfaces [8,9,10,11], QAlb is now considered a measure of the overall BCSFB [12, 13, 15]. QAlb represents a measure of predominantly blood-derived albumin within the CSF in relation to blood albumin concentration. The CSF albumin content determined from lumbar CSF, can potentially be influenced by factors other than plasma concentration. The exchange of CSF and its content with the tissues surrounding the brain ventricles and subarachnoid spaces could alter the composition. Also, variable CSF flow dynamics passing through the subarachnoid spaces to the lumbar puncture site, which lies further down the spine, appears to have an influence.
Integration of our results into the context of current studies
This finding is consistent with the results of two samples of patients with neurological disorders, including some healthy controls [18, 19]. In a first large study including 27,263 patients with neurological diseases, elevated QAlb values were observed more frequently in male patients [19]. However, details about the syndromes, diagnostic findings, and medication were not available. The study also included data from 335 healthy controls (although the definition of “healthy” was not specified), who displayed the same sex differences as well [19]. A similar study including 1209 patients with different neurological disorders also reported similar sex differences [18]. In the present study, these findings could be confirmed in a more homogeneous, well-characterized psychiatric cohort including diagnostic findings and information about potential confounding factors, such as comorbidity or psychopharmacological medication. Another study investigating a large cohort of 1,079,193 participants with unknown characteristics evaluated serum albumin and found sex differences depending on age [24]. In the current study, protein concentrations were also higher in male than in female patients, similar to previous studies [25, 26]. Overall, the sex difference in QAlb levels appears to be independent of neurological or psychiatric diagnoses, which therefore strongly suggests bias from some general factors associated with sex, which remain to be elucidated.
Possible explanations for the sex differences in albumin quotients
Sex-related differences in body height, specifically spine length, may constitute a plausible supporting factor for this bias, given that sex appears to be predominantly associated with a systematic difference in average height. Since body height was unfortunately not recorded in the present cohort, the average heights of the German population were used as an approximate comparison. Measures for the German population from a microcensus of 2017 reported a mean height for ages 18–40 of 181 cm for males and of 167 cm for females, and for ages 40–65, 179 cm for males and 166 cm for females, respectively (Statistisches Bundesamt, https://www.destatis.de). Thus, extrapolating from a general German population broadly comparable to our patient group (female mean age 43.77 years; male mean age 41.33 years) indicates a systematic height difference between the sexes by approximately 13.5 cm. This height difference implies an analogous difference of spine length between the sexes, although leg length might represent a confounder. The assumed sex differences in body height of > 10 cm and, as a consequence, in spine length between men and women, would be able to explain sex-related increases of QAlb in males as compared to females because of the longer CSF flow distance down the spinal cord in men and a comparably shorter CSF flow distance in women because of shorter female spine size in related subarachnoid spaces. This explanatory approach is in line with replicated findings of a rostro-caudal gradient of QAlb with increasing QAlb values in CSF taken at sites down the neuraxis from the site of production in the ventricles to the most distal sites [14, 15, 27]. It also agrees in principle with a decrease in QAlb when CSF was taken in sequential portioned volumes at the lumbar site [15, 28], which led to the general recommendation to always take fixed total volumes of CSF for examination to avoid this volume bias in routine diagnostics [12, 13, 21]. In addition, the suggested explanation matches with the flow-diffusion-model of BCSFB [14, 15]. The slow net flow of CSF down the neuraxis within the subarachnoid spaces is apparently related to and determined by the slow outflow of CSF along spinal nerves [29, 30], first described by Quincke in 1872 but widely forgotten until recently [31, 32].
Further influences could be caused by differences in body mass index (BMI), hormonal factors, and other sex-related factors. Although BMI was also not assessed in the present patient cohort, we expect a similar distribution in our patient population as BMI distribution was similar between the sexes in the general German population (ages 18–40: 25.4 for males vs. 23.4 for females; ages 40–65: 27.3 for males vs. 25.3 for females; Statistisches Bundesamt, https://www.destatis.de). Regarding hormonal factors, it is for example known that estrogens may provide parallel neuroprotective benefits on the stability of the BBB/BCSFB by preventing tight-junction breakdown through the up-regulation of anti-inflammatory annexin 1 (ANXA1) expression and by limiting lymphocyte migration through the modulation of the endothelial intercellular adhesion molecule 1 (ICAM-1) [33], which could indeed contribute to a better functioning of the BBB/BCSFB in females. Other hypothetical causes for the observed sex differences could include a sex-specific expression of secretion molecules, tight junctions or endothelial cells, but also sex-differences in the number of patients with consumption of alcohol or (illegal) drugs in the past.
Clinical consequences
Irrespective of the cause, the QAlb findings should be corrected for sex ratio in future CSF studies in psychiatric and neurological patients. In addition to the established age-dependent correction, the present results suggest adapting or stratifying the reference values of QAlb for sex. If our considerations regarding body height are confirmed in future studies, a correction based on body size or spine length would be preferable. Nevertheless, it should be recognized that even with corrected QAlb a relevant subgroup with BBB/BCSFB dysfunction will persist, which cannot be explained by the bias and thus may relate to yet undefined brain pathologies as suggested from findings of abnormal QAlb in various psychiatric and neurological disorders [20, 34,35,36,37,38,39]. Additionally, it should be noted that a number of other CSF abnormalities have been reported in psychiatric patient cohorts [20, 38, 40,41,42,43,44,45,46]. Progress in this research area for psychiatry will require new and more sensitive methods of CSF analysis and an increased understanding of the complex interactions of biochemical and mechanistic influences on the dynamics of CSF contents under normal and pathological conditions.
Limitations
The limitation of the present study is its retrospective, open, and uncontrolled design. Patients with affective syndromes received an LP only in selected cases because the diagnostic approach has evolved through the years [20, 43,44,45]. Therefore, the sex differences are not representative of all patients with affective syndromes. Due to the open design, patients with neurological comorbidity were also included. Therefore, BBB/BCSFB dysfunctions could, theoretically, also be caused by pathophysiology related to comorbid conditions. However, the QAlb values remained significantly different between sexes even when restricting analyses to patients without neurological comorbidity. Examinations of large, healthy control groups are lacking so far, so the present findings cannot be generalized to neurologically and/or mentally healthy populations because LPs of a large group of healthy volunteers is difficult to justify on ethical grounds. In contrast to previous studies [18, 19], the authors examined a more homogenous, well-characterized group of psychiatric patients. Another limitation is that the QAlb levels were not corrected for blood admixture and this could have influenced the results; however, this effect would apply equally to female and male patients. Unfortunately, the influences of other possible contributing factors, such as body weight, alcohol/nicotine consumption, or diabetes mellitus on BBB/BCSFB function, could not be analyzed in the present study, and body height was only estimated based on the mean heights of the current German population. In addition, exact measures of spine length or suboccipital-to-puncture-site distance for our physiological explanation of the bias were not available. A correction for the potential influence of psychotropic drugs was not performed, because equal numbers of male and female patients had been treated.
Conclusions
The current study reveals a significant sex difference in QAlb levels, with higher levels in males than in females. The present results confirm and extend previous findings of a systematic sex difference in QAlb, which exists not only in neurological patient cohorts but also in psychiatric patients, independent of diagnosis. Irrespective of the underlying pathophysiology, which remains to be elucidated, the present findings suggest the need for an extended correction mode of QAlb reference values in the future.
Availability of data and materials
All necessary information is displayed descriptively in the results section.
References
Deisenhammer F, Bartos A, Egg R, Gilhus NE, Giovannoni G, Rauer S, Sellebjerg F. Guidelines on routine cerebrospinal fluid analysis. Report from an EFNS task force. Eur J Neurol. 2006;13(9):913–22. https://doi.org/10.1111/j.1468-1331.2006.01493.x.
Pollak TA, Lennox BR, Muller S, Benros ME, Pruss H, Tebartz van Elst L, Klein H, Steiner J, Frodl T, Bogerts B, Tian L, Groc L, Hasan A, Baune BT, Endres D, Haroon E, Yolken R, Benedetti F, Halaris A, Meyer JH, Stassen H, Leboyer M, Fuchs D, Otto M, Brown DA, Vincent A, Najjar S, Bechter K. Autoimmune psychosis: an international consensus on an approach to the diagnosis and management of psychosis of suspected autoimmune origin. Lancet Psychiatry. 2020;7(1):93–108. https://doi.org/10.1016/s2215-0366(19)30290-1.
Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37(1):26–322. https://doi.org/10.1016/j.nbd.2009.07.031.
Carvey PM, Hendey B, Monahan AJ. The blood-brain barrier in neurodegenerative disease: a rhetorical perspective. J Neurochem. 2009;111(2):291–314. https://doi.org/10.1111/j.1471-4159.2009.06319.x.
Serlin Y, Shelef I, Knyazer B, Friedman A. Anatomy and physiology of the blood–brain barrier. Semin Cell Dev Biol. 2015;38:2–6. https://doi.org/10.1016/j.semcdb.2015.01.002.
Spieler D, Namendorf C, Namendorf T, von Cube M, Uhr M. Donepezil, a cholinesterase inhibitor used in Alzheimer's disease therapy, is actively exported out of the brain by abcb1ab p-glycoproteins in mice. J Psychiatr Res. 2020;124:29–33. https://doi.org/10.1016/j.jpsychires.2020.01.012 (Epub 2020 Jan 27).
Pollak TA, Drndarski S, Stone JM, David AS, McGuire P, Abbott NJ. The blood-brain barrier in psychosis. Lancet Psychiatry. 2018;5(1):79–92. https://doi.org/10.1016/s2215-0366(17)30293-6.
Brochner CB, Holst CB, Mollgard K. Outer brain barriers in rat and human development. Front Neurosci. 2015;9:75. https://doi.org/10.3389/fnins.2015.00075.
Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol. 2009;31(4):497–511. https://doi.org/10.1007/s00281-009-0177-0.
Erickson MA, Banks WA. Neuroimmune axes of the blood-brain barriers and blood-brain interfaces: bases for physiological regulation, disease states, and pharmacological interventions. Pharmacol Rev. 2018;70(2):278–314. https://doi.org/10.1124/pr.117.014647.
Erickson MA, Nicolazzo JA, Banks WA. Commentary on the 2018 Named Series on blood-brain interfaces: roles of neuroimmunomodulation in health and disease. Brain Behav Immun. 2018;74:3–6. https://doi.org/10.1016/j.bbi.2018.08.016.
Tumani H, Huss A, Bachhuber F. The cerebrospinal fluid and barriers - anatomic and physiologic considerations. Handb Clin Neurol. 2017;146:21–322. https://doi.org/10.1016/b978-0-12-804279-3.00002-2.
Wildemann B, Oschmann P, Reiber H. Laboratory diagnosis in neurology 1st. edition ed. Stuttgart: Thieme; 2010.
Reiber H. Flow rate of cerebrospinal fluid (CSF)–a concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J Neurol Sci. 1994;122(2):189–203. https://doi.org/10.1016/0022-510x(94)90298-4.
Reiber H, Uhr M. Physiologie des Liquors. In: Berlit P, editor. Klinische Neurologie. Berlin: Springer Berlin Heidelberg; 2018. p. 1–19.
Ahn SM, Byun K, Cho K, Kim JY, Yoo JS, Kim D, Paek SH, Kim SU, Simpson RJ, Lee B. Human microglial cells synthesize albumin in brain. PLoS ONE. 2008;3(7):e2829. https://doi.org/10.1371/journal.pone.0002829.
Park JH, Park JA, Ahn JH, Kim YH, Kang IJ, Won MH, Lee CH. Transient cerebral ischemia induces albumin expression in microglia only in the CA1 region of the gerbil hippocampus. Mol Med Rep. 2017;216(1):661–5. https://doi.org/10.3892/mmr.2017.6671(Epub 2017 May 31).
Castellazzi M, Morotti A, Tamborino C, Alessi F, Pilotto S, Baldi E, Caniatti LM, Trentini A, Casetta I, Granieri E, Pugliatti M, Fainardi E, Bellini T. Increased age and male sex are independently associated with higher frequency of blood–cerebrospinal fluid barrier dysfunction using the albumin quotient. Fluids Barriers CNS. 2020. https://doi.org/10.1186/s12987-020-0173-2.
Parrado-Fernandez C, Blennow K, Hansson M, Leoni V, Cedazo-Minguez A, Bjorkhem I. Evidence for sex difference in the CSF/plasma albumin ratio in ~20 000 patients and 335 healthy volunteers. J Cell Mol Med. 2018;22(10):5151–4. https://doi.org/10.1111/jcmm.13767.
Endres D, Meixensberger S, Dersch R, Feige B, Stich O, Venhoff N, Matysik M, Michel M, Runge K, Nickel K, Urbach H, Domschke K, Prüss H, Tebartz van Elst L. Cerebrospinal fluid, antineuronal autoantibody, EEG, and MRI findings from 992 patients with schizophreniform and affective psychosis. Transl Psychiatry. 2020;10(1):279. https://doi.org/10.1038/s41398-020-00967-3.
Engelborghs S, Niemantsverdriet E, Struyfs H, Blennow K, Brouns R, Comabella M, Dujmovic I, van der Flier W, Frolich L, Galimberti D, Gnanapavan S, Hemmer B, Hoff E, Hort J, Iacobaeus E, Ingelsson M, Jan de Jong F, Jonsson M, Khalil M, Kuhle J, Lleo A, de Mendonca A, Molinuevo JL, Nagels G, Paquet C, Parnetti L, Roks G, Rosa-Neto P, Scheltens P, Skarsgard C, Stomrud E, Tumani H, Visser PJ, Wallin A, Winblad B, Zetterberg H, Duits F, Teunissen CE. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimers Dement. 2017;8:111–26. https://doi.org/10.1016/j.dadm.2017.04.007.
Stich O, Rauer S, Kaiser R Liquordiagnostik. In Neurologie compact. Für Klinik und Praxis. Hufschmidt A, Lücking CH, Rauer S. 2013. 6th edition, Thieme, Stuttgart. DOI: 10.1055/b-0034-66239
R Development Core Team (2008). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3–900051–07–0, https://www.r-project.org.
Weaving G, Batstone GF, Jones RG. Age and sex variation in serum albumin concentration: an observational study. Ann Clin Biochem. 2016;53(Pt 1):106–11. https://doi.org/10.1177/0004563215593561.
McCudden CR, Brooks J, Figurado P, Bourque PR. Cerebrospinal fluid total protein reference intervals derived from 20 years of patient data. Clin Chem. 2017;63(12):1856–65. https://doi.org/10.1373/clinchem.2017.278267 (Epub 2017 Oct 11).
Castellazzi M, Pizzicotti S, Lombardo I, Alfiero S, Morotti A, Pellegatti P, Negri G, Natali L, Ferri C, Fainardi E, Bellini T, Pugliatti M. Sexual dimorphism in the cerebrospinal fluid total protein content. Clin Chem Lab Med. 2020. https://doi.org/10.1515/cclm-2020-0419 (Online ahead of print).
Reiber H. Proteins in cerebrospinal fluid and blood: barriers, CSF flow rate and source-related dynamics. Restor Neurol Neurosci. 2003;21(3–4):79–96.
Seyfert S, Faulstich A. Is the blood-CSF barrier altered in disease? Acta Neurol Scand. 2003;108(4):252–6. https://doi.org/10.1034/j.1600-0404.2003.00119.x.
Bechter K. The peripheral cerebrospinal fluid outflow pathway - Physiology and pathophysiology of CSF recirculation: A review and hypothesis. Neurol Psychiatry Brain Res. 2011. https://doi.org/10.1016/j.npbr.2011.06.003ss.
Bechter K, Schmitz B. Cerebrospinal fluid outflow along lumbar nerves and possible relevance for pain research: case report and review. Croatian Med J. 2014;55(4):399–404. https://doi.org/10.3325/cmj.2014.55.399.
Bechter K, Hof PR, Benveniste H. On the flow dynamics of cerebrospinal fluid. Neurol Psychiatry Brain Res. 2015;21(2):96–103. https://doi.org/10.1016/j.npbr.2015.01.001.
Benveniste H, Hof PR, Nedergaard M, Bechter K. Modern cerebrospinal fluid flow research and Heinrich Quincke's seminal 1872 article on the distribution of cinnabar in freely moving animals. J Comp Neurol. 2015;523(12):1748–55. https://doi.org/10.1002/cne.23758.
Maggioli E, McArthur S, Mauro C, Kieswich J, Kusters DH, Reutelingsperger CP, Yaqoob M, Solito E. Estrogen protects the blood-brain barrier from inflammation-induced disruption and increased lymphocyte trafficking. Brain Behav Immun. 2016;51:212–22. https://doi.org/10.1016/j.bbi.2015.08.020.
Brettschneider J, Claus A, Kassubek J, Tumani H. Isolated blood-cerebrospinal fluid barrier dysfunction: prevalence and associated diseases. J Neurol. 2005;252(9):1067–73. https://doi.org/10.1007/s00415-005-0817-9.
Gudmundsson P, Skoog I, Waern M, Blennow K, Zetterberg H, Rosengren L, Gustafson D. Is there a CSF biomarker profile related to depression in elderly women? Psychiatry Res. 2010;176(2–3):174–8. https://doi.org/10.1016/j.psychres.2008.11.012 (Epub 2010 Feb 4).
Zetterberg H, Jakobsson J, Redsäter M, Andreasson U, Pålsson E, Ekman CJ, Sellgren C, Johansson AG, Blennow K, Landén M. Blood-cerebrospinal fluid barrier dysfunction in patients with bipolar disorder in relation to antipsychotic treatment. Psychiatry Res. 2014;217(3):143–6. https://doi.org/10.1016/j.psychres.2014.03.045 (Epub 2014 Apr 5).
Skillbäck T, Delsing L, Synnergren J, Mattsson N, Janelidze S, Nägga K, Kilander L, Hicks R, Wimo A, Winblad B, Hansson O, Blennow K, Eriksdotter M, Zetterberg H. CSF/serum albumin ratio in dementias: a cross-sectional study on 1861 patients. Neurobiol Aging. 2017;59:1–9. https://doi.org/10.1016/j.neurobiolaging.2017.06.028 (Epub 2017 Jul 11).
Orlovska-Waast S, Köhler-Forsberg O, Brix SW, Nordentoft M, Kondziella D, Krogh J, Benros ME. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(6):869–87. https://doi.org/10.1038/s41380-018-0220-4.
Musaeus CS, Gleerup HS, Høgh P, Waldemar G, Hasselbalch SG, Simonsen AH. Cerebrospinal fluid/plasma albumin ratio as a biomarker for blood-brain barrier impairment across neurodegenerative dementias. J Alzheimers Dis. 2020;75(2):429–36. https://doi.org/10.3233/JAD-200168.
Maxeiner HG, Rojewski MT, Tumani H, Herzog S, Fuchs D, Schmitt A, Schmitt M, Bechter K. Immunological and histochemical analyses of cerebrospinal fluid and peripheral blood from patients with neurological and psychiatric disorders. Acta Neuropsychiatr. 2009;21(Suppl 2):51–7. https://doi.org/10.1017/s0924270800032737.
Bechter K, Reiber H, Herzog S, Fuchs D, Tumani H, Maxeiner HG. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J Psychiatr Res. 2010;44(5):321–30. https://doi.org/10.1016/j.jpsychires.2009.08.008.
Bechter K. CSF diagnostics in psychiatry–present status–future projects. Neurol Psychiatry Brain Res. 2016. https://doi.org/10.1016/j.npbr.2016.01.008.
Endres D, Dersch R, Hottenrott T, Perlov E, Maier S, van Calker D, Hochstuhl B, Venhoff N, Stich O, van Elst LT. Alterations in cerebrospinal fluid in patients with bipolar syndromes. Front Psychiatry. 2016;7:194. https://doi.org/10.3389/fpsyt.2016.00194.
Endres D, Perlov E, Baumgartner A, Hottenrott T, Dersch R, Stich O, Tebartz van Elst L. Immunological findings in psychotic syndromes: a tertiary care hospital's CSF sample of 180 patients. Front Hum Neurosci. 2015;9:476. https://doi.org/10.3389/fnhum.2015.00476.
Endres D, Perlov E, Dersch R, Baumgartner A, Hottenrott T, Berger B, Stich O, Tebartz van Elst L. Evidence of cerebrospinal fluid abnormalities in patients with depressive syndromes. J Affect Disord. 2016;198:178–84. https://doi.org/10.1016/j.jad.2016.03.030.
Bechter K, Deisenhammer F. Psychiatric syndromes other than dementia. Handb Clin Neurol. 2017;146:285–96. https://doi.org/10.1016/b978-0-12-804279-3.00017-4.
Acknowledgements
DS and DE were funded by the Berta-Ottenstein-Programme for Advanced Clinician Scientists, Faculty of Medicine, University of Freiburg.
Funding
Open Access funding enabled and organized by Projekt DEAL. The article processing charge was funded by the Baden-Wuerttemberg Ministry of Science, Research and Art and the University of Freiburg in the funding programme Open Access Publishing.
Author information
Authors and Affiliations
Contributions
SMe, KB, and DE wrote the paper. LTvE, SMe, and DE created the study design. RD performed and interpreted the CSF basic analyses. BF supported EEG analyses and statistical analyses. SMa created Fig. 1 and supported statistical analyses. KB worked out the interpretation about the sex bias by CSF flow principles and beyond. HU was responsible for MRI analyses and interpretation. MAS, KR, DD, KN, DS, HU, HP, KD and LTvE supported the clinical interpretation and critically revised the manuscript. All authors were critically involved in the theoretical discussion and composition of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This retrospective analysis received approval from the local ethics committee of the University of Freiburg (EK Fr 396/18). The patients gave their written informed consent before the lumbar puncture.
Consent for publication
Not applicable.
Competing interests
SMe: None. KB: None. RD: Lecture fees from Roche and travel grants from Biogen. BF: None. SMa: None. MAS: None. KR: None. DD: None. KN: None. DS: None. HU: Shareholder of the Veobrain: HP: None. KD: Steering Committee Neurosciences, Janssen. LTvE: Advisory boards, lectures, or travel grants within the last three years: Roche, Eli Lilly, Janssen-Cilag, Novartis, Shire, UCB, GSK, Servier, Janssen and Cyberonics. DE: None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Meixensberger, S., Bechter, K., Dersch, R. et al. Sex difference in cerebrospinal fluid/blood albumin quotients in patients with schizophreniform and affective psychosis. Fluids Barriers CNS 17, 67 (2020). https://doi.org/10.1186/s12987-020-00223-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12987-020-00223-2