Abstract
Background
The principal function of iodine acts on thyroid function, but in recent years, the role of iodine deficiency in metabolism has also been gradually revealed. We aimed to investigate the current status of iodized salt consumption and urinary iodine concentration (UIC) in an urban Chinese population with type 2 diabetes, and to further explore whether UIC was associated with diabetic microvascular complications.
Methods
Four thousand five hundred fifty-nine subjects with diabetes from 7 communities in downtown Shanghai were enrolled in the cross-sectional Metal Study in 2018. UIC was detected using an inductively coupled plasma-mass spectrometer. Diabetic kidney disease (DKD) was defined as urinary albumin-to-creatinine ratio (UACR) > 30 mg/g or estimated glomerular filtration rate < 60 mL/min/1.73 m2. Diabetic retinopathy (DR) was evaluated by high-quality fundus photographs and was remotely read by ophthalmologist.
Results
The median UIC of subjects with diabetes was 115.4 μg/L (78.9–170.8) in downtown Shanghai. Among all the subjects, 52.7% consumed non-iodized salt and 40.4% were iodine deficient. Iodine deficiency (UIC < 100 μg/L) was associated with an increased odds of DKD (OR 1.17; 95%CI 1.01–1.37) after adjustment for age, sex, education, current smokers, BMI, HbA1c, duration of diabetes, dyslipidemia, thyroid-stimulating hormone and free thyroxine. No association was observed between UIC and DR after multivariable adjustment.
Conclusions
A concerning number of subjects with diabetes consumed non-iodized salt and suffered from iodine deficiency in coastal regions of China. Low UIC might be a risk factor for DKD, which should be further confirmed by longitudinal prospective studies.
Similar content being viewed by others
Introduction
Type 2 Diabetes Mellitus (T2DM) has become a serious global health care burden, causing microvascular complications which are associated with increased disability, reduced quality of life and life expectancy [1, 2]. It was estimated that there were 451 million cases of adult diabetes worldwide in 2017 and the number was projected to increase to 693 million by 2045 [3], leading to high incidence and prevalence of microvascular complications. Approximately one third with T2DM will develop diabetic retinopathy (DR), and one quarter will develop diabetic kidney disease (DKD) [4]. Thus, it is critical to identify and control some novel modifiable risk factors contributing to microvascular complications in patients with T2DM.
Iodine is an indispensible micronutrient for the synthesis of thyroid hormones. Iodine deficiency (ID) in early life impairs neurodevelopment, and also has many adverse effects throughout various life stages [5]. Over the past 20 years, substantial progress has been achieved in the worldwide effort to eliminate iodine deficiency disorders (IDD) by salt iodization program. However, this program is vulnerable and requires a long-term commitment from governments. In several countries where ID had been once eliminated, salt iodization programs were discontinued and ID has now reappeared [6].
The principal function of iodine acts on thyroid function, but in recent years, the role of ID in metabolism has also been gradually revealed. Analysis of data from the National Health and Nutrition Survey (NHANES) 2007–2012 found that in US adults, low urinary iodine concentration (UIC) was associated with dyslipidemia [7] and coronary artery disease [8]. Moreover, O. S. Al-Attas et al. [9] reported that UIC is markedly decreased in T2DM and urinary iodine was negatively associated with insulin resistance in patients with T2DM. Most recently, Mingyue Jin et al. [10] also found that at a lower UIC (< 100 μg/L), the prevalence of diabetes significantly increased relative to an UIC of 100–299 μg/L. Animal studies also showed that iodine supplementation could reduce the blood glucose levels and improve the insulin sensitivity in goats [11]. However, the role of iodine nutrition on diabetic microvascular complications has not been studied.
The aim of this study was to investigate the current status of iodized salt consumption and UICs in an urban Chinese population with T2DM, and additionally whether UIC is associated with diabetic microvascular complications including DKD and DR.
Methods
Study population
The cross-sectional METAL study (Environmental Pollutant Exposure and Metabolic Diseases in Shanghai, www.chictr.org.cn, ChiCTR1800017573) was launched to investigate the association between iodine nutrition and microvascular complications in Chinese adults with diabetes. We recruited study participants from seven communities in Huangpu and Pudong new district, Shanghai, China. Huangpu district, located in downtown Shanghai, is the administrative, economic and cultural center of the metropolitan coastal city [12]. Pudong new district is the symbol of China’s reform and opening-up [13]. We randomly selected half of patients with diabetes from the registration platform in each community healthcare center. Chinese citizens ≥18 years old who had lived in their current area for ≥6 months were included. In August 2018, a total of 4813 subjects with T2DM who were 23–99 years of age received an examination. Participants with missing UIC values (n = 254) were excluded. Finally, 4559 participants were involved in the present analysis.
The study received ethical approval from the Ethics Committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine. All procedures followed were abided by the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the appropriate institutional review committee. Informed consent was received from all participants included in the study prior to the data collection.
Measurements
The same well-trained and experienced personnel in SPECT-China study [14,15,16] used a questionnaire to collect information on socio-demographic characteristics, education, medical history, family history, and lifestyle risk factors. Weight and height were measured using a balance beam and a vertical ruler in light clothing and without shoes. Body mass index (BMI) was calculated as the ratio of weight in kilograms divided by height in meters squared. Current smoking was defined as having smoked at least 100 cigarettes in one’s lifetime and currently smoking cigarettes [17]. Especially, “For the past three years, which type of salt was used in your family” was applied to collect information about type of salt. Three options for this item were provided: (1) only iodized salt; (2) only non-iodized salt; (3) both.
Blood samples were drawn between 6:00 am and 9:00 am after an overnight fast. Blood was refrigerated immediately after phlebotomy, and in 2 hours it was centrifugated and the serum was aliquoted and frozen in a central laboratory. Glycated hemoglobin (HbA1c) was measured by high-performance liquid chromatography (MQ-2000PT, Medconn, Shanghai, China). Fasting plasma glucose, serum creatinine, triglycerides, total cholesterol, high (HDL) and low-density lipoprotein (LDL) were performed with a Beckman Coulter AU 680 (Brea, USA). Serum thyroid-stimulating hormone (TSH) and free thyroxine (FT4) were measured by electrochemiluminescence (Roche, E601, Germany).
Morning fasting spot urine samples collected were refrigerated immediately and frozen at − 20 °C in 2 hours. UIC was detected using an inductively coupled plasma-mass spectrometer (ICP-MS, No. 7700x, Agilent Technologies Inc., USA). The concentrations of urine albumin and creatinine were determined with a Beckman Coulter AU 680 (Brea, USA) using a turbidimetric immunoassay and an enzymatic method, respectively. Urinary albumin-to-creatinine ratio (UACR) was calculated as the urinary albumin concentrations divided by the urinary creatinine concentrations and expressed in mg/g.
DR screening was evaluated by mydriatic binocular indirect ophthalmoscopy (Topcon TRC-NW400 Non-Mydriatic Retinal Camera, Oakland, USA). Fundus photographs were read by an experienced ophthalmologist specialized in retina.
Outcome definition
Dyslipidemia was defined as total cholesterol ≥6.22 mmol/L (240 mg/dL), triglycerides ≥2.26 mmol/L (200 mg/dL), LDL ≥ 4.14 mmol/L (160 mg/dL), HDL < 1.04 mmol/L (40 mg/dL), or self-reported previous diagnosis of hyperlipidemia by physicians, according to the modified National Cholesterol Education Program-Adult Treatment Panel III.
The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation for “Asian origin” [18]. As suggested by American Diabetes Association (ADA), high UACR was defined as UACR ≥30 mg/g, reduced eGFR as eGFR < 60 ml/min/1.73m2, and DKD as UACR > 30 mg/g or eGFR < 60 mL/min/1.73 m2 [19].
The internationally accepted DR classification by the “Global Diabetic Retinopathy Project Group” in 2003 was applied [20]. The classification was: no retinopathy, non-proliferative DR (intraretinal micro-aneurysms, hemorrhages, venous beading, prominent microvascular abnormalities) and proliferative DR (neovascularization or vitreous/preretinal hemorrhages).
Statistical analysis
Statistical analysis was run with SPSS 24.0 (IBM Corporation, Armonk, NY, USA). General characteristics are presented as median with the interquartile range (IQR) for continuous variables or as proportion for categorical variables. Mann-Whitney U test and the Kruskal-Wallis test were used for comparison of two or more groups of non-normally distributed data. Pearson’s χ2 tests were performed to compare categorical variables.
The associations of UIC with elevation of UACR, reduction of eGFR, DKD and DR were analyzed with logistic regression analyses. The results are presented as odds ratio (OR) and 95% confidence intervals (CIs). Model 1 was unadjusted. Model 2 was adjusted for age, sex, education, current smokers, BMI, HbA1C, duration of diabetes, dyslipidemia, TSH and FT4.
Results
General characteristics of the study population
The general characteristics are presented in Table 1. The mean age of the study population was 67.0, nearly one half (53.9%) were women. About 45.5% of the study population were overweight or obese (BMI ≥25 kg/m2), and 17.9% were current smokers. The percentage of an educational level beyond high school was 50.9% and the average duration of diabetes was 9 years.
Compared to those with adequate iodine nutrition, subjects with ID were slightly older and were more likely to be women. These subjects also had higher TSH, lower BMI and a lower percentage of current smokers. In addition, subjects with more than adequate and excess iodine nutrition were slightly younger and had higher BMI, but comparable TSH.
Urinary iodine concentration and type of salt intake in the population
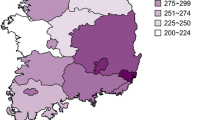
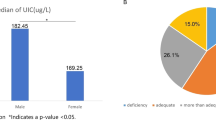
The distribution of UICs in the study population is presented in Fig. 1. The median (25th–75th percentile) UIC of subjects with diabetes was 115.4 μg/L (78.9–170.8) in downtown Shanghai, which falls within the range of 100-199 μg/L that WHO/UNICEF/ICCIDD categorize as adequate. Urinary iodine measurements indicative of ID (UIC < 100 μg/L) were present in 40.4% of the study population. Meanwhile, 10.0 and 6.2% of the population showed more than adequate (UIC 200–299.9 μg/L) and excess iodine intake (UIC ≥ 300 μg/L), respectively.
The distribution of type of salt intake is presented in Fig. 2. As high as 52.7% of the study population consumed non-iodized salt, 27.0% consumed iodized salt, and 20.3% consumed both. Logistic regression analysis showed that compared to those who consumed iodized salt, subjects consumed non-iodized salt were more likely to be women (OR 1.27, 95%CI 1.10–1.47) and have a higher educational attainment (OR 1.29, 95%CI 1.12–1.50), but a comparable age (OR 0.99, 95%CI 0.98–1.01).
Association of urinary iodine concentration with elevation of UACR, reduction of eGFR and DKD
The association of UIC with elevation of UACR, reduction of eGFR and DKD is shown in Table 2. Compared with those with adequate iodine nutrition, subjects with ID had an increased risk of elevation of UACR (OR 1.17, 95%CI 1.02–1.36), reduction of eGFR (OR 1.35, 95%CI 1.03–1.79) and DKD (OR 1.19, 95%CI 1.04–1.37). Adjustment for age, sex, education, current smokers, BMI, HbA1C, duration of diabetes, dyslipidemia, TSH and FT4 did not attenuate the association of ID with UACR and DKD. However, multivariable adjustment weakened the association between ID and reduction of eGFR further such that it was no longer significant. Meanwhile, subjects with more than adequate and excess iodine nutrition did not have an increased risk of elevation of UACR, reduction of eGFR and DKD after multivariable adjustment.
Association of urinary iodine concentration with DR
Table 3 presents the association of UIC with non-proliferative and proliferative DR. Compared with those with adequate iodine nutrition, the ORs of non-proliferative and proliferative DR in subjects with more than adequate iodine nutrition were 0.73 (95%CI 0.54–0.99) and 0.35 (95%CI 0.08–1.49), respectively. Multivariable adjustment weakened the association between more than adequate iodine nutrition and non-proliferative DR further such that the association was no longer significant. There was no significant association observed between DR and ID and excess iodine nutrition.
Discussion
In this study among over 4500 community-dwelling Chinese adults with diabetes, we found that 52.7% of the subjects consumed non-iodized salt, and 40.4% had ID. Iodine deficiency was significantly associated with a higher prevalence of elevated UACR and DKD, independently of age, sex, education, current smokers, BMI, HbA1C, duration of diabetes, dyslipidemia, TSH and FT4. To the best of our knowledge, this is the first study to investigate the current status of iodized salt consumption and iodine nutrition status in a relatively large population with diabetes, and further investigate the association between UIC and diabetic microvascular complications.
China was once severely affected by IDD, and hence, a mandatory Universal Salt Iodization (USI) program was introduced in 1996, and was successful in eliminating IDD. However, since the prevalence of thyroid diseases has markedly increased in recent years, some concerns about the USI have circulated, especially among coastal residents in urban areas [21]. In the present analysis, the median UIC of residents with diabetes in downtown Shanghai has fallen to marginal levels of iodine sufficiency (115.4 μg/L), and more surprisingly, more than half (52.7%) of the subjects consumed non-iodized salt and 40.4% were iodine deficient. Compared with a study conducted by the Shanghai Municipal Center for Disease Control and Prevention (CDC) in 2009, 95.3% of participants used iodized salt and 28.6% were iodine deficient at that time [22]. During 2011–2012, Zhongyan Shan and her colleagues performed a cross-sectional study in eastern and central China and reported that the median UIC was 197 μg/L in school-aged children and 205 μg/L in the total cohort population [23]. Our previous study conducted on the general population found that 46.4% consumed non-iodized salt in the urban area of Shanghai in 2016 [24].
The present study indicates that an increasing number of urban residents in downtown Shanghai prefer to use non-iodized salt in recent years and suffer from ID. Women and those with a higher educational level were more tended to consume non-iodized salt. Why? Changes in the reported spectrum and growing incidence of thyroid disorders have been linked to the increased iodine intake resulting from USI in the local media and international medical literature [25]. Those with high educational attainment were more likely to be confused by these information and worry about their thyroid health, and call for liberalizing provincial control of sales of non-iodized salt. Formerly needed a prescription, the sale of non-iodized salt has now been unofficially allowed by some coastal city authorities [26]. In addition, T2DM is associated with an increased risk of multiple thyroid disorders such as thyroid nodule [27], thyroid cancer [28], and autoimmune thyroid diseases [29]. Since the prevalence of thyroid abnormalities were found to be much higher in females than males [30], it is reasonable to deduce that these women would have a higher tendency towards consuming non-iodized salt after diagnosis of thyroid abnormalities.
Considering that Shanghai is a coastal city, local residents believe that they should never suffer from IDD with high iodine-enriched aquatic products consumption. However, it may not be true to rely on consumption of seafood alone to provide sufficient iodine. Actually, the environmental levels of iodine in Shanghai are deficient (< 10 μg/L) [23]. Furthermore, based on the research initiated by Shanghai Municipal CDC, iodized salt contributed 63.5% of the total dietary iodine in Shanghai [22]. Aquatic products, which residents thought to be rich in iodine, accounted for only 5.03% of the total dietary iodine [22].Thus, iodized salt is still the main source for iodine supplementation in coastal populations.
DKD was commonly defined by ADA as “UACR≥30mg/g or eGFR<60 ml/min per 1.73 m2” [31]. We found that ID was associated with elevated UACR and DKD. The mechanism underlying the association of ID with DKD is not yet fully understood. Deficiency of iodine could reduce thyroid hormone production and elevate TSH. It has been reported that lower free triiodothyronine and elevated TSH have significant association with risk for albuminuria in T2DM [32, 33]. Moreover, inadequate iodine intake is significantly correlated with an increase in oxidative stress [34]. Recent evidence has shown that inflammatory cytokines such as tumor necrosis factor-alpha and interleukin-1 play a pivotal role in the pathogenesis of DKD [35]. Therefore, we suppose the possible mechanism may be via inflammatory response.
In view of the ever-growing prevalence of T2DM and DKD all over the world, successive intervention in this large population can have important impact on public health. ID, unlike most micronutrient deficiencies, is not restricted to people in developing countries with poor diets. Since salt iodization is simple, effective and inexpensive, the best strategy to control ID is addition of iodine into salt in nearly all countries [36]. Monitoring iodine situation of people with diabetes is of critical significance and education programs to diabetes, especially women with high academic background, may also include information of adequate iodine intake in our clinical practice. Our study shed light on the possible beneficial effect of iodine supplementation in reducing albuminuria in T2DM, which warrants further investigation in well-designed randomized controlled trial.
Our study benefited from its well-defined community-based participants with a relatively large sample size. Second, regarding the novelty, our study is the first to provide iodine status, and linked iodine insufficiency to an increased risk of albuminuria in people with diabetes. Third, we used ICP-MS to detect UIC in the present analysis, which was considered as the gold-standard method [37]. There were also some limitations we should acknowledge. First, no causal relationship could be determined due to the cross-sectional design of the study, and thus our findings need to be validated by longitudinal prospective studies. Second, although UIC is recommended by the WHO/ICCIDD/UNICEF for evaluation of iodine status at the population level and widely used in large-scale epidemiological studies [38, 39], the single spot urine measurement may not accurately assess long-term iodine status at the individual level. Actually, inter- and intra-individual variability exists in UIC [40]. However, the application of a large sample size (from 100 to 500 subjects per sub-group) may counteract the bias related to the use of only one casual urine sample [41]. Future follow-up studies collecting 24-h urine specimens twice are needed to replicate the present results.
Conclusion
A large proportion of diabetic patients in downtown Shanghai consumed non-iodized salt and had ID. ID may increase the risk of DKD independent of thyroid function in diabetic patients. Maintaining USI at an appropriate level is indispensable for diabetic patients. Cohort and intervention studies as well as basic research exploring the effect and mechanism of iodine supplementation on renal function are warranted.
Availability of data and materials
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- T2DM:
-
Type 2 Diabetes Mellitus
- DKD:
-
Diabetic kidney disease
- DR:
-
Diabetic retinopathy
- ID:
-
Iodine deficiency
- UIC:
-
Urinary iodine concentration
- TSH:
-
Thyroid-stimulating hormone
- BMI:
-
Body mass index
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- UACR:
-
Urinary albumin-to-creatinine ratio
- eGFR:
-
Estimated glomerular filtration rate
References
Kahm K, Laxy M, Schneider U, Rogowski WH, Lhachimi SK, Holle R. Health care costs associated with incident complications in patients with type 2 diabetes in Germany. Diabetes Care. 2018;41:971–8.
Chen C, Chen Q, Nie B, Zhang H, Zhai H, Zhao L, et al. Trends in bone mineral density, osteoporosis, and osteopenia among U.S. adults with prediabetes, 2005-2014. Diabetes Care. 2020;43:1008–15.
Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81.
Valencia WM, Florez H. How to prevent the microvascular complications of type 2 diabetes beyond glucose control. Bmj. 2017;356:i6505.
Zimmermann MB, Boelaert K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015;3:286–95.
Zimmermann MB, Andersson M. Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev. 2012;70:553–70.
Lee KW, Shin D, Song WO. Low urinary iodine concentrations associated with dyslipidemia in US adults. Nutrients. 2016;8:171.
Tran HV, Erskine NA, Kiefe CI, Barton BA, Lapane KL, Do VTH, et al. Is low iodine a risk factor for cardiovascular disease in Americans without thyroid dysfunction? Findings from NHANES. Nutr Metab Cardiovasc Dis. 2017;27:651–6.
Al-Attas OS, Al-Daghri NM, Alkharfy KM, Alokail MS, Al-Johani NJ, Abd-Alrahman SH, et al. Urinary iodine is associated with insulin resistance in subjects with diabetes mellitus type 2. Exp Clin Endocrinol Diabetes. 2012;120:618–22.
Jin M, Zhang Z, Li Y, Teng D, Shi X, Ba J, et al. U-shaped associations between urinary iodine concentration and the prevalence of metabolic disorders: a cross-sectional study. Thyroid. 2020;30:1053–65.
Nudda A, Battacone G, Bomboi G, Floris B, Decandia M, Pulina G. Effect of dietary iodine on thyroid hormones and energy blood metabolites in lactating goats. Animal. 2013;7:60–5.
Qu Y, Zhuo L, Li N, Hu Y, Chen W, Zhou Y, et al. Prevalence of post-stroke cognitive impairment in China: a community-based, cross-sectional study. PLoS One. 2015;10:e0122864.
Sun X, Li Y, Liu S, Lou J, Ding Y, Liang H, et al. Enhanced performance of community health service centers during medical reforms in Pudong New District of Shanghai, China: A longitudinal survey. PLoS One. 2015;10:e0125469.
Wang N, Wang X, Li Q, Han B, Chen Y, Zhu C, et al. The famine exposure in early life and metabolic syndrome in adulthood. Clin Nutr. 2017;36:253–9.
Zhai H, Chen C, Wang N, Chen Y, Nie X, Han B, et al. Blood lead level is associated with non-alcoholic fatty liver disease in the Yangtze River Delta region of China in the context of rapid urbanization. Environ Health. 2017;16:93.
Chen C, Zhao L, Ning Z, Li Q, Han B, Cheng J, et al. Famine exposure in early life is associated with visceral adipose dysfunction in adult females. Eur J Nutr. 2019;58:1625–33.
Chen C, Zhai H, Cheng J, Weng P, Chen Y, Li Q, et al. Causal link between vitamin D and Total testosterone in men: a Mendelian randomization analysis. J Clin Endocrinol Metab. 2019;104:3148–56.
Stevens LA, Claybon MA, Schmid CH, Chen J, Horio M, Imai E, et al. Evaluation of the chronic kidney disease epidemiology collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011;79:555–62.
Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, et al. Diabetic kidney disease: a report from an ADA consensus conference. Diabetes Care. 2014;37:2864–83.
Wilkinson CP, Ferris FL 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–82.
Zou Y, Lou X, Ding G, Mo Z, Zhu W, Mao G. Iodine nutritional status after the implementation of the new iodized salt concentration standard in Zhejiang Province, China. BMC Public Health. 2014;14:836.
Zou S, Wu F, Guo C, Song J, Huang C, Zhu Z, et al. Iodine nutrition and the prevalence of thyroid disease after salt iodization: a cross-sectional survey in Shanghai, a coastal area in China. PLoS One. 2012;7:e40718.
Shan Z, Chen L, Lian X, Liu C, Shi B, Shi L, et al. Iodine status and prevalence of thyroid disorders after introduction of mandatory universal salt iodization for 16 years in China: a cross-sectional study in 10 cities. Thyroid. 2016;26:1125–30.
Chen C, Xu H, Chen Y, Chen Y, Li Q, Hu J, et al. Iodized salt intake and its association with urinary iodine, thyroid peroxidase antibodies, and thyroglobulin antibodies among urban Chinese. Thyroid. 2017;27:1566–73.
Teng X, Shan Z, Chen Y, Lai Y, Yu J, Shan L, et al. More than adequate iodine intake may increase subclinical hypothyroidism and autoimmune thyroiditis: a cross-sectional study based on two Chinese communities with different iodine intake levels. Eur J Endocrinol. 2011;164:943–50.
Wu Y, Li X, Chang S, Liu L, Zou S, Hipgrave DB. Variable iodine intake persists in the context of universal salt iodization in China. J Nutr. 2012;142:1728–34.
Chen Y, Zhu C, Chen Y, Wang N. The Association of Thyroid Nodules with metabolic status: A cross-sectional SPECT-China study. Int J Endocrinol. 2018;2018:6853617.
Qi J, He P, Yao H, Song R, Ma C, Cao M, et al. Cancer risk among patients with type 2 diabetes: a real-world study in Shanghai, China; 2019.
Sarfo-Kantanka O, Sarfo FS, Ansah EO, Yorke E, Akpalu J, Nkum BC, et al. Frequency and determinants of thyroid autoimmunity in Ghanaian type 2 diabetes patients: a case-control study. BMC Endocr Disord. 2017;17:2.
M B, T G, M P, A P, PC W. Gender differences in thyroid system function: relevance to bipolar disorder and its treatment. Bipolar Disord. 2014;16:58–71.
10. Microvascular Complications and Foot Care. Standards of medical Care in Diabetes-2018. Diabetes Care. 2018;41:S105–s118.
Yasuda T, Kaneto H, Kuroda A, Yamamoto T, Takahara M, Naka T, et al. Subclinical hypothyroidism is independently associated with albuminuria in people with type 2 diabetes. Diabetes Res Clin Pract. 2011;94:e75–7.
Wu J, Li X, Tao Y, Wang Y, Peng Y. Free triiodothyronine levels are associated with diabetic nephropathy in Euthyroid patients with type 2 diabetes. Int J Endocrinol. 2015;2015:204893.
Vidal ZE, Rufino SC, Tlaxcalteco EH, Trejo CH, Campos RM, Meza MN, et al. Oxidative stress increased in pregnant women with iodine deficiency. Biol Trace Elem Res. 2014;157:211–7.
Jha JC, Banal C, Chow BS, Cooper ME, Jandeleit-Dahm K. Diabetes and kidney disease: role of oxidative stress. Antioxid Redox Signal. 2016;25:657–84.
Pearce EN, Andersson M, Zimmermann MB. Global iodine nutrition: where do we stand in 2013? Thyroid. 2013;23:523–8.
Ittermann T, Johner S, Below H, Leiterer M, Thamm M, Remer T, et al. Interlaboratory variability of urinary iodine measurements. Clin Chem Lab Med. 2018;56:441–7.
Jeon MJ, Kim WG, Kwon H, Kim M, Park S, Oh HS, et al. Excessive iodine intake and thyrotropin reference interval: data from the Korean National Health and nutrition examination survey. Thyroid. 2017;27:967–72.
Inoue K, Leung AM, Sugiyama T, Tsujimoto T, Makita N, Nangaku M, et al. Urinary iodine concentration and mortality among U.S. adults. Thyroid. 2018;28:913–20.
Chen W, Li X, Guo X, Shen J, Tan L, Lin L, et al. Urinary iodine excretion (UIE) estimated by iodine/creatinine ratio from spot urine in Chinese school-age children. Clin Endocrinol (Oxf). 2017;86:628–33.
Andersen S, Karmisholt J, Pedersen KM, Laurberg P. Reliability of studies of iodine intake and recommendations for number of samples in groups and in individuals. Br J Nutr. 2008;99:813–8.
Acknowledgements
The authors thank Xiaojin Wang and Bingshun Wang from the Department of Biostatistics and Shanghai Jiaotong University School of Medicine for data processing.
Funding
This study was supported by National Natural Science Foundation of China (81800694, 81600609); Science and Technology Commission of Shanghai Municipality (18410722300); the Fourth Round of Three-Year Public Health Action Plan of Shanghai by the Shanghai Municipal Commission of Health and Family Planning (20164Y0079); Municipal Human Resources Development Program for Outstanding Young Talents in Medical and Health Sciences in Shanghai (2017YQ053); Fundamental research program funding of Ninth People’s Hospital affiliated to Shanghai Jiao Tong university School of Medicine (JYZZ099); Shanghai Sailing Program (20YF1423500).
Author information
Authors and Affiliations
Contributions
Y.L. and N.W. designed the study; C.C., Y.C., H.Z., F.X., B.H., W.Z., Y.W., H.W., and N.W. conducted the research; C.C. and Y.C. analyzed the data and wrote the manuscript. C.C. and Y.C. contributed equally. The final manuscript was read and approved by all authors.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethical approval was obtained from the Ethics Committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine. Written informed consent was received from all participants.
Consent for publication
N/A
Competing interests
No potential conflicts of interest relevant to this article were reported.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, C., Chen, Y., Zhai, H. et al. Iodine nutrition status and its association with microvascular complications in urban dwellers with type 2 diabetes. Nutr Metab (Lond) 17, 70 (2020). https://doi.org/10.1186/s12986-020-00493-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12986-020-00493-5