Abstract
Background
Considering the lack of data on the association between habitual dietary intakes of nitrate (NO3−) and nitrite (NO2−) and cardiovascular events, we assessed possible effects of dietary NO3− and NO2−, in the context of total antioxidant capacity (TAC) of the diet, with the risk of cardiovascular (CVD) outcomes.
Methods
Adult men and women without CVD (n = 2369) were recruited from the Tehran Lipid and Glucose Study and were followed for a mean of 6.7 years. Dietary NO3− and NO2− intakes, as well as dietary TAC and nitric oxide (NO) index were assessed at baseline (2006–2008). Multivariable-adjusted Cox proportional hazards regression models were used to estimate risk of CVD above and below median of dietary intakes of NO3−/NO2− and dietary TAC and NO index. Due to a significant interaction between NO3−/NO2− intake and TAC, stratified analyses were done for < and ≥ median dietary TAC.
Results
Daily mean (SD) dietary NO3− and NO2− intakes were 460 (195) and 9.5 (3.9) mg; mean (SD) dietary TAC and NO index was 1406 (740) and 338 (197) μmol trolox equivalent (TE)/100 g. In subjects with lower dietary TAC, higher intake of NO3− (≥ 430 mg/d) was accompanied with an increased risk of CVD (HR = 3.28, 95% CI = 1.54–6.99). There were no significant associations between dietary intakes of NO2−, TAC of the diet and NO index with the occurrence of CVD events during the study follow-up.
Conclusion
High habitual intake of NO3−, in the context of low TAC of the food, may be associated with the risk of CVD outcomes.
Similar content being viewed by others
Background
After the discovery of nitric oxide (NO) production from endogenous reduction of inorganic nitrate (NO3−) and nitrite (NO2−), the old perceptions regarding potential hazardous effects of NO3− and NO2− have changed [1, 2]. Several experimental and clinical studies have been conducted to reveal potential health benefits of these anions in different pathological conditions including hypertension and cardiovascular disease (CVD) [3,4,5]. Currently, clinical evidence demonstrate that supplementation with NO3− and NO2−, using either inorganic salts or nitrate-rich vegetables (e.g. spinach and beetroot) may promote cardiovascular health [6]. The beneficial effects of NO3− and NO2− ingestion described in several clinical studies include reduction in blood pressure, measures of arterial stiffness and platelet activity, as well as improvement of vascular function and lipid metabolism [7,8,9]. Dietary NO3− supplementation has also been shown to be able to reverse vascular dysfunction in older adults with moderately increased risk of CVD [10].
Although the health benefits associated with a diet rich in green-leafy vegetables, such as the Mediterranean diet or the Dietary Approaches to Stop Hypertension (DASH) diet in prevention of diabetes and CVD, have been attributed to their high content of NO3− [11, 12], there is limited data to confirm longitudinal cardioprotective effects of habitual dietary NO3− and NO2− intake in epidemiological studies. There are also several issues that need to be clarified in the case of NO3− and NO2− intake in the general population; e.g. the total amount and the sources of NO3− and NO2− intakes from the diet, and potential interaction with other dietary components such as antioxidants that may impact on the endogenous metabolic fates of these anions. Despite the beneficial properties of NO3−/NO2− on glucose and insulin homeostasis documented by several pre-clinical and experimental investigations [13], in a recent population-based cohort study we showed that higher NO2− intakes from animal-based sources, along with low intake of vitamin C, may increase the risk of type 2 diabetes [14].
Considering the recommendations for advancing knowledge of the relation between dietary NO3− and risk of CVD in populations [15], in the current study, we aimed to investigate on the potential effect of dietary NO3− and NO2− exposure on the development of CVD events during 6.7-year follow-up. It has been suggested that dietary antioxidants could facilitate direct conversion of NO2− to NO and enhance NO bioavailability [16]; we therefore hypothesized that the antioxidant capacity of the diet may modify the association between NO3−/NO2− intakes and the risk of CVD. Accordingly, we used interactions term of both NO3− and NO2− intakes with dietary total antioxidant capacity (TAC) in relation to CVD events. Furthermore, we considered dietary NO index, a novel scoring system combining the inherent NO3−/NO2− content and oxygen radical absorbance capacity (ORAC) score of the food [17].
Methods
Study population
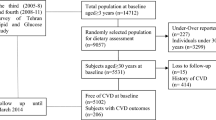
This study was conducted within the framework of the Tehran Lipid and Glucose Study (TLGS), an ongoing community-based prospective study being conducted to investigate and prevent non-communicable diseases, in a representative sample in the district 13 of Tehran, the capital city of Iran [18]. During the third phase of the TLGS (2006–2008), a total of 12,523 subjects completed the examinations, of which dietary data from 3678 subjects who agreed to participate and completed the food frequency questionnaire (FFQ) were available [19, 20] . The characteristics of participants who completed the validated FFQ were similar to those of the total population in the third phase of TLGS [21]. For the current analysis, adult men and women (≥ 19 years), free of CVD at baseline, with complete baseline data were included. Participants with under-reported or over-reported energy intake (< 800 kcal/d or > 4200 kcal/d, respectively), or specific diets were excluded. The eligible remaining participants were followed until March 2014, for a mean period of 6.7 years from the baseline examination. Participants who left the study were excluded, and final analyses were conducted on the data of 2369 adults (Fig. 1).
Demographic, anthropometric and clinical measures
Trained interviewers collected information using standard questionnaires. Detailed measurements of variables in TLGS have been reported elsewhere [18]. Smoking status was obtained using face-to-face interviews; subjects who smoked daily or occasionally were considered current smokers. Weight was measured to the nearest 100 g using digital scales, while the subjects were minimally clothed, without shoes. Height was measured to the nearest 0.5 cm, in a standing position without shoes, using a tape measure. Body mass index (BMI) was calculated as weight (kg) divided by square of the height (m2).
For measurements of systolic (SBP) and diastolic blood pressure (DBP), after a 15-min rest in upright position, two measurements of blood pressure were taken on the right arm, using a standardized mercury sphygmomanometer; the mean of the two measurements was considered as the participant’s blood pressure.
Biochemical measures
Blood samples were taken from all study participants at baseline and at follow-up phases after 12–14 h fasting. Serum glucose was measured by the enzymatic colorimetric method using glucose oxidase. The standard 2-h post-challenge serum glucose (2 h–SG) test was performed using oral administration of 82.5 g glucose monohydrate solution (equivalent to 75 g anhydrous glucose) for all individuals who were not on glucose lowering drugs.
Triglyceride (TG) level was measured by enzymatic colorimetric analysis with glycerol phosphate oxidase. High-density lipoprotein cholesterol (HDL-C) was measured after precipitation of the apolipoprotein B containing lipoproteins with phosphotungstic acid. Analyses were performed using commercial kits (Pars Azmoon Inc., Tehran, Iran) and a Selectra 2 auto-analyzer (Vital Scientific, Spankeren, Netherlands). Both inter- and intra-assay coefficients of variation of all assays were < 5%.
Habitual dietary assessment
A validated 168-item FFQ was used to assess typical food intakes over the previous year. Trained dietitians, with at least 5 years of experience in the TLGS survey, asked participants to designate their intake frequency for each food item consumed during the past year on a daily, weekly, or monthly basis. Portion sizes of consumed foods reported in household measures were then converted to grams [21]. The validity of the FFQ has been previously evaluated by comparing food groups and nutrient values determined from the questionnaire with values estimated from the average of twelve 24-h dietary recall surveys and the reliability has been assessed by comparing energy and nutrient intakes from two FFQ; Pearson correlation coefficients and intra-class correlation for energy and nutrients showed acceptable agreements between FFQ and twelve 24-h dietary recall surveys, and FFQ1 and FFQ2 [20].
Since the Iranian Food Composition Table is incomplete, and has limited data on nutrient content of raw foods and beverages, to analyze foods and beverages for their energy and nutrient content, the US Department of Agriculture Food Composition Table was used [22].
Estimation of NO3 −/NO2 − intake
To estimate dietary intake of NO3− and NO2−, a local database was used [23]. In a recent survey conducted on frequently consumed food items among Iranians, NO3− and NO2− contents of 87 food items including grains, legumes, fruits and vegetables, dairy products, meats and processed meats were measured. Briefly, a relatively high NO3− concentration was observed in breads (~ 50.0 mg 100 g− 1). Mean ranges of NO3− and NO2− in fruits were 7.50–46.8 and 0.15–0.71 mg 100 g− 1, respectively. Vegetables with the highest NO3− concentrations included radish (626 mg 100 g− 1), beetroot (495 mg 100 g− 1), tarragon (424 mg 100 g− 1), lettuce (365 mg 100 g− 1), mint (279 mg 100 g− 1), and celery (261 mg 100 g− 1). The levels of NO2− in vegetables ranged 0.21–0.74 mg 100 g− 1. In dairy products, mean NO3− and NO2− content ranged 0.14–0.45 and 1.26–5.75 mg 100 g− 1. Mean NO3− and NO2− concentrations in meats and processed meats were 5.56–19.4 and 2.93–13.9 mg 100 g− 1, respectively [23].
Validity of NO3 − and NO2 − estimation by FFQ
Serum and urinary NO3− and NO2− concentrations are considered as reliable biomarkers of exogenous exposure to NO3− and NO2− and the majority of the urinary nitrate can be accounted for by dietary sources [24, 25]. To ensure that estimation of NO3−/NO2− ingestion was associated with empirical measures of exposure [15], in a biomarker study we assessed the correlation between estimated dietary NO3− and NO2− intake and urinary and serum NO3− and NO2− levels, in a subsample (n = 251) of our cohort population. After adjustment for intra- to inter-individual variance and other potential confounders including age, body mass index and serum creatinine levels, a good agreement was observed between dietary intakes of NO3− and NO2− and urinary NO3− and NO2− values (r = 0.59, 95% CI = 0.49–0.67, and r = 0.29, 95% CI = 0.17–0.41, respectively). The correlations between dietary NO3−/NO2− and serum levels were weak (r = 0.19, 95% CI = 0.07, 0.32 and r = 0.09, 95% CI = − 0.03, 0.23, respectively).
Calculation of dietary TAC and NO index
Dietary TAC was estimated based on the ORAC of selected foods reported by Nutrient Data Laboratory of USDA, and expressed as μmol of Trolox Equivalents (TE) per 100 g of foods (μmol TE/100 g) [26]. Dietary NO index was calculated by applying an algorithm considering the NO3−/NO2− concentrations of foods as well as their reported ORAC values: Nitric Oxide Index = [NO3− + NO2− (mg/100 g) × ORAC (μmol/100 g)] / 1000] [17].
Definition of terms and outcomes
Details of the collection of CVD outcome data have been described elsewhere [27]. Cardiovascular disease was defined as any coronary heart disease (CHD) events, stroke (a new neurological deficit that lasted ≥24 h), or CVD death (definite fatal myocardial infarction (MI), definite fatal CHD, and definite fatal stroke) [28]. CHD events included cases of definite MI (diagnostic ECG and biomarkers), probable MI (positive ECG findings plus cardiac symptoms or signs plus missing biomarkers or positive ECG findings plus equivocal biomarkers), and angiographic proven CHD. History of CVD was defined as previous ischemic heart disease and/or cerebrovascular accidents. Hypertension (HTN) was defined as SBP ≥ 140 or mm Hg DBP ≥ 90 mmHg, or taking blood pressure lowering medications [29]. Type 2 diabetes (T2D) was defined as fasting serum glucose ≥126 mg/dL or 2 h–SG ≥ 200 mg/dL, or taking anti-diabetic medications [30]. The CVD risk score was calculated according to the sex-specific “general CVD” algorithms and based on age, total cholesterol, HDL-C, SBP, treatment for HTN, smoking, and T2D status [31], which has been validated among Iranian population [32]. This score is the main predictor of CVD events in our population [32].
Statistical analysis
Dietary intakes were adjusted for total energy intake, according to residuals methods [33]. The incidence of CVD events during the study follow-up was considered as a dichotomous variable (yes/no) in the models.
Median intake of dietary NO3− and NO2− and NO3− + NO2− (NOx) was 430, 8.9, and 439 mg/d, respectively. Mean and standard deviation (SD) values and the frequency (%) of baseline characteristics of the study participants were compared above and below median dietary NOx using independent t test or chi square test.
Cox proportional hazards regression models with person-year as the underlying time metric were used to estimate hazards ratios (HRs) and 95% confidence intervals (CIs) for the association between median of dietary NO3−, NO2−, NOx, TAC and nitric oxide index and incidence of CVD outcomes. The multivariable-adjusted model was included total energy intakes (kcal/d), dietary total fat (g/d), dietary fiber (g/d), and CVD risk score. Adjustment of CVD risk score, as a continuous potential risk factor of CVD events, improved the stability of our models due to the limited number of events during the study follow-up.
The proportional hazards assumption of the multivariable Cox model was assessed using Schoenfeld’s global test of residuals.
Time to event was defined as time to end of follow-up (censored cases) or time to having an event, whichever occurred first. We censored participants at the time of death due to non-CVD causes, at time of leaving the district, or study follow-up end time of March 2014.
Due to a significant interaction cross-product terms of dietary TAC with both NO2− and NO3− in the multivariable-adjusted model (P < 0.05), stratified analyses were done for dietary NO2−/NO3− and NOx intakes above and below the median dietary TAC (< or ≥1284 μmol TE/ 100 g).
All analyses were performed using IBM SPSS for Windows version 20 and STATA version 12 SE (StataCorp LP, TX, USA), with a two-tailed P value< 0.05 being considered significant.
Results
Mean (SD) age of the study participants was 38.1 (13.3) years and 43.5% were men. Mean (SD) BMI was 26.6 (4.8) kg/m2 at baseline. During an average of 6.7 ± 1.4 y of follow-up, 79 participants experienced CVD outcomes. Daily mean (SD) dietary NO3− intake in the study population was 460 (195) mg/d, which came almost entirely from plant sources (94.3%); the major contributors to NO3− intakes were vegetables (46.1%) and grains (28.8%). Dietary intakes of NO2− from animal sources accounted for 42.4% of daily mean intake of NO2− and the remainder of NO2− intake was derived from plant sources. The major contributors to NO2− intake were white rice (17.1%), chicken meat (11.7%), yogurt (6.6%), tomato (5.3%), sausages (4.7%), lamb meat (3.5%), cucumber (3.3%). Mean (SD) dietary TAC and NO index was 1406 (740) μmol TE/100 g and 338 (197).
Baseline characteristics of the cohort population above and below median intake of dietary NOx, are summarized in Table 1. Subjects who had higher intake of NOx were more likely to be older (P < 0.05). Mean TAC of diet (1621 vs. 1190 μmol TE/100 g, P = 0.001) and dietary NO index (440 vs. 236, P = 0.001) was also significantly higher in subjects with higher NOx intakes. There was no significant difference in other baseline characteristics between two groups. Dietary intakes of NO3−and NO2− from vegetables and fruits was significantly higher in subjects who had high-TAC diet (P for all < 0.05).
The association of dietary NO3−, NO2− and NOx intakes with the incidence of CVD events, above and below median TAC of diet, are reported in Table 2. In subjects with lower dietary TAC, higher intake of NO3− was accompanied with an increased risk of CVD (HR = 3.28, 95% CI = 1.45–6.99). There were no significant association between dietary intakes of NO2− with the occurrence of CVD events during the study follow-up.
The associations of dietary TAC and NO index with the incidence of CVD after 6.7 years of follow-up are reported in Table 3. After adjustment of all potential confounders, there were no significant associations between TAC of the diet (HR = 0.92, 95% CI = 0.59–1.59) and dietary NO index (HR = 1.12, 95% CI = 0.69–1.83) with the occurrence of CVD events during the study follow-up.
Discussion
In this longitudinal population-based study, we are the first to demonstrate the potential impact of habitual dietary NO3− and NO2− intake along with dietary antioxidant capacity on the risk of CVD. Independent of other risk factors, higher intake of dietary NO3− was significantly associated with an increased risk of CVD in subjects consuming food with low antioxidant capacity, dietary NO2− intake was not related to incidence of CVD after a median 6.7 years of follow-up. We also assessed probable association of NO index (i.e. combines inherent NO3−/NO2− content and ORAC score of the food), with the development of CVD events; by using this novel scoring system we did not observed any significant association between dietary NO index and the incidence of CVD events. Recently, it was shown that serum NO3−/NO2− are independent risk factors for the development of CVD among a Tehranian adult population [34]; in the current study, we extended our previous work by showing the positive association of dietary NO3− with development of CVD in subjects who had a low-TAC diet.
Although accumulating evidence supports biological cardioprotective effects of inorganic NO3−/NO2− following a short-time supplementation, in both experimental and clinical studies, [3, 35, 36], cardiovascular responses to habitual dietary intake of NO3−/NO2− in a long-term period still remains unknown and seems to be a challenging issue [37]. Lack of improvement in blood pressure or atherogenesis as well as dropped levels of serum NO2− during a prolonged inorganic NO3− supplementation, has been recently showed in low density lipoprotein receptor knockout mice [38], suggests a possible negative feedback mechanism in long-term consumption of inorganic NO3− which seems to rebalance the NO3−-NO2−-NO pathway [37].
Our findings in the current study also reinforce possible importance of antioxidant compounds in modification of cardiovascular outcomes in longitudinal exposure of dietary NO3−. In agreement with our idea, Blekkenhorst et al. in a 15-years follow-up of older adults reported that NO3− intake from vegetables, as a high-TAC food group, was inversely associated with atherosclerotic vascular disease (ASVD) mortality independent of lifestyle and CVD risk factors; whereas, non-vegetable NO3− intake was not related to ASVD (HR = 1.03, 95% CI = 0.85–1.25), P = 0.754) [39]. The usual dietary consumption of NO3− and NO2− in our study was approximately twofold of the acceptable daily intake (ADI) values and higher than the other population. In a recent experimental study we demonstrated that long-term dietary supplementation with high dose of inorganic NO3− was associated with down-regulation of endothelial NO synthase function and elevated blood pressure [40]. The existence of similar crosstalk between the NO3−-NO2−-NO pathway and the NO synthase pathway was not assessed in the present study, but may contribute to the increased CVD risk in the population consuming food with high NO3− and low TAC. Our observations may indicate that beneficial effects of NO3−/NO2− can be expected in its acceptable range of intakes and from the specific food sources. It should be noted that high intake of NO3− with low TAC was related to high intake of grains, meats and processed meats along with low consumption of fruits and vegetables. Increased risk of CVD events in participants with higher NO3−/NO2− along with a low-TAC diet, therefore may be attributed to higher intake of meats and processed meats, regardless of dietary NO3−/NO2− intakes.
Difference between short-term effects of rich NO3−/NO2− extracts compared to its regular intake in a long-term period, in the context of diverse dietary patterns may be another explanation for our findings. In our previous studies, we also observed that higher intakes of NO2−, especially from animal sources, was accompanied with an elevated incidence of type 2 diabetes in subjects who consumed a lower vitamin C [14]. It seems that antioxidant components of our daily diet could modulate endogenous pathways of NO3−/NO2− metabolism and modify the risk-benefit outcomes of these anions in the body [41, 42].
Our study had some strengths and limitations. This study was conducted in the framework of a prospective population-based design, with a high rate of follow-up completeness. We used a validated comprehensive FFQ to assess regular dietary intakes of the participants, and estimated NO3−/NO2− exposure based on a national database of NO3−/NO2− content [23]. Use of validated CVD risk score in the multivariable-adjusted models allowed us to account for major CVD confounders without adding many variables that would lead to instability of our models.
Use of ORAC values to calculate NO index is a major limitation of this study since the values of antioxidant capacity have no relevance to the effects of specific bioactive compounds in foods; in addition, ORAC assay using different substrates do not provide comparable results. Based on these evidence, USDA’s Nutrient Data Laboratory removed the ORAC Database for selected foods from the website in 2012 [43]. Lack of data on income level, alcohol consumption, and dietary supplement use, as potential moderator of CVD risk, and also lack of data on serum TAC and inflammatory markers should be considered as limitations of this analysis. Lack of data on dietary supplements could also affect our estimation of dietary TAC. Another main limitation in this study was a lack of information regarding water intake and NO3−/NO2− concentration of drinking water, at baseline. However, our recent estimation of intake from drinking water in a subsample of TLGS population, showed a relatively low contribution of drinking water in overall NO3−/NO2− exposure compared to its dietary sources, in our population (6.7% and 26.6% for NO3− and NO2−, respectively) [14]. Due to potential changes in an individual’s diet and NO3−/NO2− content of food items, as well as changes in other CVD risk factors during the study follow-up, some degree of misclassification might have occurred which could lead to biased estimated hazards ratios towards the null, as inherent in any prospective study.
It should be noted that our findings may not be generalized to other populations due to different NO3−/NO2− content of the food items especially vegetables and different dietary patterns and food habits among different populations.
Conclusion
The results of our investigation suggest that high habitual intake of NO3− in the context of low TAC diet may be associated with CVD events, independent of other known risk factors. A null association of dietary NO index, which is calculated from NO3− and NO2− content and ORAC score of the diet, also indicates that the suggested adverse effect of NO3− may be modified by the antioxidant capacity of the foods.
Abbreviations
- 2 h–SG:
-
2-h serum glucose
- ASVD:
-
Atherosclerotic vascular disease
- BMI:
-
Body mass index
- CHD:
-
Coronary heart disease
- CVD:
-
Cardiovascular disease
- DASH:
-
Dietary approach to stop hypertension
- DBP:
-
Diastolic blood pressure
- FFQ:
-
Food frequency questionnaire
- HDL-C:
-
High-density lipoprotein cholesterol
- HR:
-
Hazards ratio
- HTN:
-
Hypertension
- NO:
-
Nitric oxide
- NO2 - :
-
Nitrite
- NO3 - :
-
Nitrate
- ORAC:
-
Oxygen radical absorbance capacity
- SBP:
-
Systolic blood pressure
- SD:
-
Standard deviation
- TAC:
-
Total antioxidant capacity
- TG:
-
Triglycerides
- TLGS:
-
Tehran Lipid and Glucose Study
References
Bahadoran Z, Ghasemi A, Mirmiran P, Azizi F, Hadaegh F. Nitrate-nitrite-nitrosamines exposure and the risk of type 1 diabetes: a review of current data. World J Diabetes. 2016;7:433–40.
Bahadoran Z, Mirmiran P, Ghasemi A, Kabir A, Azizi F, Hadaegh F. Is dietary nitrate/nitrite exposure a risk factor for development of thyroid abnormality? A systematic review and meta-analysis. Nitric Oxide. 2015;47:65–76.
Lundberg JO, Carlstrom M, Larsen FJ, Weitzberg E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc Res. 2011;89:525–32.
Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37:395–400.
Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–67.
Kapil V, Weitzberg E, Lundberg JO, Ahluwalia A. Clinical evidence demonstrating the utility of inorganic nitrate in cardiovascular health. Nitric Oxide. 2014;38:45–57.
Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med. 2006;355:2792–3.
Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–90.
Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320–7.
Rammos C, Hendgen-Cotta UB, Sobierajski J, Bernard A, Kelm M, Rassaf T. Dietary nitrate reverses vascular dysfunction in older adults with moderately increased cardiovascular risk. J Am Coll Cardiol. 2014;63:1584–5.
Rathod KS, Velmurugan S, Ahluwalia A. A 'green' diet-based approach to cardiovascular health? Is inorganic nitrate the answer? Mol Nutr Food Res. 2016;60:185–202.
Gilchrist M, Benjamin N: Vegetables and diabetes. Is nitrate the answer? Bmj 2010, 341:c5306.
Bahadoran Z, Ghasemi A, Mirmiran P, Azizi F, Hadaegh F. Beneficial effects of inorganic nitrate/nitrite in type 2 diabetes and its complications. Nutr Metab (Lond). 2015;12:16.
Bahadoran Z, Mirmiran P, Ghasemi A, Carlstrom M, Azizi F, Hadaegh F. Vitamin C intake modify the impact of dietary nitrite on the incidence of type 2 diabetes: a 6-year follow-up in Tehran lipid and glucose study. Nitric Oxide. 2016
Ahluwalia A, Gladwin M, Coleman GD, Hord N, Howard G, Kim-Shapiro DB, Lajous M, Larsen FJ, Lefer DJ, McClure LA, et al. Dietary nitrate and the epidemiology of cardiovascular disease: report from a National Heart, Lung, and Blood Institute workshop. Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease. 2016;5:e003402.
Bondonno CP, Croft KD, Ward N, Considine MJ, Hodgson JM. Dietary flavonoids and nitrate: effects on nitric oxide and vascular function. Nutr Rev. 2015;73:216–35.
Bryan NS, Loscalzo J: Nitrite and nitrate in human health and disease. Springer Science & Business Media; 2011.
Azizi F, Ghanbarian A, Momenan AA, Hadaegh F, Mirmiran P, Hedayati M, Mehrabi Y, Zahedi-Asl S. Prevention of non-communicable disease in a population in nutrition transition: Tehran lipid and glucose study phase II. Trials. 2009;10:5.
Esfahani FH, Asghari G, Mirmiran P, Azizi F. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran lipid and glucose study. J Epidemiol. 2010;20:150–8.
Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 2010;13:654–62.
Hosseini-Esfahani F, Jessri M, Mirmiran P, Bastan S, Azizi F. Adherence to dietary recommendations and risk of metabolic syndrome: Tehran lipid and glucose study. Metabolism. 2010;59:1833–42.
Fewtrell L. Drinking-water nitrate, methemoglobinemia, and global burden of disease: a discussion. Environ Health Perspect. 2004:1371–4.
Bahadoran Z, Mirmiran P, Jeddi S, Azizi F, Ghasemi A, Hadaegh F. Nitrate and nitrite content of vegetables, fruits, grains, legumes, dairy products, meats and processed meats. J Food Compos Anal. 2016;51:93–105.
Pannala AS, Mani AR, Spencer JP, Skinner V, Bruckdorfer KR, Moore KP, Rice-Evans CA. The effect of dietary nitrate on salivary, plasma, and urinary nitrate metabolism in humans. Free Radic Biol Med. 2003;34:576–84.
Bondonno CP, Liu AH, Croft KD, Ward NC, Puddey IB, Woodman RJ, Hodgson JM. Short-term effects of a high nitrate diet on nitrate metabolism in healthy individuals. Nutrients. 2015;7:1906–15.
Haytowitz DB, Bhagwat S. USDA database for the oxygen radical absorbance capacity (ORAC) of selected foods, release 2. US Department of Agriculture. 2010;
Hadaegh F, Harati H, Ghanbarian A, Azizi F. Association of total cholesterol versus other serum lipid parameters with the short-term prediction of cardiovascular outcomes: Tehran lipid and glucose study. Eur J Cardiovasc Prev Rehabil. 2006;13:571–7.
Nejat A, Mirbolouk M, Mohebi R, Hasheminia M, Tohidi M, Saadat N, Azizi F, Hadaegh F. Changes in lipid measures and incident coronary heart disease: Tehran Lipid & Glucose Study. Clin Biochem. 2014;47:1239–44.
Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, et al. Guidelines for the Management of Arterial Hypertension: the task force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;2007(25):1105–87.
Standards of medical care in diabetes--2014. Diabetes Care 2014, 37 Suppl 1:S14–S80.
RB D’A Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation. 2008;117:743–53.
Khalili D, Hadaegh F, Soori H, Steyerberg EW, Bozorgmanesh M, Azizi F. Clinical usefulness of the Framingham cardiovascular risk profile beyond its statistical performance: the Tehran lipid and glucose study. Am J Epidemiol. 2012;176:177–86.
Yilmaz B, Sahin K, Bilen H, Bahcecioglu IH, Bilir B, Ashraf S, Halazun KJ, Kucuk O. Carotenoids and non-alcoholic fatty liver disease. Hepatobiliary Surg Nutr. 2015;4:161–71.
Hadaegh F, Asgari S, Bozorgmanesh M, Jeddi S, Azizi F, Ghasemi A. Added value of total serum nitrate/nitrite for prediction of cardiovascular disease in middle east caucasian residents in Tehran. Nitric Oxide. 2016;54:60–6.
Kevil CG, Kolluru GK, Pattillo CB, Giordano T. Inorganic nitrite therapy: historical perspective and future directions. Free Radic Biol Med. 2011;51:576–93.
Tang Y, Jiang H, Bryan NS. Nitrite and nitrate: cardiovascular risk-benefit and metabolic effect. Curr Opin Lipidol. 2011;22:11–5.
Ramms B, Gordts PL. Dietary nitrate struggles in atherosclerosis. Atherosclerosis. 2016;245:71–3.
Marsch E, Theelen TL, Janssen BJ, Briede JJ, Haenen GR, Senden JM, van Loon LJ, Poeze M, Bierau J, Gijbels MJ, et al. The effect of prolonged dietary nitrate supplementation on atherosclerosis development. Atherosclerosis. 2016;245:212–21.
Blekkenhorst LC, Bondonno CP, Lewis JR, Devine A, Woodman RJ, Croft KD, Lim WH, Wong G, Beilin LJ, Prince RL, Hodgson JM. Association of dietary nitrate with atherosclerotic vascular disease mortality: a prospective cohort study of older adult women. Am J Clin Nutr. 2017;
Carlstrom M, Liu M, Yang T, Zollbrecht C, Huang L, Peleli M, Borniquel S, Kishikawa H, Hezel M, Persson AE, et al. Cross-talk between nitrate-nitrite-NO and NO synthase pathways in control of vascular NO homeostasis. Antioxid Redox Signal. 2015;23:295–306.
Tannenbaum SR. Preventive action of vitamin C on nitrosamine formation. Int J Vitam Nutr Res Suppl. 1989;30:109–13.
Tannenbaum SR, Wishnok JS, Leaf CD. Inhibition of nitrosamine formation by ascorbic acid. Am J Clin Nutr. 1991;53:247S–50S.
United States Department of Agriculture. Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods, Release 2 (2010) [https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/nutrient-data-laboratory/docs/oxygen-radical-absorbance-capacity-orac-of-selected-foods-release-2-2010/].
Acknowledgments
This study was supported by the Research Institute for Endocrine Sciences of Shahid Beheshti University of Medical Sciences. We thank the Tehran Lipid and Glucose Study participants and the field investigators of the Tehran Lipid and Glucose Study for their cooperation and assistance in physical examinations, biochemical evaluation and database management.
Funding
Not applicable.
Availability of data and materials
Please contact author for data requests.
Author information
Authors and Affiliations
Contributions
ZB, PM, AG, FH and FA designed research (project conception, development of overall research plan, and study oversight). ZB and PM conducted research; ZB and FH analyzed data or performed statistical analysis; ZB, MC, AG, and FH wrote paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consents were obtained from all participants. The ethics research council of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences in Tehran approved the study protocol.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bahadoran, Z., Carlström, M., Ghasemi, A. et al. Total antioxidant capacity of the diet modulates the association between habitual nitrate intake and cardiovascular events: A longitudinal follow-up in Tehran Lipid and Glucose Study. Nutr Metab (Lond) 15, 19 (2018). https://doi.org/10.1186/s12986-018-0254-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12986-018-0254-2