Abstract
Background
This study was conducted to estimate the prevalence, determinants of hepatitis B, hepatitis C and the survival of tuberculosis patients until drug-induced hepatitis.
Methods
Prospective cohort study design was implemented. The data were collected from September 2016 – May 2019. Systematic random sampling was used to select the study participants. Baseline data were collected before the patient starts DOTS, the sign of liver toxicity was assessed every week. Tuberculosis treatment outcomes and WHO clinical stage was recorded at the end of 6th months. Descriptive statistics were used to estimate the prevalence of hepatitis B, hepatitis C viral infections and their effect on tuberculosis treatment outcomes. Binary logistic regression was used to identify the determinants of hepatitis B and C infections. The Kaplan Meier survival curve was used to estimate the survival of tuberculosis patient and Cox regression was used to identify the predictors of drug-induced hepatitis.
Results
A total of 3537 tuberculosis patients were followed. The prevalence of hepatitis B and C viral infection among tuberculosis patients were 15.1 and 17.3% respectively. Hepatitis B viral infection among tuberculosis patients was associated with alcohol, sex, HIV, chronic illness. Hepatitis C viral infection among tuberculosis patients was associated with alcohol, sex, HIV, chronic illness. The incidence density for liver toxicity among tuberculosis patients was 843/15707 person-months and liver toxicity was determined by HIV, Hepatitis B, Hepatitis C, the severity of tuberculosis and chronic illnesses.
Conclusion
Decision-makers should consider incorporating screening for hepatitis B and C viral infection during tuberculosis treatment.
Similar content being viewed by others
Background
Hepatitis is an inflammation of the liver cells called hepatocyte and, most commonly caused by hepatitis viruses. Hepatitis A hepatitis B, hepatitis C, hepatitis D, and hepatitis E viruses are responsible for injuring the hepatocyte. The severe form of hepatitis is caused by hepatitis B and hepatitis C viruses [1]. More than 2 billion people were infected with hepatitis B and annually killing 800,000 people [2,3,4]. The global prevalence of hepatitis C virus infection ranges from 2.5, − 3% [5, 6]. Globally, each year, 10 million new cases of tuberculosis and 1.6 million death of tuberculosis were reported by world health organization [7].
The prevalence of hepatitis B viral infection among tuberculosis patients ranges from 0.5 to 44% [8,9,10,11]. Hepatitis C virus burden among tuberculosis patients ranges from 3.4 - 44.6% [12,13,14].
In Ethiopia, 8% of the total population was infected with hepatitis B and 1.9% of the community was infected with hepatitis C virus [15, 16]. Ethiopia labeled as one of the high burden countries for tuberculosis [17]. The prevalence of smear positive tuberculosis was 108/100000 population and the prevalence of bacteriologically confirmed tuberculosis was 277/100000 population [18]. There is no evidence on tuberculosis hepatitis B and C co-infection rate in Ethiopia.
Hepatitis B or hepatitis C co-infection with tuberculosis increase the risk of treatment failure [19] activates latent tuberculosis [20,21,22], increase the risk of mortality [23], and drug-induced liver injury [24,25,26,27,28].
There is no updated evidence of tuberculosis, hepatitis B and hepatitis C co-infection in resource-limited setting like Ethiopia and the objectives of this research work were
-
1.
To estimate the prevalence of hepatitis B viral infection among tuberculosis patients
-
2.
To estimate the prevalence of hepatitis C viral infection among tuberculosis patients
-
3.
To assess the determinants of hepatitis B and hepatitis C viral infections among tuberculosis patients
-
4.
To describe the effects of hepatitis B and hepatitis C infection on tuberculosis treatment outcomes
-
5.
To estimate the survival of tuberculosis patients until drug-induced hepatitis
Methods
A prospective cohort study design was implemented among tuberculosis patients on directly observed treatment strategy (DOTS) following their treatment in West Gojam health facilities, Amhara regional state, Ethiopia. West Gojam is one of the 11 zones of Amhara regional state, Ethiopia. The data were collected from September 2016 – May 2019. Tuberculosis patients on DOTS were followed for 6 months. Transferred out tuberculosis patients and patient with incomplete medical records were excluded from the study.
Data were collected using an interview technique, document review, collecting the stool and blood samples. Clinical nurses were recruited to conduct the interview and extract necessary data from the medical records. Baseline data were collected before the patient start DOTS and the sign of liver toxicity were assessed every week. Tuberculosis treatment outcomes and WHO clinical stages were recorded at the end of 6th months. Laboratory technologists were recruited to analyze the stool and blood samples. For each tuberculosis patient, 1 g stool sample was collected and analyzed using concentration techniques to identify the presence of intestinal parasites. For each tuberculosis patient, 5 ml (ML) venous blood was collected using aseptic technique and Enzyme-linked immune Sorbent assay (ELIZA) test was performed to screen the presence of hepatitis B and hepatitis C. The standard operating procedures (SOP) were followed. CAGE tool was used to detect problematic alcohol use; viral load suppression was detected when HIV positive patient record less than 1000 copies of virus per ML of blood. Good treatments outcome was declared if the patient completed the treatments or become smear negative at the end of DOTS. To maintain the quality of the data: pre-test was performed on 50 tuberculosis patients, training was given for all data collectors and supervisors and the data collection procedures were closely supervised.
Epi-info software was used to calculate the sample size, the following assumptions were considered: 95% confidence interval, power of 90%, HIV positive to negative patient’s proportion of 1:3 and 10% loss to follow up rate. The estimated sample size was 912 HIV positive and 2731 HIV negative tuberculosis patients.
These samples were representative of tuberculosis population taking DOTS. The systematic random sampling technique was used to recruit HIV positive tuberculosis patients and HIV negative tuberculosis patients.
Epi-info software was used to enter the data and SPSS software was used for the analysis. Descriptive statistics were used to estimate the prevalence of hepatitis B, hepatitis C viral infections and their effects on tuberculosis treatment outcomes. Binary logistic regression was used to identify the determinants of hepatitis B and hepatitis C viral infections. The Kaplan Meier survival curve was used to estimate the survival of tuberculosis patient until drug-induced hepatitis. Predictors of drug induced hepatitis were identified using Cox regression.
Ethical clearance was obtained from Bahir Dar University ethical review board. Permission letter was obtained from Amhara national regional, state health bureau ethics committee and the respective health facility heads. Written informed consent was obtained from each study participant. The confidentiality of the data was kept at all stages. Study participants with hepatitis B or hepatitis C viral infections were linked to the available services in the health facilities.
Results
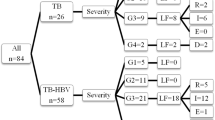
A total of 3537 tuberculosis patients were followed, giving for a response rate of 97%, 106 tuberculosis patients were excluded from the study due to incomplete medical records and death. The mean age of the study participants was 34.49 years (standard deviation [SD] ± 15.6 years), 51.6% of the study participants were married and 80% of the study participants were from the rural areas (Table 1).
The prevalence of hepatitis B viral infection among tuberculosis patients was 15.1% [95% CI: 13.92 - 16.28%]. The prevalence of hepatitis B viral infection among HIV positive tuberculosis patients was 35.27%. The burden of hepatitis C viral infection among tuberculosis patients was 17.3% [95% CI: 16.06 - 18.55%], but this prevalence inflated to 46.09% of HIV infected tuberculosis patients.
After adjusting for residence, alcohol, smoking, sex, HIV, chronic illness, intestinal parasitic infections, and age; hepatitis B viral infection was associated with problematic alcohol use, sex, HIV, and chronic illnesses (Table 2).
After adjusting for residence, alcohol, smoking, sex, HIV, chronic illness, intestinal parasitic infections, and age; hepatitis C viral infection was associated with problematic alcohol use, sex, HIV, and chronic illness. (Table 3).
The treatment outcome was good in 89.5% of hepatitis B negative TB patients, and among Hepatitis B positive patients the treatment success rate was 68.9%. The severity of tuberculosis presentation among hepatitis B positive TB patients was 80%. Good treatment outcome was observed in 92.6% of hepatitis C negative tuberculosis patients, and 56.9% of hepatitis C negative patients. The prevalence of severe tuberculosis was 58% among hepatitis C positive TB patients, and 23.5% among hepatitis C negative TB patients (Tables 4 and 5).
Hepatitis B and C significantly affected the efficacy of highly active anti-retroviral therapy (HAART); viral load suppression was 26.4% for hepatitis B positive TB patients, and 73.6% among hepatitis B negative TB patients. Among hepatitis C positive TB patients with viral load suppression were only 37.8%, but viral load suppression was 62.2% among hepatitis C negative TB patients (Tables 6 and 7).
The incidence density for the liver toxicity among tuberculosis patients was 843/15707 person-months. The median time of hepato-toxicity was 24 days (Fig. 1).
After adjusting for age, HIV, hepatitis B, hepatitis C, the severity of tuberculosis, chronic illness, smoking, and alcohol; hepatotoxicity was predicted by HIV, Hepatitis B, Hepatitis C, the severity of tuberculosis and other chronic illnesses (Table 8).
Discussion
The odds of hepatitis B infection among HIV positive TB patients were 4.95 times higher; HIV infection increases the odds of hepatitis C viral infection by 10 folds. This finding agrees with a 2016 published systematic review [29]. This occurs because both hepatitis B and Hepatitis C virus share the same route of transmission with HIV/AIDS [30].
Hepatitis infection was higher in female, the odds of hepatitis B infection in female were 13.4 times higher and hepatitis C viral infection was 6.8 folds higher in the female. This finding agrees with other scholarly works [31, 32]. This is due to the anatomy and physiology of females reproductive organs which expose them to acquire these hepatitis viruses more easily [33, 34].
The presence of chronic illnesses increases the odds of hepatitis B by 1.6 folds and hepatitis C infection by 4.9 folds higher. This finding was in line with previous scholarly results [35,36,37]. This is because of the unhealthy lifestyle of the groups like substance abuse, unsafe sexual practices, and alcohol consumption habits which predisposes these patients to acquire the diseases [38].
Problematic alcohol use increases the odds of hepatitis B infection by 4 folds. The odds of hepatitis C viral infection were 1.5 folds higher among tuberculosis patients with problematic alcohol use. This finding was in line with previous work [39, 40]. This is because people with problematic alcohol use can commit unprotected sexual intercourse that exposes them to acquire the virus more easily [41].
Hepatitis B infection decreased the treatment success rate of tuberculosis by 20.6%; Hepatitis C viral infection decreased the treatment success rate of tuberculosis by 35.7%. This finding agrees with 2018 finding from China that indicates having hepatitis B or C decreased the treatment success rate [19]. This might be due to poor adherence of anti-TB drugs, poor bioavailability and metabolism of the drugs due to repeated vomiting as a result of hepatitis virus infections [42].
Hepatitis B infection increased the severity of tuberculosis by 59.5%, and hepatitis C increases the severity of tuberculosis by 34.5%. This finding agrees with previous findings [43]. This is because hepatitis viruses reactivate tuberculosis and lead to severe clinical presentations [44].
Viral load suppression was 47.2% lower among hepatitis B infected HIV patients and 19% lower among hepatitis C infected HIV patients. At the end of DOTS, 95% of the hepatitis C negative HAART patients had less than II WHO clinical stages, however, only 75% of hepatitis C infected HAART patients had less than II clinical stages. At the end of tuberculosis treatment, 76% of hepatitis B infected HAART patients had less than II clinical stages, but 90% of hepatitis B free HAART patient had less than II clinical stages. The finding was in line with the 2016 systematic review report [45]. This is because of the effects of hepatitis B and hepatitis C viral infection on the immune system of the host that leads them to the poor response to treatments [46, 47].
The incidence density for the liver toxicity among tuberculosis patients was 843/15707 person-months. This figure is higher as compared to the previous finding [48]. This might be due to the high alcohol consumption rate and adherence to traditional medicine in the study area.
HIV infection increases the hazard of liver toxicity by 2.25 folds. This result was in line with previously published findings [49]. This is due to the extra pill burden of HIV positive patients like Nevirapine, which causes additional hepatotoxicity [50, 51].
The risk of liver toxicity was 6 folds higher among hepatitis B infected DOTS patients, liver toxicity was 3 folds higher among hepatitis C infected tuberculosis patients. This finding agrees with finding from South Korea [24]. This is because the hepatitis virus accelerates the injury of liver cells as a result of anti-tuberculosis drugs [52].
The hazard of liver toxicity was 1.5 times higher in severe tuberculosis patients. This finding agrees with previous research work [53]. This might be due to the treatment regimen given to this group of tuberculosis patients with extra-anti-tuberculosis drugs.
Chronic illness increases the risk of liver toxicity by 1.2 folds during tuberculosis treatment. This finding was in line with other work [54]. This is because of the additional treatment given to the chronic diseases which increase the workload of the liver, causing additional injury to the liver cells [55, 56].
The limitation of this study was the failure to identify the specific serotypes for hepatitis B and hepatitis C viral infection. However, the main objective of this study was to identify the prevalence of all the serotypes and this limitation will not impose significant concern for this research.
Conclusion
The prevalence of hepatitis B and hepatitis C viral infection was higher in tuberculosis patients. Hepatitis B and hepatitis C viral infection among tuberculosis patients were determined by HIV, alcohol, sex, and chronic illnesses. Hepatitis B and C co-infection lead to bad tuberculosis and HIV treatment outcomes.
Recommendation
Decision-makers should consider screening for hepatitis B and hepatitis C viral infection to obtain good HIV and tuberculosis treatment outcomes.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AIDS:
-
Acquired immune deficiency syndrome
- AOR:
-
Adjusted odds ratio
- CI:
-
Confidence interval
- COR:
-
Crude odds ratio
- DOTS:
-
Directly observed treatment strategy
- ELIZA:
-
Enzyme-linked immune Sorbent assay
- HAART:
-
Highly active anti-retroviral therapy
- HIV:
-
Human immune deficiency syndrome
- ML:
-
Milliliter
- SD:
-
Standard deviation
- SOP:
-
Standard operating procedures
- TB:
-
Tuberculosis
- WHO:
-
World health organization
References
WHO. What is Hepatitis? Switzerland. Geneva: World Health Organization; 2019.
Trepo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384(9959):2053–63.
Lavanchy D, Kane M. Global epidemiology of hepatitis B virus infection. Hepatitis B virus in human diseases. Switzerland: Springer; 2016. p. 187–203.
Aires RS, Matos MA, Lopes CL, Teles SA, Kozlowski AG, Silva AM, et al. Prevalence of hepatitis B virus infection among tuberculosis patients with or without HIV in Goiania City, Brazil. J Clin Virol. 2012;54(4):327–31.
Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: an up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22(34):7824–40.
Blach S, Zeuzem S, Manns M, Altraif I, Duberg A-S, Muljono DH, et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(3):161–76.
WHO. Global Tuberculosis Report 2018. Swetherland: World Health Organization; 2018.
Iglecias LM, Puga MA, Pompilio MA, Teles SA, Croda J, Lima LA, et al. Epidemiological study of hepatitis B virus among prisoners with active tuberculosis in Central Brazil. Int J Tuberc Lung Dis. 2016;20(11):1509–15.
Franco E, Bagnato B, Marino MG, Meleleo C, Serino L, Zaratti L. Hepatitis B: Epidemiology and prevention in developing countries. World J Hepatol. 2012;4(3):74.
Mo P, Zhu Q, Teter C, Yang R, Deng L, Yan Y, et al. Prevalence, drug-induced hepatotoxicity, and mortality among patients multi-infected with HIV, tuberculosis, and hepatitis virus. Int J Infect Dis. 2014;28:95–100.
Farhoudi B, SeyedAlinaghi S, Mohraz M, Hosseini M, Farnia M. Tuberculosis, hepatitis C and hepatitis B co-infections in patients with HIV in the great Tehran prison, Iran. Asian Pac J Trop Dis. 2016;6(1):82–3.
Reis NR, Lopes CL, Teles SA, Matos MA, Carneiro MA, Marinho TA, et al. Hepatitis C virus infection in patients with tuberculosis in Central Brazil. Int J Tuberc Lung Dis. 2011;15(10):1397–402.
Behzadifar M, Heydarvand S, Behzadifar M, Bragazzi NL. Prevalence of hepatitis C virus in tuberculosis patients: a systematic review and meta-analysis. Ethiop J Health Sci. 2019;29(1):945–56.
Araujo-Mariz C, Lopes EP, Ximenes RA, Lacerda HR, Miranda-Filho DB, Montarroyos UR, et al. Serological markers of hepatitis B and C in patients with HIV/AIDS and active tuberculosis. J Med Virol. 2016;88(6):996–1002.
Woldegiorgis AE, Erku W, Medhin G, Berhe N, Legesse M. Community-based sero-prevalence of hepatitis B and C infections in South Omo Zone, Southern Ethiopia. PloS one. 2019;14(12):e0226890.
Abera B, Adem Y, Yimer M, Mulu W, Zenebe Y, Mekonnen Z. Community seroprevalence of hepatitis B, C and human immunodeficiency virus in adult population in gojjam zones, Northwest Ethiopia. Virol J. 2017;14(1):21.
Organization WH. Global tuberculosis report 2013. Switzerland: World Health Organization; 2013.
Kebede A, Alebachew Z, Tsegaye F, Lemma E, Abebe A, Agonafir M, et al. The first population-based national tuberculosis prevalence survey in Ethiopia, 2010-2011. Int J Tuberc Lung Dis. 2014;18(6):635–9.
Chen L, Bao D, Gu L, Gu Y, Zhou L, Gao Z, et al. Co-infection with hepatitis B virus among tuberculosis patients is associated with poor outcomes during anti-tuberculosis treatment. BMC Infect Dis. 2018;18(1):295.
Pedrosa M, Nogales S, Vergara M, Miquel M, Casas M, Dalmau B, et al. Reactivation of peritoneal and pleural tuberculosis during hepatitis C treatment with direct-acting antivirals. Gastroenterol Hepatol. 2019;42(3):174–5.
de Oliveira Uehara SN, Emori CT, Perez RM, Mendes-Correa MC. de Souza Paiva Ferreira a, de Castro Amaral Feldner AC, et al. high incidence of tuberculosis in patients treated for hepatitis C chronic infection. Braz J Infect Dis. 2016;20(2):205–9.
Wu PH, Lin YT, Hsieh KP, Chuang HY, Sheu CC. Hepatitis C virus infection is associated with an increased risk of active tuberculosis disease: a Nationwide population-based study. Medicine (Baltimore). 2015;94(33):e1328.
Ladep NG, Agbaji OO, Agaba PA, Muazu A, Ugoagwu P, Imade G, et al. Hepatitis B Co-Infection is Associated with Poorer Survival of HIV-Infected Patients on Highly Active Antiretroviral Therapy in West Africa. J AIDS Clin Res. 2013;2013(Suppl 3):1-7.
Kim WS, Lee SS, Lee CM, Kim HJ, Ha CY, Kim HJ, et al. Hepatitis C and not hepatitis B virus is a risk factor for anti-tuberculosis drug induced liver injury. BMC Infect Dis. 2016;16:50.
Pukenyte E, Lescure F, Rey D, Rabaud C, Hoen B, Chavanet P, et al. Incidence of and risk factors for severe liver toxicity in HIV-infected patients on anti-tuberculosis treatment. Int J Tuberc Lung Dis. 2007;11(1):78–84.
Abera W, Cheneke W, Abebe G. Incidence of antituberculosis-drug-induced hepatotoxicity and associated risk factors among tuberculosis patients in Dawro zone, South Ethiopia: a cohort study. Int J Mycobacteriol. 2016;5(1):14–20.
Lomtadze N, Kupreishvili L, Salakaia A, Vashakidze S, Sharvadze L, Kempker RR, et al. Hepatitis C virus co-infection increases the risk of anti-tuberculosis drug-induced hepatotoxicity among patients with pulmonary tuberculosis. PLoS One. 2013;8(12):e83892.
Zheng Y, Ma S, Tan D, Lu M. A meta-analysis of liver lesions in hepatitis B patients undergoing anti-tuberculosis therapy. Zhonghua Gan Zang Bing Za Zhi. 2014;22(8):585–9.
Hughes E, Bassi S, Gilbody S, Bland M, Martin F. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness: a systematic review and meta-analysis. Lancet Psychiatry. 2016;3(1):40–8.
Control CfD, Prevention. HIV and viral hepatitis. South Carolina State Documents Depository. 2017.
vom Steeg LG, Klein SL. SeXX matters in infectious disease pathogenesis. PLoS pathogens. 2016;12(2):e1005374.
Wang SH, Chen PJ, Yeh SH. Gender disparity in chronic hepatitis B: mechanisms of sex hormones. J Gastroenterol Hepatol. 2015;30(8):1237–45.
Shakour M, Salehi K, Yamani N. Reproductive health need assessment of adolescent boys and girls during puberty: a qualitative study. Int J Pediatr. 2018;6(9):8195–205.
Arnold KB, Burgener A, Birse K, Romas L, Dunphy LJ, Shahabi K, et al. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV-susceptible target cells. Mucosal Immunol. 2016;9(1):194.
Chen Y-C, Su Y-C, Li C-Y, Wu C-P, Lee M-S. A nationwide cohort study suggests chronic hepatitis B virus infection increases the risk of end-stage renal disease among patients in Taiwan. Kidney Int. 2015;87(5):1030–8.
Fabrizi F, Verdesca S, Messa P, Martin P. Hepatitis C virus infection increases the risk of developing chronic kidney disease: a systematic review and meta-analysis. Dig Dis Sci. 2015;60(12):3801–13.
Chen Y-C, Su Y-C, Li C-Y, Hung S-K. 13-year nationwide cohort study of chronic kidney disease risk among treatment-naive patients with chronic hepatitis B in Taiwan. BMC Nephrol. 2015;16(1):110.
Huygens MW, Vermeulen J, Swinkels IC, Friele RD, Van Schayck OC, De Witte LP. Expectations and needs of patients with a chronic disease toward self-management and eHealth for self-management purposes. BMC Health Serv Res. 2016;16(1):232.
Krajden M, Cook DA, Wong S, Yu A, Butt ZA, Rossi C, et al. What is killing people with hepatitis C virus infection? Analysis of a population-based cohort in Canada. Int J Drug Policy. 2019;2019:5.
Binka M, Butt ZA, Wong S, Chong M, Buxton JA, Chapinal N, et al. Differing profiles of people diagnosed with acute and chronic hepatitis B virus infection in British Columbia, Canada. World J Gastroenterol. 2018;24(11):1216.
Kahler CW, Wray TB, Pantalone DW, Kruis RD, Mastroleo NR, Monti PM, et al. Daily associations between alcohol use and unprotected anal sex among heavy drinking HIV-positive men who have sex with men. AIDS Behav. 2015;19(3):422–30.
Kirby BJ, Symonds WT, Kearney BP, Mathias AA. Pharmacokinetic, pharmacodynamic, and drug-interaction profile of the hepatitis C virus NS5B polymerase inhibitor sofosbuvir. Clin Pharmacokinet. 2015;54(7):677–90.
Al-Khazraji A, Alkhawam H, Garrido B. Id: 30: hepatitis b virus reactivation in an inactive carrier of chronic HBV AFTER the initiation of treatment for TIBERCULOSIS. Great Britain: BMJ Publishing Group Limited; 2016.
Pillai AA, Anania FA, Pearlman BL. Caution: reactivation of hepatitis B during hepatitis C treatment with direct-acting antiviral therapy. Am J Gastroenterol. 2016;111(12):1854.
Petersdorf N, Ross JM, Weiss HA, Barnabas RV, Wasserheit JN, HCV and HIV Transmission Working Group, et al. Systematic review and meta-analysis of hepatitis C virus infection and HIV viral load: new insights into epidemiologic synergy. J Int AIDS Soc. 2016;19(1):20944.
Large MK, Kittlesen DJ, Hahn YS. Suppression of host immune response by the core protein of hepatitis C virus: possible implications for hepatitis C virus persistence. J Immunol. 1999;162(2):931–8.
Wieland S, Thimme R, Purcell RH, Chisari FV. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci. 2004;101(17):6669–74.
Khalili H, Dashti-Khavidaki S, Rasoolinejad M, Rezaie L, Etminani M. Anti-tuberculosis drugs related hepatotoxicity; incidence, risk factors, pattern of changes in liver enzymes and outcome. DARU J Pharm Sci. 2015;17(3):163–7.
Hoffmann CJ, Charalambous S, Thio CL, Martin DJ, Pemba L, Fielding KL, et al. Hepatotoxicity in an African antiretroviral therapy cohort: the effect of tuberculosis and hepatitis B. Aids. 2007;21(10):1301–8.
Osei E, Der J, Owusu R, Kofie P, Axame WK. The burden of HIV on tuberculosis patients in the Volta region of Ghana from 2012 to 2015: implication for tuberculosis control. BMC Infect Dis. 2017;17(1):504.
Teschke R. Hepatotoxicity: molecular mechanisms and pathophysiology. Switzerland: Multidisciplinary Digital Publishing Institute; 2019.
Bao Y, Ma X, Rasmussen TP, Zhong X-B. Genetic Variations associated with anti-tuberculosis drug-induced liver injury. Curr Pharmacol Rep. 2018;4(3):171–81.
Fernandez-Villar A, Sopena B, Fernandez-Villar J, Vazquez-Gallardo R, Ulloa F, Leiro V, et al. The influence of risk factors on the severity of anti-tuberculosis drug-induced hepatotoxicity. Int J Tuberc Lung Dis. 2004;8(12):1499–505.
Tipayamongkholgul M, Marin W, Sujirarat D, Pokaew P, Pungrassami P, et al. Trop Biomed. 2016;33(1):1–7.
Mosedale M, Watkins PB. Drug-induced liver injury: advances in mechanistic understanding that will inform risk management. Clin Pharmacol Ther. 2017;101(4):469–80.
Fisher K, Vuppalanchi R, Saxena R. Drug-induced liver injury. Arch Pathol Lab Med. 2015;139(7):876–87.
Acknowledgments
We would like to acknowledge Bahir Dar University and ministry of health for sponsoring this research work. Our heartfelt acknowledgment goes to Amhara national, regional, state for their unreserved efforts during the fieldwork. We would like to acknowledge all organizations and individuals that had an input for this research work.
Funding
This research work was financially supported by the University of Bahir dar and Federal democratic republic of Ethiopia ministry of health. The funder has no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
BEF conceived the experiment; BEF and TEF performed the experiment, BEF, TEF, WGA and AG plan the data collection process, BEF, WGA and TEF analyzed and interpreted the data. BEF, TEF, WGA, and AG wrote the manuscript and approved the final draft for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical clearance was obtained from Bahir Dar University ethical review board. Permission letter was obtained from Amhara national regional, state health bureau ethics committee and the respective health facility heads. Written informed consent was obtained from each study participant. Study participants the right to withdraw from this research at any point was respected. The confidentiality of the data was kept at all stages. Study participants with hepatitis B or hepatitis C viral infections were linked to the available services in the health facilities.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Feleke, B.E., Feleke, T.E., Adane, W.G. et al. Impacts of hepatitis B and hepatitis C co-infection with tuberculosis, a prospective cohort study. Virol J 17, 113 (2020). https://doi.org/10.1186/s12985-020-01385-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-020-01385-z