Abstract
Background
Serum antibodies against myelin-oligodendrocyte-glycoprotein (MOG-IgG) are detectable in a proportion of patients with acute or relapsing neuroinflammation. It is unclear, if neuro-axonal damage occurs only in an attack-dependent manner or also progressively. Therefore, this study aimed to investigate longitudinally intra-retinal layer changes in eyes without new optic neuritis (ON) in MOG-IgG-seropositive patients.
Methods
We included 38 eyes of 24 patients without ON during follow-up (F/U) [median years (IQR)] 1.9 (1.0–2.2) and 56 eyes of 28 age- and sex-matched healthy controls (HC). The patient group’s eyes included 18 eyes without (EyeON-) and 20 eyes with history of ON (EyeON+). Using spectral domain optical coherence tomography (OCT), we acquired peripapillary retinal nerve fiber layer thickness (pRNFL) and volumes of combined ganglion cell and inner plexiform layer (GCIP), inner nuclear layer (INL), and macular volume (MV). High-contrast visual acuity (VA) was assessed at baseline.
Results
At baseline in EyeON-, pRNFL (94.3 ± 15.9 μm, p = 0.36), INL (0.26 ± 0.03 mm3, p = 0.11), and MV (2.34 ± 0.11 mm3, p = 0.29) were not reduced compared to HC; GCIP showed thinning (0.57 ± 0.07 mm3; p = 0.008), and VA was reduced (logMAR 0.05 ± 0.15 vs. − 0.09 ± 0.14, p = 0.008) in comparison to HC. Longitudinally, we observed pRNFL thinning in models including all patient eyes (annual reduction − 2.20 ± 4.29 μm vs. − 0.35 ± 1.17 μm, p = 0.009) in comparison to HC. Twelve EyeON- with other than ipsilateral ON attacks ≤ 6 months before baseline showed thicker pRNFL at baseline and more severe pRNFL thinning in comparison to 6 EyeON- without other clinical relapses.
Conclusions
We observed pRNFL thinning in patients with MOG-IgG during F/U, which was not accompanied by progressive GCIP reduction. This effect could be caused by a small number of EyeON- with other than ipsilateral ON attacks within 6 months before baseline. One possible interpretation could be a reduction of the swelling, which could mean that MOG-IgG patients show immune-related swelling in the CNS also outside of an attack’s target area.
Similar content being viewed by others
Background
Antibodies against conformation-dependent epitopes of myelin-oligodendrocyte-glycoprotein (MOG-IgG) have been described in patients with central nervous system (CNS) inflammation of putative autoimmune etiology [1,2,3,4]. MOG is also the dominant antigen for demyelinating antibodies in experimental autoimmune encephalomyelitis (EAE), the predominant animal model of multiple sclerosis (MS), and MOG-IgG can augment demyelination by cell-mediated and humoral immune responses [1]. In neuropathology studies, MOG-IgG are associated with MS-like pathology directed against myelin and oligodendrocytes and biopsies present a MS pattern II [5, 6]. MOG-IgG affinity-purified from the blood of patients with optic neuritis (ON) enhanced inflammation and induced demyelination upon transfer into experimental animals indicating the pathogenic potential of MOG-IgG detected in the blood of these patients [7]. It is discussed whether MOG-IgG define a separate disease entity tentatively called MOG-IgG-associated diseases, MOG-IgG autoimmunity or MOG-IgG seropositive encephalomyelitis rather than being part of several autoimmune disorders, especially neuromyelitis optica spectrum disorders (NMOSD) [1, 3, 8, 9]. However, the bouquet of clinical phenotypes in MOG-IgG-associated diseases at clinical onset is not easy to differentiate and overlaps with aquaporin-4-IgG (AQP4-IgG)-seropositive NMOSD and in rare cases with MS [2, 10,11,12], although distinct clinical features such as seizures have been described [13,14,15]. ON is the most common manifestation and can lead to substantial neuro-axonal damage after multiple relapses, as shown in different cohorts [11, 16]. The pattern of retinal degeneration after ON seems to be similar in all MOG-IgG-seropositive cohorts as shown by optical coherence tomography (OCT) studies [11, 16]. OCT proved to be a precise and reproducible method for non-invasive visualization and quantification of retinal layers and plays a crucial role in analyzing retinal changes in various neuroinflammatory disorders [17,18,19,20]. In a cross-sectional study, MOG-IgG-related OCT features indicated subclinical pathology in eyes without a history of ON (EyeON-) [16]. However, no longitudinal OCT data is reported in MOG-IgG-associated diseases so far and the pattern of longitudinal retinal damage still remains elusive. Using OCT, we assessed retinal layer thinning as a marker of neuro-axonal damage in a cohort of MOG-IgG-seropositive patients without ON during follow-up (F/U). We aimed to investigate at baseline and longitudinally microstructural changes in MOG-IgG-seropositive patients, extending previous work in AQP4-IgG-seropositive NMOSD [21, 22].
Methods
Study populations
Twenty-four patients were seen and followed [F/U (years; median (inter-quartile-range (IQR))) 1.9 (1.0–2.2)] at four university tertiary care centers specialized in neuroimmunological diseases (Institute of Clinical Neuroimmunology, Ludwig Maximilians University (LMU), Munich, Germany, N = 11; NeuroCure Clinical Research Center, Charité – Universitätsmedizin Berlin, Germany, N = 10; Department of Neurology, University of Lille Hospital, Lille, France, N = 1; Department of Neurology, Klinikum Rechts der Isar, Technische Universität München (TUM), Munich, Germany, N = 2). Written informed consent was obtained from all patients participating in the study. The local ethics committees approved the study protocol in accordance with the Declaration of Helsinki (1964) in its currently applicable version. All patients were matched by age (W = 370, p = 0.542) and sex (χ2 = 0,937, p = 0.333) to 56 eyes of 28 healthy controls (HC; F/U) [years; median (IQR)] 1.9 (1.9–2.3) from the NeuroCure Clinical Research Center, Charité – Universitätsmedizin Berlin, Germany. Inclusion criteria were the detection of MOG-IgG, complete longitudinal clinical and OCT imaging data with minimum F/U of 8 months and age between 15 and 75 years at baseline. Only eyes without concomitant potentially confounding diseases (glaucoma, diabetes mellitus, retinal surgery, retinal disease, ametropia > 6 diopters) were included. Eyes with a history of ON ≤ 5 months before baseline were excluded. Clinical data (diagnosis, disease onset, number of ON, date last ON, brain attacks, myelitis, EDSS, relapses, treatment history) were collected for all patients. For detection of MOG-IgG, serum samples from all patients were analyzed at least once by established cell-based assays at the discretion of each center using the laboratory’s cutoffs (MOG IFT, EUROIMMUN, Laboratory Stöcker, Germany; Molecular Neuroimmunology Group, University Heidelberg, Heidelberg, Germany; Reindl Lab, Medical University of Innsbruck, Innsbruck, Austria; Meinl Lab, LMU, Munich, Hemmer Lab, TUM, Munich) [3, 7, 23].
Optical coherence tomography
All centers used SPECTRALIS spectral-domain OCT (Heidelberg Engineering, Heidelberg, Germany) with automatic real-time (ART) function for image averaging. We acquired peripapillary retinal nerve fiber layer thickness (pRNFL) and volumes of combined ganglion cell and inner plexiform layer (GCIP), inner nuclear layer (INL) and macular volume (MV) by OCT. GCIP, INL and MV were calculated as a 3 mm diameter cylinder around the fovea from a macular volume scan (25° × 30°, 61 vertical B-scans, 12 ≤ ART ≤ 18; 20° × 20°, 25 vertical B-scans, 27 ≤ ART ≤ 49). The peripapillary RNFL (pRNFL) was measured with activated eye tracker using ring scans around the optic nerve (12°, 1536 A-scans, 57 ≤ ART ≤ 100) or the most inner ring of a star-and-ring scan around the optic nerve (12°, 768 A-scans, 27 ≤ ART ≤ 33). For two patients (8.3%), the ring scan protocol changed during the acquisition period (ring scan to inner ring of a star-and-ring scan). Segmentation of all layers was performed semi-automatically using software provided by the OCT manufacturer (Eye Explorer 1.9.10.0 with viewing module 6.3.4.0, Heidelberg Engineering, Heidelberg, Germany). Experienced raters (BK for TU Munich data, JH for all other data) carefully checked all scans for sufficient quality and segmentation errors and corrected if necessary. OCT data in this study is reported and analyzed according to the APOSTEL and OSCAR-IB recommendations [24, 25]. Macular microcysts were defined as the presence of cystic lesions on at least one scan detected by experienced raters (BK for TU Munich scans, JH for all other scans). Additionally, we collected habitually corrected monocular high-contrast visual acuity (VA) using ETDRS (Early Treatment Diabetic Retinopathy Study) charts at baseline in 20 ft distance for a subset of patients (N = 15).
Statistical methods
Group differences between MOG-IgG patients and HC were tested by chi-squared test for sex and Wilcoxon rank-sum test for age. Main outcomes were change of GCIP, pRNFL, INL and MV and VA over F/U. Cross-sectional differences of OCT values and VA between all groups were analyzed pairwise by generalized estimating equation (GEE) models to account for inter-eye within-patient correlations of monocular measurements. Longitudinal analyses of OCT and VA were performed with linear mixed effects models using time from baseline and group as fixed effects and patient-ID and eye-ID as random effects; results are reported for effect “Time from Baseline * Group”, which reflects the group-specific change over time. Annual loss was estimated for each individual as change to baseline at last visit divided by F/U time in years. All tests and graphical representations were performed with R version 3.3.1 [http://www.R-project.org]. Statistical significance was established at p < 0.05, and all results were interpreted in the context of an exploratory analysis and therefore not adjusted for multiple comparison.
Results
Cohort description and follow-up
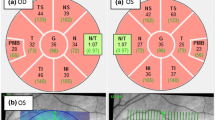
We included 38 eyes of 24 patients without ON during F/U. 70% of the patients from Berlin [7/10] and 64% of the patients from LMU Munich [7/11] have been included in previous cross-sectional studies [7, 10, 16]. MOG-IgG-seropositive patients had the following diagnosis: recurrent ON (N = 7), MOG-IgG-seropositive NMOSD (N = 12) meeting the 2015 IPND (International Panel for Neuromyelitis Optica Diagnosis) criteria for seronegative NMOSD [26], MOG-IgG-seropositive MS (N = 3) and MOG-IgG-seropositive meningoencephalomyelitis (N = 2). All patients had ≥ 1 F/U visit(s) [median (range) 2 visits (2–7)]. The MOG-IgG-seropositive cohort included 18 eyes without (EyeON-) and 20 eyes with a history of ON (EyeON+) (number of ONs [median (range)] 0 (0 – 8); time since ON in years [median (range)] 2.2 (0.4 – 14.9)). From the 18 EyeON-, we identified 12 eyes with other than ipsilateral ON attacks within 6 months before baseline (five eyes of three patients with a myelitis, four eyes of two patients with myelitis and brainstem attacks, one eye of one patient with myelitis and contralateral ON and 2 eyes of 2 patients with contralateral ON; age 40 ± 9, male/female 5/3, EDSS 2.5, median follow-up 14 ± 5.9 months)) and six eyes without other attacks (age 39.0 ± 21.0, male/female 1/4, EDSS 3.5, median follow-up 26 ± 4.5 months). Retrospectively, one patient (2 eyes) could not be included in the study analysis because he had ONs on both sides during F/U and another patient (2 eyes) could not be included because he had insufficient follow-up less than 8 months. Data of further 8 eyes had to be excluded (five eyes with ON during F/U, one eye with ON less than 5 months before study inclusion, one eye with missing data, one eye with OCT-confounding disease). Clinical characteristics of all included patients are shown in Table 1.
Group differences at baseline
First, we analyzed group differences at baseline between MOG-IgG-seropositive patient eyes with a history of ON (EyeON+), patient eyes without previous ON (EyeON-) and eyes from HC. At baseline, in EyeON-, pRNFL, INL and MV were not significantly different, but GCIP was significantly thinner in comparison to HC (p = 0.008) (Table 2, Fig. 1). VA was lower in EyeON- in comparison to HC (p = 0.013).
Baseline data: bee swarm plots of cross-sectional OCT data for HC (gray, left), MOG-IgG-seropositive EyeON- (blue, middle) and MOG-IgG-seropositive Eye ON+ (red, right) (median ± IQR, single eyes as dots) for a pRNFL, b GCIP, c INL, and d MV. Abbreviations: Eye ON-: MOG-IgG-seropositive eyes without a history of ON; Eye ON+: MOG-IgG-seropositive eyes with a history of ON; GCIP: combined ganglion cell and inner plexiform layer; HC: Healthy control; INL: inner nuclear layer; IQR: inter-quartile range; OCT: Optical coherence tomography; p: p value; pRNFL: peripapillary retinal nerve fiber layer; MV: macular volume
In EyeON+ at baseline pRNFL, GCIP and MV were significantly lower in comparison to HC (pRNFL p < 0.0001, GCIP p < 0.0001, MV p < 0.0001). In contrast, INL was significantly thicker in EyeON+ (INL 0.30 ± 0.05 mm3 vs. 0.27 ± 0.03 mm3 (p = 0.046)). VA was also lower in EyeON+ (0.55 ± 0.81) in comparison to HC [− 0.09 (0.14), p = 0.01) and EyeON- [0.05 (0.15), p = 0.058).
One EyeON- showed a massive thinness of the pRNFL at baseline despite a missing history of ON. We found macular microcysts within the INL in 6/20 (30%) EyeON+.
OCT changes during F/U
Longitudinally, we observed pRNFL thinning, which was not accompanied by progressive GCIP reduction, in eyes without ON during F/U (annual loss: − 2.20 ± 4.29 μm vs. HC -0.35 ± 1.17 μm, p = 0.009) (Fig. 2; individual changes in Additional file 1). There were no longitudinal group differences between EyeON+ and EyeON- for GCIP, pRNFL, INL and MV as well as between MOG-IgG-seropositive NMOSD and other MOG-IgG-seropositive patients (Table 3). In a previous study investigating spinal cord changes in MOG-IgG patients, we suspected edematous changes in patients close to a clinical attack [27]. We therefore investigated patients with a non-ipsilateral ON attack within 6 months of the baseline visit in a subgroup analysis. At baseline, the pRNFL in 12 EyeON- with a non-ipsilateral ON attack within the 6 months before baseline was thicker in comparison to 6 EyeON- without a non-ipsilateral ON attack within the 6 months before baseline (pRNFL 100.2 ± 12.7 μm vs. 82.7 ± 16.2 μm (p = 0.019)) (Fig. 3A). Reduction of pRNFL thickness was seen mainly in 3 eyes of the subgroup analysis. Two of the 3 eyes had no clinical evidence of unilateral ON attacks of the contralateral eye within the 6 months prior to inclusion in the study. One of the 3 eyes had a relapse complex with myelitis, brain attack and contralateral ON within 6 months prior to baseline. An ON-affection of these 12 EyeON- with a non-ipsilateral ON attack was further ruled out by a stable high-contrast visual acuity (HCVA) without a change during F/U (HCVA as decimal, median (range): at baseline 1.0 (0.6–1.1); at last visit 1.0 (0.6–1.6)). A longitudinal graphical display of EyeON- showed the pRNFL thinning to be predominantly present in EyeON- with an attack before baseline (Fig. 3B). However, due to the small sample size, no statistical analysis could be performed.
Bar graphs of longitudinal OCT data. Plotted change (mean ± standard error) for rounded time since baseline in years for a pRNFL and b GCIP, c MV for eyes of MOG-IgG-seropositive patients (blue, dashed), and HC (gray, continuous), displayed until median F/U time (2 years). Abbreviations: F/U: follow-up; GCIP: combined ganglion cell and inner plexiform layer; HC: healthy control; INL: inner nuclear layer; Eye ON-: MOG-IgG-seropositive eyes without a history of ON; ON: optic neuritis; OCT: optical coherence tomography; pRNFL: peripapillary retinal nerve fiber layer; SE: standard error, MV: macular volume. F/U investigations were rounded up or down to the year 0, 1, or 2 and follow-up visits with a time since baseline < 6 months were excluded from the graphical display. N rounded for timepoints: T0: N (MOG) = 38 eyes, T1: N (MOG) = 27 eyes, T2: N (MOG) = 26 eyes, T0: N (HC) = 56 eyes, T1: N (HC) = 41 eyes, T2: N (HC) = 40 eyes
a Bee swarm plots of cross-sectional OCT data for HC (gray, left), EyeON- with non-ipsilateral ON attacks ≤ 6 months before baseline (blue, middle), and EyeON- with no attacks ≤ 6 months before baseline (blue, right) (median ± IQR, single eyes as dots) for pRNFL. b Bar graphs of longitudinal OCT data. Plotted change (mean ± standard error) for rounded time since baseline for pRNFL of EyeON- with other attacks (blue, dashed) and EyeON- without other attacks (blue, continuous), displayed until median F/U time. F/U investigations were rounded up or down to the year 0, 1, or 2 and follow-up visits with a time since baseline < 6 months were excluded from the graphical display. EyeON-attack- T0/1/2: N = 6 eyes, EyeON-attack- T0: N = 12 eyes, T1: N = 11 eyes, T2: N = 3 eyes. Abbreviations: F/U: follow-up; HC: healthy control; Eye ON-: MOG-IgG-seropositive eyes without a history of ON; ON: optic neuritis; OCT: optical coherence tomography; pRNFL: peripapillary retinal nerve fiber layer; N: number of eyes that contributed to the analysis
Discussion
In this study, we investigated longitudinally, MOG-IgG-seropositive patients for potential progressive or covert damage in the retina in the absence of new clinical ON. We could not detect progressive GCIP thinning during F/U in MOG-IgG-seropositive patients, which is in contrast to progressive GCIP reduction in AQP4-IgG-seropositive NMOSD and MS [22, 27]. Instead, we observed a longitudinal pRNFL reduction, which in a consequent subgroup analysis appeared to primarily occur in patients with non-ipsilateral ON attacks within 6 months before baseline. A hypothetical explanation of this finding could be a remission of pRNFL edema.
Cross-sectional retinal imaging studies have shown conflicting results as to whether MOG-IgG-associated diseases have a more favorable outcome compared to patients with ON in other disease contexts [28,29,30,31,32,33,34]. The presumed higher relapse rates in MOG-IgG-seropositive patients could be associated with a severe retinal neuroaxonal loss and an unfavorable visual outcome [11]. Although OCT data regarding MOG-IgG-associated retinal damage are inconsistent [11, 16, 30, 35], neuroaxonal retinal damage may occur as a consequence of clinical episode(s) of ON or of subclinical involvement [11, 16]. ON was associated with macular microcysts, a biomarker suggestive of severe optic neuropathy [16, 36, 37]. A previous study investigating a smaller cohort of MOG-IgG-positive patients showed a significant reduction of the pRNFL and the ganglion cell layer in EyeON- compared to HC cross-sectionally [16]. By contrast, in our current study, we could only confirm a significant GCIP reduction in EyeON- at baseline but no significant reduction of the pRNFL as a hint towards subclinical retinal pathology. However, pRNFL edema as a marker of immune-related swelling in the CNS after relapses and also outside of relapses could have contributed to this finding. The GCIP reduction at baseline could be discussed as progressive neurodegenerative retinal involvement, subclinical optic nerve pathology, chiasmal crossover of ON in contralateral eyes, or as an expression of subclinical ON in the previous patient’s history. However, according to Ramanathan et al., only 5% of ONs in MOG-IgG-seropositive patients shows chiasmal involvement [38].
Longitudinally, we observed pRNFL but not GCIP thinning. We hypothesize that this can be explained not only by subclinical retinal or optic nerve involvement or drug-induced retinal damage related to immunosuppressive treatment, but also by a remission of non-ipsilateral ON attacks that has occurred in EyeON- within 6 months before baseline since patients without clinical attacks ≤ 6 months before baseline did not present significant pRNFL or GCIP loss during F/U. This is clearly in contrast to our recently published data about longitudinal GCIP thinning in AQP4-IgG-seropositive NMOSD [22] or earlier studies reporting GCIP loss in MS [27] and might be an important hint towards the differentiation of MOG-IgG-associated diseases from AQP4-IgG-seropositive NMOSD. AQP4-IgG-seropositive NMOSD is an astrocytopathy, and a primary retinopathy caused by antibody-mediated damage is supported by animal studies and recently also clinical studies [22, 39]. In contrast, the retina does not harbor myelin-producing oligodendrocytes and an expression of MOG has not been shown, making a primary retinopathy unlikely.
Further, data showing clear differences between AQP4-IgG-seropositive NMOSD and MOG-IgG-associated diseases were presented recently by Chien et al. [40]. Spinal cord imaging data showed differences in spinal cord affection patterns and disability accumulation. A higher prevalence of myelitis with clinical attacks and chronic spinal cord lesions was detected for AQP4-IgG-seropositive NMOSD patients in comparison to MOG-IgG-associated diseases [40]. Interestingly, MOG-IgG-seropositive patients showed a swelling of the upper cervical cord area during other non-myelitis attacks, also pointing towards a systemic inflammatory affection in MOG-IgG-associated diseases as potentially shown here in the pRNFL during different attacks [40]. Our data is in line with the conclusion that AQP4-IgG-seropositive NMOSD and MOG-IgG-associated diseases are distinct immunological disorders, but share common clinical patterns [22, 40,41,42].
Limitations of our study are the heterogeneity of MOG-IgG-seropositive patients with different clinical phenotypes in our cohort, the heterogeneity of immunosuppressive treatments of our patients, and due to the rarity of MOG-IgG-seropositive patients in Europe, the small sample size, which leads to outliers possibly having a larger effect on the results, short and variable F/U, and the evaluation of MOG-IgG by different labs using different assays. Additionally, our study lacks magnetic resonance imaging data on optic nerve lesion lengths and lesion volumes of the afferent visual system as well as whole-brain lesion volume to further evaluate subclinical retinal atrophy in MOG-IgG-associated diseases.
Conclusions
We report in this small explorative study of MOG-IgG-associated diseases no evidence of GCIP thinning during F/U. Additionally, we found pRNFL reduction without GCIP loss during F/U predominantly in EyeON- with other than ipsilateral ON attacks ≤ 6 months before baseline. We will investigate in a planned longitudinal study involving more centers, whether this reduction is actually due to a remission of edema or reflects retinal neurodegenerative processes or drug-induced retinal damage related to aggressive immunosuppressive treatment.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AQP4-IgG:
-
Aquaporin-4 antibodies
- ART:
-
Automatic real time
- B:
-
Estimate
- CNS:
-
Central nervous system
- EAE:
-
Experimental autoimmune encephalomyelitis
- EDSS:
-
Expanded disability status scale
- EyeON- :
-
Eyes without a history of optic neuritis
- EyeON+ :
-
Eyes with a history of optic neuritis
- F/U:
-
Follow-up
- GCIP:
-
Combined ganglion cell and inner plexiform layer
- GEE:
-
Generalized estimated eq.
- HC:
-
Healthy control
- INL:
-
Inner nuclear layer
- IQR:
-
Inter-quartile range
- LMU:
-
Ludwig-Maximilians University Munich
- MOG-IgG:
-
Myelin-oligodendrocyte glycoprotein antibodies
- MS:
-
Multiple sclerosis
- N:
-
Number
- NCRC:
-
NeuroCure Clinical Research Center Berlin
- NMOSD:
-
Neuromyelitis optica spectrum disorders
- OCT:
-
Optical coherence tomography
- ON:
-
Optic neuritis
- p :
-
p value
- pRNFL:
-
Peripapillary retinal nerve fiber layer
- SD:
-
Standard deviation
- SE:
-
Standard error
- MV:
-
Macular volume
- TUM:
-
Technical University Munich
- VA:
-
Visual acuity
References
Hohlfeld R, Dornmair K, Meinl E, Wekerle H. The search for the target antigens of multiple sclerosis, part 1: autoreactive CD4+ T lymphocytes as pathogenic effectors and therapeutic targets. Lancet Neurol. 2016;15:198–209.
Dos Passos GR, Oliveira LM, da Costa BK, Apostolos-Pereira SL, Callegaro D, Fujihara K, et al. MOG-IgG-associated optic neuritis, encephalitis, and myelitis: lessons learned from neuromyelitis optica spectrum disorder. Front Neurol. 2018;9:217.
Jarius S, Paul F, Aktas O, Asgari N, Dale RC, de Seze J, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation. 2018;15:134.
Peschl P, Bradl M, Höftberger R, Berger T, Reindl M. Myelin oligodendrocyte glycoprotein: deciphering a target in inflammatory demyelinating diseases. Front Immunol. 2017;8:529.
Spadaro M, Gerdes LA, Mayer MC, Ertl-Wagner B, Laurent S, Krumbholz M, et al. Histopathology and clinical course of MOG-antibody-associated encephalomyelitis. Ann Clin Transl Neurol. 2015;2:295–301.
Jarius S, Metz I, König FB, Ruprecht K, Reindl M, Paul F, et al. Screening for MOG-IgG and 27 other anti-glial and anti-neuronal autoantibodies in “pattern II multiple sclerosis” and brain biopsy findings in a MOG-IgG-positive case. Mult Scler. 2016;22:1541–9.
Spadaro M, Winklmeier S, Beltrán E, Macrini C, Höftberger R, Schuh E, et al. Pathogenicity of human antibodies against myelin oligodendrocyte glycoprotein. Ann Neurol. 2018;84:315–28.
Narayan R, Simpson A, Fritsche K, Salama S, Pardo S, Mealy M, et al. MOG antibody disease: a review of MOG antibody seropositive neuromyelitis optica spectrum disorder. Mult Scler Relat Disord. 2018;25:66–72.
Borisow N, Mori M, Kuwabara S, Scheel M, Paul F. Diagnosis and treatment of NMO spectrum disorder and MOG-encephalomyelitis. Front Neurol. 2018;9:888.
Cobo-Calvo A, Ruiz A, Maillart E, Audoin B, Zephir H, Bourre B, et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: the MOGADOR study. Neurology. 2018;90:e1858–69.
Pache F, Zimmermann H, Mikolajczak J, Schumacher S, Lacheta A, Oertel FC, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 4: afferent visual system damage after optic neuritis in MOG-IgG-seropositive versus AQP4-IgG-seropositive patients. J Neuroinflammation. 2016;13:282.
Zamvil SS, Slavin AJ. Does MOG Ig-positive AQP4-seronegative opticospinal inflammatory disease justify a diagnosis of NMO spectrum disorder? Neurol Neuroimmunol Neuroinflamm. 2015;2:e62.
Hamid SHM, Whittam D, Saviour M, Alorainy A, Mutch K, Linaker S, et al. Seizures and encephalitis in myelin oligodendrocyte glycoprotein IgG disease vs aquaporin 4 IgG disease. JAMA Neurol. 2018;75:65–71.
Ogawa R, Nakashima I, Takahashi T, Kaneko K, Akaishi T, Takai Y, et al. MOG antibody-positive, benign, unilateral, cerebral cortical encephalitis with epilepsy. Neurol Neuroimmunol Neuroinflamm. 2017;4:e322.
Pandit L, Mustafa S, Uppoor R, Nakashima I, Takahashi T, Kaneko K. Reversible paraspinal muscle hyperintensity in anti-MOG antibody-associated transverse myelitis. Neurol Neuroimmunol Neuroinflamm. 2018;5:e412.
Havla J, Kümpfel T, Schinner R, Spadaro M, Schuh E, Meinl E, et al. Myelin-oligodendrocyte-glycoprotein (MOG) autoantibodies as potential markers of severe optic neuritis and subclinical retinal axonal degeneration. J Neurol. 2017;264:139–51.
Bennett JL, de Seze J, Lana-Peixoto M, Palace J, Waldman A, Schippling S, et al. Neuromyelitis optica and multiple sclerosis: seeing differences through optical coherence tomography. Mult Scler. 2015;21:678–88.
Galetta SL, Villoslada P, Levin N, Shindler K, Ishikawa H, Parr E, et al. Acute optic neuritis: unmet clinical needs and model for new therapies. Neurol Neuroimmunol Neuroinflamm. 2015;2:e135.
Oberwahrenbrock T, Traber GL, Lukas S, Gabilondo I, Nolan R, Songster C, et al. Multicenter reliability of semiautomatic retinal layer segmentation using OCT. Neurol Neuroimmunol Neuroinflamm. 2018;5:e449.
Oertel FC, Zimmermann H, Paul F, Brandt AU. Optical coherence tomography in neuromyelitis optica spectrum disorders: potential advantages for individualized monitoring of progression and therapy. EPMA J. 2018;9:21–33.
Oertel FC, Kuchling J, Zimmermann H, Chien C, Schmidt F, Knier B, et al. Microstructural visual system changes in AQP4-antibody–seropositive NMOSD. Neurol Neuroimmunol Neuroinflamm. 2017;4:e334.
Oertel FC, Havla J, Roca-Fernández A, Lizak N, Zimmermann H, Motamedi S, et al. Retinal ganglion cell loss in neuromyelitis optica: a longitudinal study. J Neurol Neurosurg Psychiatry. 2018;89(12):1259-1265.
Mader S, Gredler V, Schanda K, Rostasy K, Dujmovic I, Pfaller K, et al. Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J Neuroinflammation. 2011;8:184.
Schippling S, Balk LJ, Costello F, Albrecht P, Balcer L, Calabresi PA, et al. Quality control for retinal OCT in multiple sclerosis: validation of the OSCAR-IB criteria. Mult Scler. 2015;21:163–70.
Cruz-Herranz A, Balk LJ, Oberwahrenbrock T, Saidha S, Martinez-Lapiscina EH, Lagreze WA, et al. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2016;86:2303–9.
Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–89.
Balk LJ, Cruz-Herranz A, Albrecht P, Arnow S, Gelfand JM, Tewarie P, et al. Timing of retinal neuronal and axonal loss in MS: a longitudinal OCT study. J Neurol. 2016;263:1323–31.
Sato DK, Callegaro D, Lana-Peixoto MA, Waters PJ, Jorge FM de H, Takahashi T, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology. 2014;82:474–81.
Höftberger R, Sepulveda M, Armangue T, Blanco Y, Rostásy K, Cobo Calvo A, et al. Antibodies to MOG and AQP4 in adults with neuromyelitis optica and suspected limited forms of the disease. Mult Scler. 2015;21:866–74.
Akaishi T, Sato DK, Nakashima I, Takeshita T, Takahashi T, Doi H, et al. MRI and retinal abnormalities in isolated optic neuritis with myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies: a comparative study. J Neurol Neurosurg Psychiatry. 2016;87:446–8.
Nakajima H, Motomura M, Tanaka K, Fujikawa A, Nakata R, Maeda Y, et al. Antibodies to myelin oligodendrocyte glycoprotein in idiopathic optic neuritis. BMJ Open. 2015;5:e007766.
Akaishi T, Nakashima I, Takeshita T, Mugikura S, Sato DK, Takahashi T, et al. Lesion length of optic neuritis impacts visual prognosis in neuromyelitis optica. J Neuroimmunol. 2016;293:28–33.
Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation 27;13(1):280.
Jitprapaikulsan J, Chen JJ, Flanagan EP, Tobin WO, Fryer JP, Weinshenker BG, et al. Aquaporin-4 and myelin oligodendrocyte glycoprotein autoantibody status predict outcome of recurrent optic neuritis. Ophthalmology. 2018;125:1628–37.
Ramanathan S, Reddel SW, Henderson A, Parratt JDE, Barnett M, Gatt PN, et al. Antibodies to myelin oligodendrocyte glycoprotein in bilateral and recurrent optic neuritis. Neurol Neuroimmunol Neuroinflamm 2014;1(4):e40.
Brandt AU, Oberwahrenbrock T, Kadas EM, Lagrèze WA, Paul F. Dynamic formation of macular microcysts independent of vitreous traction changes. Neurology. 2014;83:73–7.
Kaufhold F, Zimmermann H, Schneider E, Ruprecht K, Paul F, Oberwahrenbrock T, et al. Optic neuritis is associated with inner nuclear layer thickening and microcystic macular edema independently of multiple sclerosis. PLoS One 2013;6;8(8):e71145.
Ramanathan S, Prelog K, Barnes EH, Tantsis EM, Reddel SW, Henderson AP, et al. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult Scler. 2016;22:470–82.
Felix CM, Levin MH, Verkman AS. Complement-independent retinal pathology produced by intravitreal injection of neuromyelitis optica immunoglobulin G. J Neuroinflammation. 2016;13:275.
Chien C, Scheel M, Schmitz-Hübsch T, Borisow N, Ruprecht K, Bellmann-Strobl J, et al. Spinal cord lesions and atrophy in NMOSD with AQP4-IgG and MOG-IgG associated autoimmunity. Mult Scler. 2018:1352458518815596.
Spadaro M, Gerdes LA, Krumbholz M, Ertl-Wagner B, Thaler FS, Schuh E, et al. Autoantibodies to MOG in a distinct subgroup of adult multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2016;3:e257.
Körtvélyessy P, Breu M, Pawlitzki M, Metz I, Heinze H-J, Matzke M, et al. ADEM-like presentation, anti-MOG antibodies, and MS pathology: TWO case reports. Neurol Neuroimmunol Neuroinflamm. 2017;4:e335.
Acknowledgements
We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Funds of Charité-Universitätsmedizin Berlin. JH and FCO like to thank Charlotte Bereuter, Angelika Bamberger, Luise Böhm, and Ivonne Hinz for their excellent technical support. SJ would like to thank Mrs. Anna Eschlbeck and the Nikon Imaging Center at the University of Heidelberg for excellent technical assistance. MR would like to thank Mrs. Kathrin Schanda for excellent technical assistance.
Funding
The project was supported with grants from the German Ministry for Education and Research (BMBF/KKNMS; Competence Network Multiple Sclerosis (to FP, KR, EM, BK), from the Deutsche Forschungsgemeinschaft (DFG, grant Exc. 257 to FP, AUB; DFG SFB TR128 to EM, RH), from the German Federal Ministry of Economic Affairs and Energy (EXIST 03EFEBE079 to AUB), from the German Ministry of Education and Research (N2-ADVISIMS 16GW0079 to FP and AUB), from the National Multiple Sclerosis Society (to FP), from the Guthy-Jackson Charitable Foundation (to FP, AUB), from the German Federal Ministry of Education and Research under 01ZZ1603[A-D] and 01ZZ1804[A-H] (DIFUTURE) and Friedrich-Baur-Stiftung (to JH) and from Novartis (to HZ, BK), from intramural funding of the Technical University of Munich (to BK), from the Verein zur Therapieforschung für MS Kranke (to EM).
Author information
Authors and Affiliations
Contributions
FCO participated in the design of the study, collected data, performed OCT quality check (BERLIN), performed the statistical analysis, contributed to the interpretation of the results, and drafted the manuscript. OO acquired OCT and clinical and visual function data and performed OCT quality check (LILLE). BK acquired OCT and clinical and visual function data and performed OCT quality check and segmentation (TUM). HZ acquired OCT and visual function data (BERLIN). NB and JBS acquired clinical data and participated in the coordination of the cohort study (BERLIN). AB acquired OCT and visual function data and performed OCT quality check (LMU). SJ performed the MOG-IgG assays. MR, KR, EM, RH, TK, and FP participated in the study design and coordination. AUB conceived the study and participated in its design, coordination as well as contributed to the interpretation of the results and supervised the statistical analyses. JH participated in the design of the study and the coordinaton, collected data, acquired OCT and clinical and visual function data (LMU), performed OCT segmentation (all centers apart from TUM), contributed to the interpretation of the results, and drafted the manuscript. All authors were involved in revising the manuscript for intellectual content and read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Twenty-four patients were seen at four university tertiary care centers specialized in neuroimmunological diseases (Institute of clinical Neuroimmunology, Ludwig- Maximilians University (LMU), Munich, NeuroCure Clinical Research Center, Charité – Universitätsmedizin Berlin, Germany; Department of Neurology, University of Lille Hospital, Lille, France; Department of Neurology, Klinikum Rechts der Isar, Technische Universität München (TUM), Munich, Germany). Written informed consent was obtained from all patients participating in the study. The local ethics committees approved the study protocol in accordance with the Declaration of Helsinki (1964) in its currently applicable version.
Consent for publication
Not applicable
Competing interests
JH reports a grant for OCT research from the Friedrich-Baur-Stiftung; personal fees and non-financial support from Merck, Novartis, Roche, Bayer Healthcare, Santhera, Biogen, Sanofi Genzyme; and non-financial support of the Guthy-Jackson Charitable Foundation, all outside the submitted work. FCO was an employee of Nocturne UG, unrelated to this work. OO reports grant for research from Novartis-Pharma and grants and personal fees from Biogen, Genzyme, Merck, Novartis-Pharma, and Teva-Pharma, outside the submitted work. BK reports grants from the Bundesministerium für Bildung und Forschung (Kompetenznetz Multiple Sklerose KKNMS), intramural funding from the Technical University of Munich (KKF program) and a grant from Novartis unrelated to this study. HZ reports a research grant from Novartis related to this study and speaking fees from Teva unrelated to this study. LB has nothing to disclose. JBS has received travel grants and speaking fees from Bayer Healthcare, Biogen Idec, Merck Serono, Sanofi-Aventis/Genzyme, Teva Pharmaceuticals unrelated to this study. SJ has nothing to disclose. The Neurological Research Laboratory (Medical University of Innsbruck and Tirol Kliniken, MR) receives payments for antibody assays (AQP4- and anti-neuronal antibodies) and for AQP4- and MOG-antibody validation experiments organized by Euroimmun (Germany). KR was supported by the German Ministry of Education and Research (BMBF/KKNMS, Competence Network Multiple Sclerosis) and has received research support from Novartis and Merck Serono as well as speaking fees and travel grants from Guthy Jackson Charitable Foundation, Bayer Healthcare, Biogen Idec, Merck Serono, Sanofi-Aventis/Genzyme, Teva Pharmaceuticals, Roche and Novartis. EM received an honorarium from Roche, Novartis and Genzyme, and grant support from Novartis and Genzyme. RH received research grants and/or speaker honoraria from Actelion, Genzyme-Sanofi, Novartis, Immunic, Roche. FP reports research grants and speaker honoraria from Bayer, Teva, Genzyme, Merck, Novartis, MedImmune and is a member of the steering committee of the OCTIMS study (Novartis), all unrelated to this work. AUB is the founder and holds shares of Motognosis and Nocturne. He is named as an inventor on several patent applications describing serum biomarkers for MS, perceptive visual computing for tracking of motor dysfunction and OCT image analysis. TK received travel expenses and personal compensations from Bayer Healthcare, Teva Pharma, Merck, Novartis Pharma, Sanofi-Aventis/Genzyme, Roche, and Biogen as well as grant support from Bayer-Schering AG, Novartis and Chugai Pharma, unrelated to this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Figure S1. Spaghetti plots of longitudinal OCT data. Plotted absolute values of all subjects for time since baseline in years for (A) pRNFL and (B) GCIP, (C) MV for eyes of MOG-IgG-seropositive patients (turquoise) and HC (red), as well as (D) plotted absolute values of all EyesON- with (green) and without (red) an attack in the 6 months before baseline. Abbreviations: GCIP: Combined ganglion cell and inner plexiform layer, HC: Healthy control, Eye ON-: MOG-IgG-seropositive eyes without a history of ON, ON: Optic neuritis, OCT: Optical coherence tomography, pRNFL: Peripapillary retinal nerve fiber layer, MV: Macular volume. (TIFF 47160 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Oertel, F.C., Outteryck, O., Knier, B. et al. Optical coherence tomography in myelin-oligodendrocyte-glycoprotein antibody-seropositive patients: a longitudinal study. J Neuroinflammation 16, 154 (2019). https://doi.org/10.1186/s12974-019-1521-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12974-019-1521-5