Abstract
Background
Drinks with higher dissolved oxygen concentrations have in recent times gained popularity as a potential ergogenic aid, despite a lack of evidence regarding their efficacy. The aim of this study was to assess effects of ingestion of an oxygen supplement (OS) on exercise performance and post-exercise recovery in a group of trained runners.
Methods
Trained male runners (n = 25, mean ± SD; age 23 ± 6 years, mass 70 ± 9 kg, BMI 21.9 ± 2.7 kg.m−2 VO2max 64 ± 6mL.kg−1.min−1), completed a randomised double blinded, crossover study to assess the effect of ingestion of OS solution on exercise performance and recovery. Trials consisted of a 30min rest period, 5min warm-up, a 5000m treadmill time-trial, and a 30min passive recovery. Participants ingested 6x15mL of either OS or a taste matched placebo during the trials (3 during the rest phase, 1 during exercise and 2 during the recovery). Muscle tissue O2 saturation was measured via near infrared spectroscopy. Blood lactate concentrations were measured prior to, mid-way and directly after the finish of the 5000m time trials and every 3-min during the post-exercise recovery.
Results
Ingestion of OS did not improve exercise performance. No significant differences were observed for muscle tissue O2 saturation at any time-points. However, lactate clearance was significantly improved during recovery in the OS trials. Both AUC (109 ± 32 vs. 123 ± 38 mmol.min, P < 0.05, d = 0.40) and lactate half-life (λ) (1127 ± 272 vs. 1223 ± 334 s, P < 0.05, d = 0.32) were significantly reduced.
Conclusions
Despite no evidence of improved exercise performance, ingestion of OS did enhance post-exercise recovery via increased lactate clearance.
Similar content being viewed by others
Background
The study of oxygen supplementation dates back to the 1940’s, when high altitude climbers fought - and in many cases died—to plant their nation’s flag on the world’s highest peaks. Climbers used bottled oxygen to supplement their breathing in order stay alive in the extreme environment above 8000m, known as “the death zone”. Many studies from this period documented the physiological effects of inspiring higher concentrations of oxygen during exercise. Such effects include increased arterial oxygen saturation [1], decreased pulmonary ventilation [2], lower submaximal heart rate and blood lactate concentration [3], and increased VO2max [4].
More recently, commercially available drinks advertising high concentrations of dissolved O2 have become popular. Despite anecdotal reports from athletes, few controlled studies have been conducted and therefore the ergogenic effects of these drinks remains questionable [5]. Of the limited studies which have examined the effects of oxygenated water, most have reported no effect on aerobic performance [6–10]. The majority of these studies have used VO2 either at sub-maximal or maximal intensity as a measure of aerobic performance [6–9]. However, it is not immediately clear why these researchers would expect to see any change in VO2 following ingestion of supplemental oxygen. VO2 is calculated using the Haldane Transformation which assumes that O2 consumption is equal to the difference between inspired O2 and expired O2. Since ingested O2 is not accounted for in this equation, any additional O2 which is theoretically utilized by the working muscles would not be detected using pulmonary gas measurement.
The primary criticism put forward by skeptics of oxygenated drinks is that ingested O2 is not readily diffused across the gastro-intestinal tract [6, 9, 11]. However, two studies have demonstrated that high-concentration oxygen solutions are capable of diffusing O2 into the bloodstream, albeit into the hepatic portal vein in rabbits [12] and kittens [13]. Neither study assessed whether this gas diffusion altered the systemic or peripheral arterial saturation, and so extrapolating a potential ergogenic effect at a muscular level is pre-mature. To date, no study has verified if the human gastro-intestinal tract has the potential to absorb O2 into the hepatic portal vein. Previous human studies have used pulse oximetry [5, 10, 14] or blood gas analysis [6, 8] to quantify systemic O2 concentrations with most reporting no change in oxygen saturation. However, muscle tissue O2 saturation has yet to be determined.
The majority of studies examining the ergogenic effects of oxygenated water report that exercise performance is not improved [6–10]. Only one study, which recruited higher trained athletes, reported improvements in performance and increased O2 saturation ([14] abstract only). However, an interesting effect of oxygenated water has been observed in two separate studies [7, 8]. Despite both studies reporting no improvement in performance, the authors did observe lower maximal lactate concentration and enhanced lactate clearance post-exercise. In both studies, this finding was statistically significant and it is curious that neither group discussed the potential implications of such a finding on recovery. In addition, neither study tracked lactate clearance kinetics for longer than 6 min, so the full effect has not been established.
Based on the limited published literature examining ingestion of oxygen supplements (OS), it appears that a more comprehensive evaluation is warranted. In addition, the effects on lactate clearance kinetics require further examination, since the two studies which previously measured this variable showed a significant effect [7, 8]. The primary aim of this study was to investigate if ingesting OS had an ergogenic effect on exercise performance. A secondary aim was to assess its effect on muscle O2 saturation and lactate concentration before, during and after exercise.
Methods
Study design
A cohort of 25 male collegiate level distance runners (see Table 1) performed double-blinded, placebo controlled trials in a counterbalanced cross-over design in order to assess the effect of OS ingestion on performance, muscle O2 saturation and blood lactate kinetics. Subjects reported a minimum of 200 min.week−1 of running and were excluded if they had any medical condition that prevented them training for more than 7 consecutive days in the previous 6 months. All subjects attended the laboratory on three occasions. During the first visit, they were familiarized with all procedures and equipment. If satisfied, informed consent was obtained and pre-trial physiological data collected. The second and third visits comprised of ingestion of either OS or placebo, followed by a 5000m self-paced treadmill time-trial. Ethical approval for this study was granted by Indiana State University’s Institutional Review Board and informed consent was obtained from all subjects prior to data collection.
Exercise protocol
During the initial visit, subjects performed a maximal incremental test, in order to quantify VO2max. Starting velocity for the test was 10km.h−1 with an increase of 1km.h−1 every 3-min until volitional failure was reached. Pulmonary gas exchange data was collected throughout the test using a Parvomedics TrueOne 2600 analyser. VO2max was defined as the maximal VO2 recorded during any 15-s interval.
During visits 2 and 3, subjects performed a self-paced 5000m time-trial on a treadmill (Woodway Forefront) following ingestion of OS or a taste-matched placebo. All subjects were instructed not to eat or consume caffeine in the 120-min prior to testing, in order to ensure appropriate gastric emptying prior to ingestion of solutions and to reduce possible confounding effects that caffeine might have on exercise performance. Repeat tests were performed at the same time of the day in order to minimize the effect of circadian variability. Subjects ingested a series of 6 × 15mL volumes of either OS or taste matched placebo, at fixed time-points before, during and after the 5000m time trial (see Fig. 1).
The composition of 15mL of OS was ASO® solution (Activate Stabilized Oxygen), a registered dietary oxygen supplement for human consumption. The ingredients to ASO® are the following:
-
Distilled water: (62.04%)
-
Dissolved O2 (in molecular O4 form): (35.00%)
-
Salt & trace elements: (2.96%)
The taste-matched placebo comprised of 0.6mg of NaCl added to 15mL of distilled water. Taste testing was carried out prior to the initiation of the study. 50 students and faculty were asked to taste both solutions and decide which one they thought was the OS solution, with correct responses no better than chance. Following completion of both exercise trials, subjects were asked if they could differentiate between drinks based on taste. 11 of the subjects stated they could taste a difference between drinks but were unable to determine which drink contained OS. The remaining 14 subjects could not taste any difference between drinks.
Upon completion of the 30-min seated rest period, subjects performed a 5min warm-up at a self-selected pace. They then performed a self-paced 5000m time trial. Information on elapsed time and distance covered were provided continuously throughout the test via the treadmill monitor. Subjects were instructed to run the 5000m distance as fast as they possibly could. They were free to increase and decrease the treadmill velocity as necessary during the time-trial, but all subjects were encouraged to complete the distance as fast as possible. The 5000m distance was selected because the duration and intensity are such that the aerobic system is near maximally stressed. It has previously been reported that well-trained runners utilise an average of 94% of their VO2max when running a simulated 5000m race on a treadmill [15]. In addition, previous studies have demonstrated that test-restest reliability for 5000m time-trials is higher than submaximal endurance trials of a similar intensity [16]. Upon completion of the test, participants began a 30min passive recovery in a seated position (see Fig. 1).
Blood lactate data
Capillary blood samples were collected from the middle finger of the left hand using aseptic techniques, to measure blood lactate concentrations before, during and after exercise. Samples were collected immediately before, at the mid-point and immediately upon completion of the time-trial. Additional samples were collected at 3min intervals during the recovery. A 7μL sample was injected into an Analox GM7 metabolic analyzer. A new Teflon membrane was installed in the analyzer prior to the initiation of data collection. Test-retest analysis of the membrane was carried out via 10 repeat measures of 8mMol.L−1 lactate standard. The CV for this protocol was 0.6%, which falls within the acceptable range of 0–1%, set by the manufacturer. The lactate analyser was calibrated prior to each exercise test (to a resolution of ±0.1mmol.L−1), using 8mmol.L−1 lactate standard.
Oxygen saturation
Muscle tissue oxygen saturation was measured at 10Hz continuously from the right Rectus Femoris using a portable NIRS monitor (Portamon MkII, Artinis). The right Rectus Femoris was chosen over the Vastii muscles due to the observation of more stable data with less motion artifact during pilot testing in running. Since the midpoint of the Rectus Femoris is located more proximal to the hip joint than either of the Vastii muscles, the sensor movement was lower with this muscle. The recording site was shaved and cleaned with isopropyl alcohol prior to application of the NIRS sensor. In addition, the recording site was marked with permanent marker in order to ensure correct sensor placement on repeat visits. Data were recorded continuously throughout the rest, exercise and recovery phases and reported as a combined tissue saturation index (%TSI). A 6-min control period prior to fluid ingestion allowed for the measurement of baseline %TSI. Changes in %TSI were then averaged for each 3-min interval and normalized to baseline for each trial. This method of estimating tissue oxygen saturation has been validated and used for the last 10 years as a measure of muscle oxygenation [17].
Statistical analysis
All statistical tests were performed using Graphpad Prism Version 6.0 (GraphPad Software, CA, USA). Performance was quantified as time to completion (TTC) of the 5000m time-trial. Lactate clearance kinetics were quantified during recovery by measuring the area under the curve (AUC) and the time taken to reduce the peak lactate concentration by 50% (λ), also known as lactate half-life. A 4th order polynomial was fitted over the 10 blood lactate concentrations measured following completion of the time-trial. AUC and λ were subsequently computed using Matlab (V7.14 R2012a, Mathworks, MA, USA). All data sets were initially tested for normality via Kolmogorov-Smirnov tests. For comparison of data across time between the two drink trials, a 2-factor ANOVA (drink x time) with 1 repeated measure (drink) was used. Bonferoni post-hoc tests quantified significance where identified. For variables which were independent of time (TTC, final lactate, λ, AUC), paired Student’s T-tests were performed, with statistical significance inferred at P < 0.05. Where statistically significant differences between drinks was observed, effect sizes (Cohen’s D) were computed, with >0.2 indicating a small effect, >0.5 a moderate effect and >0.8 a large effect.
Results
Performance data
Group anthropometric and physiological data are presented in Table 1. The group mean (± SD) TTC data were 1096 ± 80 and 1102 ± 93 s, for OS and placebo trials, respectively. The group performed an average of 6 s faster during the OS trials; however, this difference did not attain statistical significance.
Blood lactate data
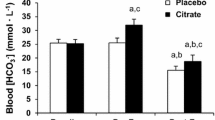
A significant time effect was observed for lactate concentrations both during exercise and recovery (P < 0.001). This time effect was statistically significant in both OS and placebo trials. Group mean lactate concentrations were lower during the OS trials at the mid-point and finish of time-trials, and at every time-point during recovery (see Fig. 2), however this effect failed to attain statistical significance. Final lactate concentrations at the end of the 5000m time-trial were 6.5 ± 1.5 vs. 6.8 ± 1.7 mmol.L−1 for OS and placebo trials respectively. Lactate clearance kinetics were significantly different comparing OS and placebo trials. Both AUC (109 ± 32 vs. 123 ± 38 mmol.min, P < 0.05, d = 0.40; see Fig. 3a) and λ (1127 ± 272 vs. 1223 ± 334, P < 0.05, d = 0.32; see Fig. 3b) were significantly reduced during the OS trials, indicating enhanced lactate clearance.
Oxygen saturation
During the rest period, tissue O2 saturation significantly increased (P < 0.001, see Fig. 4a) across time. This time effect was statistically significant in both OS and placebo trials and is most likely due to reduced cardiovascular stress and venous pooling associated with sitting in a chair for 30min. During exercise, there was a significant reduction in tissue O2 saturation across time (P < 0.001, see Fig. 4b), however no differences between drink trials were observed. Tissue O2 saturation did appear lower during OS trials however the differences to placebo were not significant (see Fig. 4b).
Discussion
The main finding from the current study is that ingestion of OS resulted in a significant improvement in post-exercise lactate clearance. However, this apparent enhancement in lactate metabolism was not detectable during exercise and did not yield an improvement in overall performance. Additionally, there was no evidence of any increase in tissue or systemic oxygen saturation measured via pulse-oximetry and NIRS. It is possible that increased oxygen saturation at a hepatic level resulted in greater lactate metabolism by the liver, however further research is required in order to test this hypothesis.
Lactate clearance
It is generally accepted that high intensity exercise results in significant production of lactate within the muscle [18] and this accumulation of lactate—or the associated muscle acidosis—is a major determinant of fatigue [19, 20]. Several studies have demonstrated that enhanced blood lactate clearance via active recovery, improves subsequent exercise performance [21, 22]. The current results suggest that ingestion of OS facilitates more rapid clearance of lactate and could therefore enhance recovery from exercise. Both the half-life and AUC data were significantly lower during recovery in the OS compared to placebo trials (see Fig. 3). To the best of our knowledge, this study is the first to evaluate the effects of OS on post-exercise lactate kinetics over a 30 min time course. Several other studies have also reported similar effects of OS ingestion on lactate concentrations—albeit over a shorter time course. Leibetseder, Strauss-Blasche [7] reported significantly lower lactate concentrations at the end of maximal incremental exercise. McNaughton, Kenney [8] also reported significant reductions in lactate concentrations. In this case, ingestion of OS resulted in significantly lower lactate concentrations at 0 and 3min after completion of a 15min cycling time-trial. The current findings represent further evidence of the effects of OS ingestion on blood lactate kinetics.
Physiological mechanisms
It is tempting to suggest that enhanced delivery of O2 to the working muscles may explain the improvements in lactate kinetics. However, no differences in tissue or peripheral O2 saturation were observed before, during or after exercise. This finding is in agreement with the majority of studies examining O2 saturation either via pulse-oximetry [10] or blood gas analysis [6, 8, 9]. The most likely explanation for these results is that an increase in hepatic metabolism via the Cori cycle resulted in greater lactate clearance. Next to the kidneys, the liver is the most important human tissue for net lactate clearance at rest [23]. This is evident in the use of lactate clearance kinetics as a reliable clinical indicator of liver transplant success [24]. The metabolic rate of liver cells is directly influenced by surrounding oxygen concentration [25, 26], with increased metabolism of lactate occurring at higher O2 concentrations. Previous studies of O2 diffusion across the gastro-intestinal tract have reported significant increases in O2 concentration within the hepatic portal vein [12, 13]. While these animal studies are not directly comparable with human physiology, they nonetheless demonstrate that enhanced O2 delivery to the liver is at least theoretically possible following OS ingestion in humans. Increased oxygen delivery to the liver via the hepatic portal vein would therefore enhance blood lactate clearance. A similar physiological effect has also been observed in liver’s metabolism of alcohol in humans. Several studies have recently reported that oxygenated alcohol beverages resulted in increased rates of blood alcohol clearance [27, 28]. Based on the combined evidence from animal, hepatocyte and human alcohol studies, we hypothesize that enhanced lactate clearance following OS ingestion is potentially mediated by increased hepatic metabolism; however this hypothesis can only be tested via in-vivo measurement of liver O2 concentrations and/or liver enzyme activity.
Applications to performance
Many sports such as track sprinting, cycling, swimming, and rowing require the athlete to perform on more than one occasion during a single day. The ability to clear lactate more efficiently and hence recover faster in early rounds of competition is of benefit to such athletes [22]. Lactate clearance is also of importance in several team based sports which involve intermittent exercise and recovery. For example, the sport of basketball involves continuous flow of play with players performing high intensity movements on average every 21 s [29]. However, basketball players receive regular recovery during the game via substitution, time-outs and breaks at each quarter. Despite these periods of recovery, it has been shown that players compete with average circulating blood lactate concentrations of 6.8 mmol.L−1 throughout the game [29]. Any intervention which enhances the clearance of lactate during a player’s recovery would therefore likely improve overall performance in such sports.
Study limitations
The main limitation of the current study is that the protocol was not designed to elicit maximal lactate concentrations. A 5000m time-trial was used, in order to assess if OS improved performance in an activity that places high demands on the oxidative energy system. While this near-maximal aerobic exercise did result in moderate accumulation of blood lactate, high-intensity repeated intermittent sprint exercise would place greater demands on the anaerobic energy systems and thus result in substantially higher lactate concentrations [30]. Based on the current results, OS ingestion may be of greater benefit during and after anaerobic exercise, especially activities which result in large accumulation of blood lactate. Secondly, the assessment of subsequent exercise performance following a period of recovery was not assessed. Therefore our statement that enhanced lactate clearance can improve subsequent performance is based on previous work [21, 22] and not the current results. Future studies should focus on the possible ergogenic effects of OS ingestion during high-intensity anaerobic exercise and/or post-recovery performance. Finally, no direct measurement of tissue specific O2 concentration or liver enzymatic activity was made. Our hypothesis that OS ingestion may enhance hepatic metabolism of lactate would require direct measurement of hepatic O2 consumption or enzymatic activity. Further research is necessary in order to elucidate the physiological mechanisms underlying the enhanced lactate clearance observed in this study.
Conclusions
Ingestion of OS resulted in enhanced post-exercise lactate clearance following a 5000m time-trial in trained distance runners. However, no improvements in performance or lactate kinetics during exercise were observed. The current findings may have important implications for optimizing post-exercise recovery strategies for athletes. Further research is warranted, in order to elucidate the physiological mechanisms underlying the apparent enhancement in lactate clearance kinetics.
References
Bannister R, Cunningham D. The effects of respiration and performance during exercise of adding oxygen to inspired air. J Physiol. 1954;125:118–37.
Asmussen E, Nielsen M. Studies on the regulation of respiration in heavy work. Acta Physiol Scand. 1946;12:171–88.
Asmussen E, Nielsen M. The effect of autotransfusion of ‘work blood’ on the pulmonary ventilation. Acta Physiol Scand. 1950;20:79–87.
Welch H. Hyperoxia and human performance: a brief review. Med Sci Sports Exerc. 1982;14(4):253–62.
Fuller P. The effects of activated stabilized oxygen on aerobic endurance in division II collegiate male soccer players, in Department of Kinesiology. Arcata: Humboldt State University; 2010.
Hampson N, Pollock N, Piantadosi C. Oxygenated water and athletic performance. J Am Med Assoc. 2003;290(18):2408–9.
Leibetseder V, Strauss-Blasche G, Marktl W, Ekmekcioglu C. Does oxygenated water support aerobic performance and lactate kinetics? Int J Sports Med. 2006;27:232–5.
McNaughton L, Kenney S, Siegler J, Midgley A, Lovell R, Bentley D. The effect of spoeroxygenated water on blood gases, lactate, and aerobic cycling performance. Int J Sports Physiol Perform. 2007;2:377–85.
Willmert N, Porcari J, Forster C, Doberstein S, Brice G. The effects of oxygenated water on exercise physiology during incremental exercise and recovery. J Exerc Physiol. 2002;5(4):16–21.
Wing-Gaia S, Subudhi A, Askew E. Effects of purified oxygenated water on exercise performance during acute hypoxic exposure. Int J Sport Nutr Exerc Metab. 2005;15(6):680.
Piantadosi C. “Oxygenated” water and athletic performance. Br J Sports Med. 2006;40(9):740–1.
Forth W, Adam O. Uptake of oxygen from the intestine - experiments with rabbits. Eur J Med Res. 2001;6(11):488–92.
Cooper EA, Smith H, Pask EA. On the efficiency of intra-gastric oxygen. 1960. Anaesthesia. 1995;50(6):535–44.
Jenkins A, Moreland M, Waddell T, Fernhall B. Effect of oxygenized water on percent oxygen saturation and performance during exercise. Med Sci Sports Exerc. 2001;33(5):S167.
Farrell PA, Wilmore JH, Coyle EF, Billing JE, Costill DL. Plasma lactate accumulation and distance running performance. Med Sci Sports. 1979;11(4):338–44.
Laursen PB, Francis GT, Abbiss CR, Newton MJ, Nosaka K. Reliability of time-to-exhaustion versus time-trial running tests in runners. Med Sci Sports Exerc. 2007;39(8):1374–9.
Ferrari M, Muthalib M, Quaresima V. The use of near-infrared spectroscopy in understanding skeletal muscle physiology: recent developments. Philos T R Roy Soc A. 2011;369:1–14.
Astrand PO, Hallback I, Hedman R, Saltin B. Blood lactates after prolonged severe exercise. J Appl Physiol. 1963;18:619–22.
Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: Cellular mechanisms. Physiol Rev. 2008;88:287–332.
Fitts RH, Metzger JM. In: Poortmans JR, editor. Mechanisms of muscular fatigue, in Principals of Exercise Biochemistry. Basel: Karger; 1988. p. 212–29.
Gupta S, Goswami A, Sadhukhan AK, Mathur DN. Comparative study of lactate removal in short term massage of extremities, active recovery and a passive recovery period after supramaximal exercise sessions. Int J Sports Med. 1996;17(2):106–10.
Monedero J, Donne B. Effect of recovery interventions on lactate removal and subsequent performance. Int J Sports Med. 2000;21(8):593–7.
van Hall G. Lactate kinetics in human tissues at rest and during exercise. Acta Physiol (Oxf). 2010;199(4):499–508.
Wu JF, Wu RY, Chen J, Ou-Yang B, Chen MY, Guan XD. Early lactate clearance as a reliable predictor of initial poor graft function after orthotopic liver transplantation. Hepatobiliary Pancreat Dis Int. 2011;10(6):587–92.
Wolfle D, Jungerman K. Long term effects of physiological oxygen concentrations on glycolysis and gluconeogenesis in hepatocyte cultures. Eur J Biochem. 1985;151:299–305.
Wolfle D, Schmidt H, Jungerman K. Short-term modulation of glycogen metabolism, glycolysis and gluconeogenesis by physiological oxygen concentrations in hepatocyte cultures. Eur J Biochem. 1983;135:405–12.
Baek IH, Lee BY, Kwon KI. Influence of dissolved oxygen concentration on the pharmacokinetics of alcohol in humans. Alcohol Clin Exp Res. 2010;34(5):834–9.
Rhee SJ, Chae JW, Song BJ, Lee ES, Kwon KI. Effect of dissolved oxygen in alcoholic beverages and drinking water on alcohol elimination in humans. Alcohol. 2013;47(1):27–30.
McInnes SE, Carlson JS, Jones CJ, McKenna MJ. The physiological load imposed on basketball players during competition. J Sports Sci. 1995;13(5):387–97.
Beneke R, Leithauser RM, Ochentel O. Blood lactate diagnostics in exercise testing and training. Int J Sports Physiol Perform. 2011;6(1):8–24.
Acknowledgements
Not applicable.
Funding
This study was sponsored by Formula Four Beverages Inc., Vancouver, BC.
Availability of data and materials
Raw data is presently unavailable for sharing due to the agreement between the researching parties and funding organizations.
Authors’ contributions
All authors contributed to the conception of the experimental design and drafting of the manuscript. NF, JV, and MF participated in data collection. Data were analyzed by NF and JV. All authors have read and approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Ethical approval for this study was granted by Indiana State University’s Institutional Review Board and informed consent was obtained from all subjects prior to data collection.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Fleming, N., Vaughan, J. & Feeback, M. Ingestion of oxygenated water enhances lactate clearance kinetics in trained runners. J Int Soc Sports Nutr 14, 9 (2017). https://doi.org/10.1186/s12970-017-0166-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12970-017-0166-y