Abstract
Background
Cigarette smoking (CS) triggers an intense and harmful inflammatory response in lungs mediated by alveolar and blood macrophages, monocytes, and neutrophils and is closely associated with prevalence of tuberculosis (TB). The risk of death in patients with long-term cigarette smoking-related pulmonary tuberculosis (LCS-PTB) is approximately 4.5 times higher than those with nonsmoking pulmonary tuberculosis (N-PTB). However, the mechanisms underlying the harmful inflammatory responses in the setting of LCS-PTB have not been well documented.
Methods
28 cases LCS-PTB patients, 22 cases N-PTB patients and 20 cases healthy volunteers were enrolled in this study. Monocytes were isolated from peripheral blood mononuclear cells. Differentiated human MDM and U937 cell were prepared with M-CSF and PMA stimulation, respectively. The miR-196b-5p, STAT1, STAT3, STAT4, STAT5A, STAT5B, STAT6, SOCS1 and SOCS3 mRNA expression were detected by qRT-PCR. Western blot was performed according to SOCS1, SOCS3, and pSTAT3 expression. The mycobacterial uptake by MDMs from different groups of patients after Bacillus Calmette–Guérin (BCG) infection and agomir-196b-5p or antagomir-196b-5p transfection were used by flow cytometry analysis. Human IL-6, IL-10 and TNF-α levels on the plasma and cell culture supernatant samples were measured using ELISA. For dual-luciferase reporter assay, the SOCS3 3′-UTR segments, containing the binding elements of miR-196b-5p or its mutant versions were synthesized as sense and antisense linkers.
Results
In this study, we found that IL-6, TNF-α production, SOCS3 mRNA expression were downregulated, while miR-196b-5p and STAT3 mRNA expression were upregulated in monocytes from LCS-PTB patients as compared to N-PTB patients. Meanwhile, we demonstrated that miR-196b-5p could target SOCS3 and activate STAT3 signaling pathway, which may possibly contribute to attenuation of BCG uptake and decrease in IL-6 and TNF-α production in macrophages.
Conclusions
Our findings revealed that CS exposure regulates inflammatory responses in monocyte/macrophages from LCS-PTB patients via upregulating miR-196b-5p, and further understanding of the specific role of miR-196b-5p in inflammatory responses mightfacilitate elucidating the pathogenesis of LCS-PTB, thus leading to the development of new therapeutic strategies for PTB patients with long-term cigarette smoking.
Similar content being viewed by others
Background
Tuberculosis (TB) caused by infection of Mycobacterium tuberculosis (MTB) is one of the top 10 causes of death in developing countries [1]. The risk factors for TB include crowding, poor nutrition, alcoholism, race/ethnicity, socioeconomic status, diabetes mellitus, human immunodeficiency virus (HIV) infection and cigarette smoking [2, 3]. Cigarette smoking (CS) has been reported to be harmful to human lungs and associated with an increase in both mortality and morbidity of TB. It has been shown that the risk of death in patients with long-term cigarette smoking-related pulmonary tuberculosis (LCS-PTB) is approximately 4.5 times higher than those with nonsmoking pulmonary tuberculosis (N-PTB) [4]. Meanwhile, LCS-PTB patients always present more severe pulmonary lesions than N-PTB patients [5]. Additionally, previous studies have shown that CS triggers an intense inflammatory response in lungs mediated by increased infiltration of alveolar and blood macrophages, monocytes, and neutrophils that release large amounts of various inflammatory cytokines and chemokines [6,7,8]. However, the molecular mechanisms underlying such pulmonary inflammatory responses in the setting of LCS have not been well documented.
Host immune system plays a critical role in the containment and cure of TB infection. The major types of innate immune cells involved in TB infection include macrophages, dendritic cells (DCs), neutrophils and natural killer (NK) cells, whereas classically activated (M1) macrophages play a central role in eliminating MTB via multiple mechanisms, such as increasing the components of free reactive oxygen and nitrogen species, production of various pro-inflammatory cytokines, phagosome acidification, autophagy induction, and among others [9]. Although M1 macrophages are known as professional killers, MTB has adopted remarkable strategies to control phagosomal processing via inhibiting phagolysosome biogenesis and acidification processes [10]. Notably, MTB survival is greatly enhanced when macrophage polarity is shifted toward alternatively activated (M2) macrophage [11]. It has been reported that treatment of monocyte-derived macrophages (MDM) by cigarette smoke extract (CSE) results in the enrichment of IL-6, TNF-α, IL-1β and MMP-9, which are commonly associated with M1 macrophages, and simultaneously, the reduction of IL-4, IL-10, and IL-13, which are associated with M2 macrophages [12,13,14,15]. It has been shown that the signal transducer and activator of transcription 1 (STAT1) plays a major role in dictating M1 macrophage phenotype, whereas STAT3/STAT6 direct M2 macrophage polarization [16,17,18].
MicroRNAs (miRNAs), small noncoding RNAs of length 22 nucleotides, are important regulatory molecules and have been shown to be involved in the regulating development and functions of monocytes/macrophages [17]. Also, they have been found to be valuable novel biomarkers for cigarette smoking [19]. MiR-196b-5p has been identified in the most primitive hematopoietic stem cells (HSCs), the precursors of of monocytes/macrophage [20]. Zhang et al. has reported that the level of miR-196b in the serum of patients with active TB was 1285.93-fold higher than that in latent TB infection (LTBI), Bacillus Calmette–Guérin (BCG)-inoculated and un-inoculated individuals [21]. Our previous results demonstrated that miR-196b-5p promotes colorectal cancer stemness and chemo-resistance via activating STAT3 signaling [22]. Accumulating data have suggested that STAT3 is a major controller of the outcome of MTB infection [23]. However, the expression of miR-196b-5p in monocytes from LCS-PTB patients and its role in the modulation of macrophage-mediated inflammatory responses during MTB infection in humans is yet to be fully elucidated. In the present study, we found that miR-196b-5p was upregulated in monocytes from LCS-PTB patients and treatment of MDM with CSE significantly increased miR-196b-5p expression. Moreover, overexpressing miR-196b-5p attenuated the uptake of BCG by MDM via targeting SOCS3 and subsequent activation of STAT3 signaling. These findings suggest that miR-196b-5p might be a valuable novel biomarker and therapeutic target for LCS-PTB patients.
Methods
Subjects
28 cases long-term cigarette smoking PTB (LCS-PTB) patients, 22 cases nonsmoking PTB (N-PTB) patients and 20 cases healthy volunteers (HV) from Dongguan sixth People’s Hospital (Dongguan, China) were enrolled in this study based on clinical symptoms, chest X radiography, acid fast bacilli (AFB) staining of sputum smears, as we previously reported [24, 25]. Subjects with HIV infection, autoimmune diseases, diabetes, cancer, immunosuppressive treatment, or pulmonary tuberculosis history were excluded.
Monocytes sorting
Peripheral blood mononuclear cells (PBMCs) were prepared to isolate monocytes using the MojoSort™ Human CD14 Nanobeads (BioLegend) according to the manufacturer’s instructions. The sorted monocytes were primary monocytes for RNA extraction.
Cultures of human MDM and U937 cells
Human MDM and U937 (a monocytic cell line, obtained from Shanghai Chinese Academy of Sciences cell bank, China) cells were cultured in RPMI 1640 medium (Gibco) supplemented with 10% FBS (heat-inactivated, mycoplasma- and endotoxin-free), 10% M-CSF, 50 U/mL penicillin, 50 µg/mL streptomycin and 2 mM l-glutamine. Monocytes were isolated from PBMC as we previously described [24,25,26]. PBMC were seeded on 24-well plates at a density of 1.5 × 106 cells/well and differentiated for 7 days, non-adherent cells were removed, and adherent cells were obtained as differentiated MDM (more than 95% of cells were CD14+, CD3−). Before experiments were performed, the cells were cultured at 5 × 105 cells/well on 24-well plates, and 50 nM phorbol myristate acetate (PMA) was used to differentiate U937 cells for 24 h.
RNA extraction and qRT- PCR
Total RNA was extracted using RNA Isolation Kit (Qiagen, USA), and reverse transcribed using the Revert Aid First Strand cDNA Synthesis Kit (Thermo, USA) according to the manufacturer’s protocol. This cDNA was amplified and quantified on CFX96 system (BIO-RAD, USA) using iQ SYBR Green (BIO-RAD, USA). The primers were provided in Additional file 1: Table S1. Primers for U6 and miR-196b-5p (Cat#: miRQ0001080) were synthesized and purified by RiboBio (Guangzhou, China) (http://www.sirna.cn/siteen/Products.aspx?id=181). QRT-PCR was performed according to a standard method, as we previously described [22]. U6 or GAPDH was used as endogenous controls. Relative expressions were calculated with the comparative threshold cycle (2−ΔΔCt) method.
Flow cytometric analysis
The isolated PBMCs were resuspended in 2% FBS-PBS, and then stained with indicated antibodies against human CD3 (HIT3α, BioLegend), CD14 (63D3, BioLegend), IL-6 (UV4, BioLegend), IL-10 (JES5-16E3, eBioscience), TNF-α (Mab11, BioLegend), and detected by flow cytometry (BD FACS Calibur II, San Jose, CA, USA) and analyzed using FlowJo 7.6 software (TreeStar Inc., USA) as we previously described [24,25,26]. Mouse IgG1 (MOPC-21), IgG2b (27–35), IgG2a (G155–178), and rat IgG2b (κ) were used as isotype control.
Exposure of cells to BCG
For testing the effects of miR-196b-5p on mycobacterial uptake in vitro, MDM or U937 macrophages were infected with green fluorescent protein (GFP) tagged BCG at the multiplicity of infection (MOI) of 10 for 6 h, then washed and cultivated for 5 days. Bacterial uptake was evaluated by measuring the percentage and median fluorescence intensity (MFI) of GFP+ MDM (BCG uptake macrophages) by flow cytometric analysis.
MiRNAs, siRNA, lentiviral particles and transfection
The agomir-196b-5p (Cat#: miR40001080), antagomir-196b-5p (Cat#: miR30001080), small interfering RNA (siRNA) for SOCS3 (SOCS3-RNAi) and respective control RNA were synthesized and purified by RiboBio, as we previously described [22]. SOCS-3 lentiviral activation particles (SOCS3-LAC, sc-400455-LAC) was purchased from Santa Cruz. Transfection of miRNAs, siRNA were performed using Lipofectamine 3000 (Life Technologies, USA) according to the manufacturer’s instructions.
Western blotting analysis
Nuclear/cytoplasmic fractionation was separated using Cell Fractionation Kit (Cell Signaling Technology, USA) and the whole cell lysates were extracted using RIPA Buffer (Cell Signaling Technology) according to the manufacturer’s instructions. Western blot was performed according to a standard method, as we previously described [22]. Proteins were visualized using ECL reagents (Pierce, USA). Antibodies against SOCS1, SOCS3, STAT3 and pSTAT3 were purchased from Cell Signaling Technology.
Dual-luciferase reporter assay
For dual-luciferase reporter assay, the SOCS3 3′-UTR segments, containing the binding elements of miR-196b-5p or its mutant versions were synthesized as sense and antisense linkers. The wild-type and mutated 3′-UTR fragments were then cloned into the downstream of luciferase reporter gene of pmirGLO vectors (Promega, Madison, WI, USA). PmirGLO-Report-WT-SOCS1 (harboring wild-type 3′-UTR) and pmirGLO-Report-Mut-SOCS1 (harboring mutant 3′-UTR) were generated. The specificity of miR-196b-5p targeting SOCS3 mRNA was ascertained by co-transfection of miR-196b-5p mimic or inhibitor (RiboBio, Guangzhou, China) and pmirGLO-Report-SOCS1/Mut into U937 cells. Additionally, as we previously described [22], relative luciferase activity of STAT3 was detected using pSTAT3 reporter luciferase plasmid (Promega). Luciferase and Renville signals were measured 36 h after transfection using a Dual Luciferase Reporter Assay Kit (Promega, Madison, WI, USA) according to the manufacturer’s protocol as we previously described [22].
Enzyme-linked immunosorbent assay (ELISA)
Cytokines were measured in the plasma and cell culture supernatant samples using human IL-6, IL-10 and TNF-α ELISA Kit (Ray Biotech, Atlanta, USA) according to the manufacturer’s instructions. Cell culture supernatant was diluted 1:10. Plasma was used as an undiluted specimen.
Statistical analysis
All analyses were performed using GraphPad Prism version 5.0 software (GraphPad Software Inc., San Diego, CA, USA). Data are presented as mean ± SEM (standard error of the mean, SEM). Student’s t-test or ANOVA (analysis of variance, ANOVA) was employed to compare the differences of measured data. A P value of less than 0.05 (95% confidence interval) was considered with statistical significance.
Results
Characteristics of the subjects included in the study
Among all prospectively enrolled subjects, 28 cases were LCS-PTB patients, 22 cases were N-PTB patients and 20 cases were healthy volunteers (HV). The demographic and clinical characteristics for all study subjects were shown in Table 1. No significant differences regarding age and gender were noted among LCS-PTB patients, N-PTB patients, and HV volunteers. Among LCS-PTB patients, 100% (28/28) were positive for acid-fast bacilli (AFB) smear-MTB and tuberculin skin test (TST) (induration diameter ≥ 10 mm), and long-term cigarette smoking (> 7 years), while 53.6% (15/28) of them were newly diagnosed LCS-PTB patients. Among N-PTB patients, all of them were AFB smear- MTB and TST (induration diameter ≥ 10 mm) positive, while 54.5% (12/22) of N-PTB patients were newly diagnosed patients. Both LCS-PTB patients and N-PTB patients had taken an ATD treatment.
Blood monocytes were elevated in LCS-PTB patients
Monocytes originated from the bone marrow represent approximately 10% of human leucocytes in the bloodstream and have traditionally been regarded as the progenitors of tissue macrophages. Monocytes could differentiate into macrophages and mediate a wide range of immunologic response processes, including reactions against TB infection [27,28,29]. Veenstra et al. and our group have previously shown that the counts and percentage of monocytes in the peripheral blood were significantly higher in active PTB patients than that in HV volunteers [25, 30]. Herein, our laboratory tests also revealed an obvious rise in the percentage and counts of monocytes in peripheral blood of LCS-PTB and N-PTB patients as compared to that in HV volunteers (Fig. 1a, b). Additionally, both percentage and counts of monocytes were even higher in peripheral blood of LCS-PTB patients than of N-PTB patients (Fig. 1a, b). However, our results showed that that the total count of lymphocytes was significantly decreased in peripheral blood of LCS-PTB patients as compared with those of both N-PTB patients and HV volunteers (Fig. 1a). These results suggest that LCS-PTB and N-PTB patients may have different systemic immune responses to TB infection.
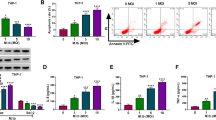
IL-6 and TNF-α were suppressed in LCS-PTB patients
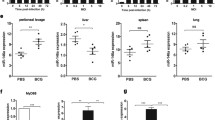
To further explore the innate immune responses in LCS-PTB patients, the IL-6, TNF-α, and IL-10 production in peripheral monocytes were detected by flow cytometric analysis. Our results showed that the percentage of CD14+ monocytes positive for both IL-6 and TNF-αwas significantly increased in peripheral blood of LCS-PTB and N-PTB patients as compared to that of HV volunteers, respectively (Fig. 2a, b). Interestingly, in comparison to N-PTB patients, LCS-PTB patients with an elevated number of monocytes in peripheral blood had a relatively lower percentage of IL-6- or TNF-α-positive CD14+ monocytes (Figs. 1a, b, 2a, b). However, no obvious differences in the percentage of IL-10-positiveCD14+ monocytes in the peripheral blood were noticed among LCS-PTB patients, N-PTB patients, and HV volunteers (Fig. 2c). In parallel, we examined the serum levels of IL-6, TNF-α, and IL-10 by ELISA. Our results showed that the serum levels of IL-6, TNF-α, and IL-10 were significantly increased in both LCS-PTB and N-PTB patients in comparison to those of HV volunteers (Fig. 2d–f), which was in consistent with previous findings by us and others [25, 31, 32]. Similarly, we found that the serum levels of IL-6 and TNF-α of LCS-PTB patients were significantly lower than those of N-PTB patients (Fig. 2d, e). These results suggest that LCS-PTB patients exhibited suppressive systemic immune responses mediated by peripheral monocytes as compared with N-PTB patients.
IL-6 and TNF-α were suppressed on monocytes from LCS-PTB patients. The production of IL-6 (a), TNF-α (b) and IL-10 (c) of monocytes among LCS-PTB patients, N-PTB patients, and HV groups were detected by FCM analysis. And the serum IL-6 (d), TNF-α (e) and IL-10 (f) levels among LCS-PTB patients, N-PTB patients, and HV groups were detected by ELISA. Data were presented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001
miR-196b-5p was upregulated in peripheral monocyte from LCS-PTB patients
Our previous results demonstrated that miR-196b-5p could activate STAT3 signaling pathway via targeting negative regulators SOCS1 and SOCS3 [22], which plays a key role in the differentiation of monocytes into macrophages [16, 23]. We then determined whether there are any changes in the expression of miR-196b-5p in primary peripheral monocytes and MDMs. Our results showed that the relative expression of miR-196b-5p was significantly upregulated in monocytes from LCS-PTB patients as compared to that of N-PTB patients or HV volunteers (Fig. 3a). However, no significant differences in miR-196b-5p expression were noticed in MDMs among LCS-PTB patients, N-PTB patients or HV groups (Fig. 3b). However, the expression of miR-196b-5p was upregulated in MDMs or differentiated U937 cells in response to CSE treatment (Fig. 3c).
miR-196b-5p was upregulated on monocyte from LSC-PTB patients. The relative expression of miR-196b-5p on sorted CD14+ monocyte (a) and MDM (b) among LCS-PTB patients, N-PTB patients and HV groups were detected by qRT-PCR. Furthermore, miR-196b-5p expression on MDM or differentiated U937 cells were detected by qRT-PCR after cigarette smoke extract (CSE) treatment (c). Data were presented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001
STAT3 mRNA expression was upregulated while SOCS3 mRNA expression was downregulated in peripheral monocytes from LCS-PTB patients
STAT pathways were among the most important signaling pathways in regulating the function of monocytes or macrophages. To dissect immune responses mediated by STAT signaling pathways in peripheral monocytes from LCS-PTB patients, N-PTB patients, and HV volunteers, the mRNA expression of STAT1, STAT3, STAT4, STAT5A, STAT5B and STAT6 was detected by qRT-PCR (Fig. 4a–f). Our results indicated that STAT3, STAT5A, STAT5B mRNAs were upregulated in monocytes from both LCS-PTB and N-PTB patients as compared to those of HV volunteers (Fig. 4b, d, e). Interestingly, only STAT3 mRNA expression was more pronounced in monocyted from LCS-PTB patients than that of N-PTB patients (Fig. 4b), whereas no significant differences in the mRNA expression of STAT1, STAT4, STAT5A, STAT5B and STAT6 were noticed in monocytes from LCS-PTB and N-PTB patients (Fig. 4a–f).
STAT3 mRNA expression was upregulated, while SOCS3 mRNA expression was downregulated on monocytes from LSC-PTB patients. The relative mRNA expression of STAT1 (a), STAT3 (b), STAT4 (c), STAT5A (d), STAT5B (e), STAT6 (f), SOCS1 (g), SOCS2 (h) and SOCS3 (i) on sorted CD14+ monocyte among LCS-PTB patients, N-PTB patients and HV groups were detected by qRT-PCR. Data were presented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001
SOCSs play a key role in the regulation of STAT3 signaling pathways. Previous studies showed that SOCS1, SOCS2, and SOCS3 were upregulated in patients with active PTB [33,34,35] and SOCS3 attenuated anti-inflammatory effects of IL-6 in macrophages [36]. We then detected SOCS1, SOCS2 and SOCS3 mRNA expressions in peripheral monocytes from LCS-PTB and N-PTB patients. Our results indicated that SOCS1 and SOCS3 mRNA expression were downregulated in monocytes from both LCS-PTB and N-PTB patients as compared to that of HV volunteers (Fig. 4g, i). However, an even lower expression level of SOCS3 mRNA was observed in monocytes from LCS-PTB patients than that in those of N-PTB patients (Fig. 4i). These data suggest that downregulation of SOCS3 and activation STAT3 may play a potential role in suppressing the pro-inflammatory responses in peripheral monocytes of LCS-PTB patients.
miR-196b-5p inhibited BCG uptake by macrophages
In order to further explore the functional role of miR-196-5b in monocytes of LCS-PTB patients, MDMs from 6 cases of LCS-PTB patients, N-PTB patients, and HV control volunteers, respectively were obtained. First, we compared mycobacterial uptake by MDMs from different groups of patients after BCG infection using flow cytometry analysis. After 5 days of BCG infection, the BCG uptake ability by MDMs had no significant differences among LCS-PTB patients, N-PTB patients, and HV volunteers (Fig. 5a). However, upregulation of miR-196b-5p with agomir-196b-5p inhibited BCG uptake, while downregulation of miR-196b-5p with antagomir-196b-5p (anti-miR-196b-5p) promoted BCG uptake in MDMs or differentiated U937 cells (Fig. 5b). These results suggested that overexpression of miR-196b-5p can attenuate the capability of macrophages to phygocytose BCG.
miR-196b-5p inhibited BCG uptake by macrophages. GFP tagged BCG was used to detect mycobacterial uptake by macrophages. The percentage and median fluorescence intensity (MFI) of GFP+ MDM (BCG uptake macrophages) among LCS-PTB patients, N-PTB patients and HV groups were detected by FCM analysis (a). And the BCG uptake by MDMs or differentiated U937 cells was detected after exogenously miR-196b-5p and anti-miR-196b-5p transfected (b). Data were presented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001
miR-196b-5p inhibited IL-6, TNF-α production in macrophages via activating STAT3 signaling pathway
Beside impairment of macrophage phagocytosis of BCG, we also found that upregulation of miR-196b-5p decreased while downregulation of miR-196b-5p increased the production of pro-inflammatory cytokines IL-6 and TNF-α, but not the anti-inflammatory cytokine IL-10, in differentiated U937 cells after BCG infection (Fig. 6a–c). In consistent with above findings, pSTAT3 was upregulated while SOCS3 was downregulated following upregulation of miR-196b-5p in macrophages (Fig. 6d). Those results suggest that upregulation of miR-196b-5p led to reduced secretion of pro-inflammatory cytokines in macrophages possibly by downregulating SOCS3 and activating STAT3 signaling pathway.
miR-196b-5p inhibited IL-6, TNF-α production on macrophages via activating STAT3 signaling pathway. The supernatant IL-6 (a), TNF-α (b) and IL-10 (c) levels on differentiated U937 cells after exogenously miR-196b-5p, anti-miR-196b-5p transfected were detected by ELISA. And SOCS1, SOCS3, STAT3 and pSTAT3 expression on differentiated U937 cells after exogenously miR-196b-5p, anti-miR-196b-5p transfected were detected by western blotting analysis (d). Data were presented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001
miR-196b-5p promoted STAT3 signaling pathway via targeting SOCS3
Our previous studies showed that miR-196b-5p targets 3′-UTRs of SOCS1 and SOCS3 mRNA to promote STAT3 signaling pathway in colorectal cancer cells [22]. We then determined whether miR-196b-5p could promote STAT3 signaling pathway in macrophages through SOCS1 or SOCS3. SOCS1 and SOCS3 mRNA expressions were detected by qRT-PCR following upregulation or downregulation of miR-196b-5p in differentiated U937 cells. Our data showed that upregulation of miR-196b-5p inhibited while downregulation of miR-196b-5p increased SOCS3 but not SOCS1 mRNA expression in macrophages, suggesting that SOCS3 may also be a target of miR-196b-5p in macrophages (Fig. 7a, b). In addition, luciferase assay also showed that upregulation of miR-196b-5p attenuated the reporter activity driven by the wildtype 3′-UTRs of SOCS3 transcripts, but not by mutant 3′-UTRs of SOCS3 transcripts, within miR-196b-5p-binding seed regions in U937 cells (Fig. 7c). Notably, we also found that silencing of SOCS3 with SOCS3-RNAi rescued the STAT3 activity repression in miR-196b-5p-silencing U937 cells (Fig. 7d). Additionally, total STAT3, especially phosphorylated STAT3 were downregulated after overexpressing of SOCS3 with SOCS3-LAC in miR-196b-5p-overexpressing U937 cells (Fig. 7e). Collectively, these results suggest that miR-196b-5p activates STAT3 signaling pathway possibly via targeting SOCS3.
miR-196b-5p promoted STAT3 signaling pathway via targeting SOCS3. The relative mRNA expression of SOCS1 (a) and SOCS3 (b) on differentiated U937 cells after exogenously miR-196b-5p, anti-miR-196b-5p transfected were detected by qRT-PCR. Luciferase assay also showed that upregulation of miR-196b-5p attenuated the reporter activity driven by the wildtype 3′-UTRs, but not by the mutant 3′-UTRs of SOCS3 transcripts within miR-196b-5p-binding seed regions in U937 cells (c). Silencing of SOCS3 with SOCS3-RNAi rescued the STAT3 activity repression in miR-196b-5p-silencing U937 cells (d). pSTAT3 were downregulated after overexpressing of SOCS3 in miR-196b-5p-overexpressing U937 cells with SOCS3-LAC (e). Data were presented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001
Discussion
Inflammatory responses are an essential part of innate immune responses to pathogens. Monocytes or macrophages were important effectors and regulators in innate immunity. Numerous studies, including our previous reports, showed an increased number of circulating blood monocytes in TB patients [25, 30, 37, 38]. However, blood monocyte-derived macrophages in PTB patients exhibit impaired phagocytic capacity [39]. MTB infection recruited the inflammatory monocytes or macrophages into lungs, which contribute to granuloma formation [40, 41] whereby IL-6, and TNF-α play an important role [25, 42,43,44]. Herein, we also found that IL-6- and TNF-α-positive CD14+ monocytes and their serum levels were significantly increased in peripheral blood of both LCS-PTB and N-PTB patients as compared with those in HV volunteers, which is in consistent with the previous results found in adult but not childhood TB patients [25, 43, 45,46,47,48]. However, the percentage of IL-6- and TNF-α-positive CD14+ monocytes were relatively lower in peripheral blood of LCS-PTB patients (cigarette smoking > 7 years) than that of N-PTB patients, although the absolute count and percentage of monocytes increased in both LCS-PTB and N-PTB patients. In parallel, we found an elevated level of IL-6 and TNF-α in serum of both LCS-PTB and N-PTB patients as compared to that of HV volunteers, while the elevation of IL-6 and TNF-α in serum of LCS-PTB was less pronounced than that of N-PTB patients. Functionally, we showed that MDMs of LCS-PTB patients exhibited a lower uptake of BCG or impaired phagocytosis of BCG. These results support the notion that long-term cigarette smoking may lead to impaired phagocytosis of BCG by macrophages due to attenuated pro-inflammatory responses.
Aberrant activation of STAT3 signaling pathway was linked to immune disorders and inflammatory response of monocytes or macrophages. Human STAT family currently consisted of seven members, STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, STAT6. In this study, we showed that STAT3, STAT5A, STAT5B mRNAs were upregulated in peripheral CD14+ monocytes from LCS- and N-PTB patients as compared with those of HV controls. Moreover, we have demonstrated, for the first time to our knowledge, that the increase in STAT3 mRNA expression and pSTAT3 expression were less pronounced in CD14+ monocytes and MDM from LCS-PTB patients than in those from N-PTB patients, which were inversely correlated with the expression levels of SOCS3 mRNA and protein. Emerging evidence indicates that deregulation of negative regulators of STAT3 signaling pathway including tyrosine phosphatase and SOCS families plays a crucial role in the activation of STAT3 signaling [49,50,51]. Therefore, our results suggest that SOCS3 is involved in the regulation of STAT3 activation in peripheral CD14+ monocytes or MDMs from LCS-PTB patients.
Most recently, the biological role and clinical significance of miR-196b-5p have been extensively studied. Overexpression of miR-196-5p has been reported in the hematologic malignancies, solid tumors, coronary artery disease and Ebola virus infection [22, 52,53,54,55]. Zhang et al. has reported that the serum level of miR-196b in active TB patients was higher than that in latent TB infection (LTBI), BCG-inoculated and un-inoculated individuals [21]. In the present study, we demonstrated that miR-196b-5p was upregulated in peripheral monocytes from LCS-PTB patients as compared with that in those from N-PTB patients. Additionally, we showed CSE and PM2.5 (unpublished data) increased miR-196b-5p expression in MDMs and differentiated U937 cells. Overexpression of miR-196b-5p downregulated the expression of SOCS3 mRNA while simultaneously activated STAT3 signaling pathway in macrophages.
Conclusions
In summary, our findings support the notion that long-term cigarette smoking may lead to upregulated expression of miR-196b-5p, which then contributes to targeted inhibition of SOCS3 and activation of STAT3 signaling pathway, and subsequently, the impaired phagocytosis or elimination of BCG by macrophages due to their attenuated pro-inflammatory responses. Therefore, miR-196b-5p may represent a novel biomarker and therapeutic target for the treatment of LCS-PTB patients.
Abbreviations
- CS:
-
cigarette smoking
- TB:
-
tuberculosis
- LCS-PTB:
-
long-term cigarette smoking-related pulmonary tuberculosis
- N-PTB:
-
nonsmoking pulmonary tuberculosis
- HV:
-
healthy volunteers
- PBMC:
-
peripheral blood mononuclear cells
- BCG:
-
Bacillus Calmette–Guérin
- MTB:
-
mycobacterium tuberculosis
- MDM:
-
monocyte-derived macrophages
- CSE:
-
cigarette smoke extract
- ELISA:
-
enzyme-linked immunosorbent assay
References
Wang T, Xue F, Chen Y, Ma Y, Liu Y. The spatial epidemiology of tuberculosis in Linyi City, China, 2005–2010. BMC Public Health. 2012;12:885.
Du PK, Mandalakas AM, Kirchner HL, Grewal HM, Schaaf HS, van Wyk SS, et al. Environmental tobacco smoke exposure increases Mycobacterium tuberculosis infection risk in children. Int J Tuberc Lung Dis. 2011;15(11):1490–6.
Bates MN, Khalakdina A, Pai M, Chang L, Lessa F, Smith KR. Risk of tuberculosis from exposure to tobacco smoke: a systematic review and meta-analysis. Arch Intern Med. 2007;167(4):335–42.
Gajalakshmi V, Peto R, Kanaka TS, Jha P. Smoking and mortality from tuberculosis and other diseases in India: retrospective study of 43000 adult male deaths and 35,000 controls. Lancet. 2003;362(9383):507–15.
Chuang HC, Su CL, Liu HC, Feng PH, Lee KY, Chuang KJ, et al. Cigarette smoke is a risk factor for severity and treatment outcome in patients with culture-positive tuberculosis. Ther Clin Risk Manag. 2015;11:1539–44.
D’hulst AI, Vermaelen KY, Brusselle GG, Joos GF, Pauwels RA. Time course of cigarette smoke-induced pulmonary inflammation in mice. Eur Respir J. 2005;26(2):204–13.
Le QC, Guénon I, Gillon JY, Valença S, Cayron-Elizondo V, Lagente V, et al. The selective MMP-12 inhibitor, AS111793 reduces airway inflammation in mice exposed to cigarette smoke. Br J Pharmacol. 2008;154(6):1206–15.
Oliveira DSC, Monte-Alto-Costa A, Renovato-Martins M, Viana NFJ, Dos SVS, Lagente V, et al. Time course of the phenotype of blood and bone marrow monocytes and macrophages in the lung after cigarette smoke exposure in vivo. Int J Mol Sci. 2017;18:9.
Liu CH, Liu H, Ge B. Innate immunity in tuberculosis: host defense vs pathogen evasion. Cell Mol Immunol. 2017;14:963.
Queval CJ, Brosch R, Simeone R. The macrophage: a disputed fortress in the battle against Mycobacterium tuberculosis. Front Microbiol. 2017;8:2284.
Ahluwalia PK, Pandey RK, Sehajpal PK, Prajapati VK. Perturbed microRNA expression by Mycobacterium tuberculosis promotes macrophage polarization leading to pro-survival foam cell. Front Immunol. 2017;8:107.
Li L, Wang Y, Gao W, Yuan C, Zhang S, Zhou H, et al. Klotho reduction in alveolar macrophages contributes to cigarette smoke extract-induced inflammation in chronic obstructive pulmonary disease. J Biol Chem. 2015;290(46):27890–900.
Fu X, Shi H, Qi Y, Zhang W, Dong P. M2 polarized macrophages induced by CSE promote proliferation, migration, and invasion of alveolar basal epithelial cells. Int Immuno Pharmacol. 2015;28(1):666–74.
Yuan F, Fu X, Shi H, Chen G, Dong P, Zhang W. Induction of murine macrophage M2 polarization by cigarette smoke extract via the JAK2/STAT3 pathway. PLoS ONE. 2014;9(9):e107063.
Kunz LI, Lapperre TS, Snoeck-Stroband JB, Budulac SE, Timens W, van Wijngaarden S, et al. Smoking status and anti-inflammatory macrophages in bronchoalveolar lavage and induced sputum in COPD. Respir Res. 2011;12:34.
Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C, et al. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014;26(2):192–7.
Roy S. miRNA in macrophage development and function. Antioxid Redox Signal. 2016;25(15):795–804.
Saleiro D, Platanias LC. Intersection of mTOR and STAT signaling in immunity. Trends Immunol. 2015;36(1):21–9.
Vrijens K, Bollati V, Nawrot TS. MicroRNAs as potential signatures of environmental exposure or effect: a systematic review. Environ Health Perspect. 2015;123(5):399–411.
Gentner B, Visigalli I, Hiramatsu H, Lechman E, Ungari S, Giustacchini A, et al. Identification of hematopoietic stem cell-specific miRNAs enables gene therapy of globoid cell leukodystrophy. Sci Transl Med. 2010;2(58):58rs84.
Zhang H, Sun Z, Wei W, Liu Z, Fleming J, Zhang S, et al. Identification of serum microRNA biomarkers for tuberculosis using RNA-seq. PLoS ONE. 2014;9(2):e88909.
Ren D, Lin B, Zhang X, Peng Y, Ye Z, Ma Y, et al. Maintenance of cancer stemness by miR-196b-5p contributes to chemoresistance of colorectal cancer cells via activating STAT3 signaling pathway. Oncotarget. 2017;8(30):49807–23.
Rottenberg ME, Carow B. SOCS3 and STAT3, major controllers of the outcome of infection with Mycobacterium tuberculosis. Semin Immunol. 2014;26(6):518–32.
Zeng JC, Lin DZ, Yi LL, Liu GB, Zhang H, Wang WD, et al. BTLA exhibits immune memory for αβ T cells in patients with active pulmonary tuberculosis. Am J Transl Res. 2014;6(5):494–506.
Zeng JC, Xiang WY, Lin DZ, Zhang JA, Liu GB, Kong B, et al. Elevated HMGB1-related interleukin-6 is associated with dynamic responses of monocytes in patients with active pulmonary tuberculosis. Int J Clin Exp Pathol. 2015;8(2):1341–53.
Zeng J, Song Z, Cai X, Huang S, Wang W, Zhu Y, et al. Tuberculous pleurisy drives marked effector responses of γδ, CD4+, and CD8+ T cell subpopulations in humans. J Leukoc Biol. 2015;98(5):851–7.
Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14(6):392–404.
Salgame P. Host innate and Th1 responses and the bacterial factors that control Mycobacterium tuberculosis infection. Curr Opin Immunol. 2005;17(4):374–80.
Mahan CS, Zalwango S, Thiel BA, Malone LL, Chervenak KA, Baseke J, et al. Innate and adaptive immune responses during acute M. tuberculosis infection in adult household contacts in Kampala, Uganda. Am J Trop Med Hyg. 2012;86(4):690–7.
Veenstra H, Baumann R, Carroll NM, Lukey PT, Kidd M, Beyers N, et al. Changes in leucocyte and lymphocyte subsets during tuberculosis treatment; prominence of CD3dimCD56+ natural killer T cells in fast treatment responders. Clin Exp Immunol. 2006;145(2):252–60.
Verbon A, Juffermans N, Van Deventer SJ, Speelman P, Van Deutekom H, Van Der Poll T. Serum concentrations of cytokines in patients with active tuberculosis (TB) and after treatment. Clin Exp Immunol. 1999;115(1):110–3.
Fiorenza G, Rateni L, Farroni MA, Bogué C, Dlugovitzky DG. TNF-alpha, TGF-beta and NO relationship in sera from tuberculosis (TB) patients of different severity. Immunol Lett. 2005;98(1):45–8.
Almeida AS, Lago PM, Boechat N, Huard RC, Lazzarini LC, Santos AR, et al. Tuberculosis is associated with a down-modulatory lung immune response that impairs Th1-type immunity. J Immunol. 2009;183(1):718–31.
Masood KI, Rottenberg ME, Salahuddin N, Irfan M, Rao N, Carow B, et al. Expression of M. tuberculosis-induced suppressor of cytokine signaling (SOCS) 1, SOCS3, FoxP3 and secretion of IL-6 associates with differing clinical severity of tuberculosis. BMC Infect Dis. 2013;13:13.
Masood KI, Rottenberg ME, Carow B, Rao N, Ashraf M, Hussain R, et al. SOCS1 gene expression is increased in severe pulmonary tuberculosis. Scand J Immunol. 2012;76(4):398–404.
Niemand C, Nimmesgern A, Haan S, Fischer P, Schaper F, Rossaint R, et al. Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J Immunol. 2003;170(6):3263–72.
La Manna MP, Orlando V, Dieli F, Di CP, Cascio A, Cuzzi G, et al. Quantitative and qualitative profiles of circulating monocytes may help identifying tuberculosis infection and disease stages. PLoS ONE. 2017;12(2):e0171358.
Zeng J, Kong B, Xiang W, Gao Y, Lu Y, Wang W, et al. The early warning and prognostic value of serum soluble TREM-1 for active pulmonary tuberculosis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2015;31(2):235–8.
Aryanpur M, Mortaz E, Masjedi MR, Tabarsi P, Garssen J, Adcock IM, et al. Reduced phagocytic capacity of blood monocyte/macrophages in tuberculosis patients is further reduced by smoking. Iran J Allergy Asthma Immunol. 2016;15(3):174–82.
Algood HM, Lin PL, Flynn JL. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin Infect Dis. 2005;41(Suppl 3):S189–93.
Ndlovu H, Marakalala MJ. Granulomas and inflammation: host-directed therapies for tuberculosis. Front Immunol. 2016;7:434.
Dorhoi A, Kaufmann SH. Tumor necrosis factor alpha in mycobacterial infection. Semin Immunol. 2014;26(3):203–9.
Law K, Weiden M, Harkin T, Tchou-Wong K, Chi C, Rom WN. Increased release of interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha by bronchoalveolar cells lavaged from involved sites in pulmonary tuberculosis. Am J Respir Crit Care Med. 1996;153(2):799–804.
Chandrashekara S, Anupama KR, Sambarey A, Chandra N. High IL-6 and low IL-15 levels mark the presence of TB infection: a preliminary study. Cytokine. 2016;81:57–62.
Torun E, Cakir E, Aktas EC, Gedik AH, Deniz G. Intracellular cytokine and cathelicidin secretion from monocytes and neutrophils in childhood tuberculosis. Pediatr Infect Dis J. 2014;33(2):224–6.
Chakraborty U, Goswami A, Saha S, Mukherjee T, Dey SK, Majumdar S, et al. Tumour necrosis factor-alpha and nitric oxide response in different categories of tuberculosis patients. Int J Tuberc Lung Dis. 2013;17(4):505–10.
Waitt CJ, Banda P, Glennie S, Kampmann B, Squire SB, Pirmohamed M, et al. Monocyte unresponsiveness and impaired IL1β, TNFα and IL7 production are associated with a poor outcome in Malawian adults with pulmonary tuberculosis. BMC Infect Dis. 2015;15:513.
Jung SB, Yang CS, Lee JS, Shin AR, Jung SS, Son JW, et al. The mycobacterial 38-kilodalton glycolipoprotein antigen activates the mitogen-activated protein kinase pathway and release of proinflammatory cytokines through Toll-like receptors 2 and 4 in human monocytes. Infect Immun. 2006;74(5):2686–96.
Lee JH, Kim C, Sethi G, Ahn KS. Brassinin inhibits STAT3 signaling pathway through modulation of PIAS-3 and SOCS-3 expression and sensitizes human lung cancer xenograft in nude mice to paclitaxel. Oncotarget. 2015;6(8):6386–405.
Robinson GW, Pacher-Zavisin M, Zhu BM, Yoshimura A, Hennighausen L. Socs 3 modulates the activity of the transcription factor Stat3 in mammary tissue and controls alveolar homeostasis. Dev Dyn. 2007;236(3):654–61.
Hong F, Jaruga B, Kim WH, Radaeva S, El-Assal ON, Tian Z, et al. Opposing roles of STAT1 and STAT3 in T cell-mediated hepatitis: regulation by SOCS. J Clin Invest. 2002;110(10):1503–13.
Díaz-Beyá M, Brunet S, Nomdedéu J, Tejero R, Díaz T, Pratcorona M, et al. MicroRNA expression at diagnosis adds relevant prognostic information to molecular categorization in patients with intermediate-risk cytogenetic acute myeloid leukemia. Leukemia. 2014;28(4):804–12.
Du Y, Yang SH, Li S, Cui CJ, Zhang Y, Zhu CG, et al. Circulating MicroRNAs as novel diagnostic biomarkers for very early-onset (≤ 40 years) coronary artery disease. Biomed Environ Sci. 2016;29(8):545–54.
Wen J, Huang Y, Li H, Zhang X, Cheng P, Deng D, et al. Over-expression of miR-196b-5p is significantly associated with the progression of myelodysplastic syndrome. Int J Hematol. 2017;105(6):777–83.
Sheng M, Zhong Y, Chen Y, Du J, Ju X, Zhao C, et al. Hsa-miR-1246, hsa-miR-320a and hsa-miR-196b-5p inhibitors can reduce the cytotoxicity of Ebola virus glycoprotein in vitro. Sci China Life Sci. 2014;57(10):959–72.
Authors’ contributions
JCZ, TC, and YQY conceived and designed the experiments; DZL, LF, YM, ZYY and HMY performed the experiments; MYH and BHL performed data analysis; YML, XLY, and YZM contributed to sample collection; JCZ wrote the paper; KYZ and QZZ assisted with writing and proofreading. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was approved by the Internal Review and the Ethics Boards of Guangdong Medical University and Dongguan sixth People’s Hospital. Informed written consent was obtained from all study subjects.
Funding
This study was supported by Grants from the National Natural Science Foundation of China (81500007); the Science and Technology Project of Guangdong Province (2016A020215003), the Medical Science Foundation of Guangdong Province (A2018123, A2015206, A2016278 and A2016395), the Science and Technology Project of Dongguan (2016105101292, 2016108101030 and 201750715005451), the National and Guangdong Provincial College Students’ innovative Entrepreneurial Training Program (201710571009, 201710571033), and the College Students’ innovative experiment projects of Guangdong Medical University (Z2016005, GDMU2016009 and GDMU2016033).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Additional file
Additional file 1: Table S1.
A list of primers used in the investigated genes.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Yuan, Y., Lin, D., Feng, L. et al. Upregulation of miR-196b-5p attenuates BCG uptake via targeting SOCS3 and activating STAT3 in macrophages from patients with long-term cigarette smoking-related active pulmonary tuberculosis. J Transl Med 16, 284 (2018). https://doi.org/10.1186/s12967-018-1654-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-018-1654-9