Abstract
Background
Certolizumab pegol, a PEGylated tumour necrosis factor (TNF)-inhibitor, improves the clinical signs and symptoms of rheumatoid arthritis (RA) when used in combination with methotrexate or as monotherapy. This study evaluatedthe cost-utility of certolizumab pegol versusTNF-inhibitors plus methotrexate in the treatment of moderate-to-severe RA in Spain.
Methods
A Markov cohort health state transition model was developed to evaluate the cost-utility (costs and quality-adjusted life years [QALYs]) of certolizumab pegol versus other TNF-inhibitors licensed in Spain in 2009. Efficacy was measured using the American College of Rheumatology (ACR) responses at 6 months, based on adjusted indirect comparisons from published clinical trials. Utilities were derived from EQ-5D data from certolizumab pegol RA clinical trials. Clinical history and resource use data came from published literature. Unit costs were taken from Spanish databases or published data (cost year 2009). Base case analyses were conducted from the payer perspective, with a lifetime horizon, 3.5 % annual discounting rates for costs and outcomes, and 3 % inflation rate for 2009 onwards. One-way sensitivity analyses were conducted.
Results
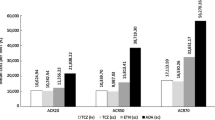
The average lifetime costs for certolizumab pegol, etanercept, adalimumab (every 2 weeks and weekly) and infliximab (3 mg/kg and 5 mg/kg) in combination with methotrexate were €140,971, €141,197, €139,148, €164,741, €136,961 and €152,561, respectively. The QALYs gained were 6.578, 6.462, 6.430 (for both adalimumab doses), 6.430, and 6.318 (for both infliximab doses), respectively. At a €30,000/QALY willingness-to-pay threshold, certolizumab pegol plus methotrexate dominated adalimumab weekly, etanercept, and infliximab 5 mg/kg, and was cost-effective versus adalimumab every 2 weeks and infliximab 3 mg/kg (all with methotrexate), with estimated ICERs of €12,346/QALY and €15,414/QALY, respectively. Certolizumab pegol monotherapy was more cost-effective versus adalimumab, and less expensive with similar health gains versus etanercept (6.416 QALYs vs 6.492). Univariate analysis showed ICERs to be sensitive to changes in time horizon, ACR response time point, baseline Heath Assessment Questionnaire (HAQ) score, and rate of HAQ-disability index deterioration after discontinuing treatment.
Conclusions
This analysis shows that certolizumab pegol is cost-effective compared with other TNF-inhibitors recommended in Spain for the treatment of RA.
Similar content being viewed by others
Background
Rheumatoid arthritis (RA) is a chronic inflammatory disease causing progressive joint destruction, deformity and disability. Although its exact aetiology is unknown, RA is believed to be an autoimmune disease stimulated by environmental factors in genetically susceptible individuals [1]. The prevalence in Spain is 0.5 % according to the EPISER study [2], with an incidence estimated at 8.3 cases per 100,000 by the Spanish Society of Rheumatology. The annual incidence of RA in adults in Spain is in the lower range for European countries, and comparable with those in other Mediterranean countries [3].
The aim of treatment is disease remission or the lowest disease activity possible. Standard treatment for RA patients in Spain with persistent disease in spite of aggressive management currently consists of disease-modifying antirheumatic drugs (DMARDs). In line with national guidelines, methotrexate, a small-molecule DMARD, is the first treatment choice in Spain for more than 80 % of patients with RA [4]. Biological DMARDs include tumour necrosis factor (TNF) inhibitors, e.g. certolizumab pegol, adalimumab, golimumab, infliximab and etanercept, which target TNFα, a proinflammatory cytokine believed to play a major role in the pathogenesis of RA [5]. TNF inhibitors or tocilizumab, an antibody directed against the interleukin-6 receptor, administered alone or in combination with methotrexate, are the first treatment option after small-molecule DMARDs [6, 7]. Other biological agents used in Spain are anakinra, abatacept and rituximab, which are used in patients with RA who do not respond to methotrexate and in patients with active RA despite treatment with TNF inhibitors [6, 7]. However, a significant proportion of patients has an unsatisfactory response to these treatments and continues to experience episodes of disease activity while receiving therapy [8–11].
Certolizumab pegol (Cimzia®, CZP) is a PEGylated Fc-free anti-TNF approved for adults with moderate to severe RA [12, 13]. It is administered by subcutaneous injection and has a relatively long elimination half-life, allowing administration once every 2 or 4 weeks. Certolizumab pegol demonstrated rapid and sustained improvements in physical function and signs and symptoms of RA, and relief in pain and fatigue and significant improvements in productivity at work and home and participation in social activities [14–17]. CZP is approved in Spain, either as monotherapy or in combination with methotrexate, for the treatment of moderate-to-severe, active RA in adult patients when the response to DMARDs, including methotrexate, has been inadequate [13].
Whilst TNF inhibitors have generally been shown to be cost-effective in the treatment of RA [18–26], data regarding the relative cost-effectiveness of the various TNF inhibitors are limited and there are few published economic evaluations for certolizumab pegol.
The aim of this study was to evaluate the cost utility of certolizumab pegol compared with other standard first-line TNF-inhibitor therapies licensed and marketed in Spain in 2009 (etanercept, adalimumab, infliximab), administered with or without methotrexate for the treatment of patients with moderate-to-severe RA who have had an inadequate response to methotrexate alone.
Methods
Cost-utility model
The economic evaluation was carried out using a theoretical cost-utility analysis framework, using a Markov model structure (cohort health state transition model) [27]. Patients entered the model at commencement of therapy with certolizumab pegol or a comparator. Two certolizumab pegol regimes were analysed: certolizumab pegol (400 mg administered on weeks 0, 2 and 4, then 200 mg every 2 weeks) in combination with methotrexate or as monotherapy. Comparators considered in the analysis were TNFα inhibitors licensed and recommended in Spain in 2009. These included etanercept (25 mg twice weekly), adalimumab (40 mg every 2 weeks or 40 mg weekly), infliximab (3 or 5 mg/kg at week 0, 2, 6 and every 8 weeks thereafter), and etanercept or adalimumab monotherapies.
The population entering the model consisted of patients that had active RA (defined as a disease activity score [DAS28] >5.1, confirmed on at least two occasions a month apart) and had failed to respond adequately to methotrexate. Baseline characteristics were reflective of those patients in clinical practice who are eligible for treatment with certolizumab pegol.
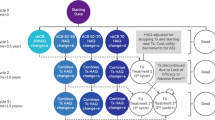
The model was developed with a 6-months or a 3-months cycle, depending on when the clinical response is assessed (Fig. 1). At the end of the first cycle, patients were assigned to 1 of 4 response groups, defined according to American College of Rheumatology (ACR) criteria: no response, ACR20, ACR50 or ACR70 response. In patients with an inadequate response (no ACR20 response), treatment was discontinued; only patients who obtained an adequate response in the first time step continued on to the modelled initial therapy. Mortality rates are also assumed during the first cycle of the model [28]. At the end of the next and following cycles, patients may have remained in the same Markov treatment health state; discontinued treatment due to lack of efficacy or due to an adverse event; or died. Patients who discontinued treatment were assumed to have moved on to alternative therapies. On discontinuation of certolizumab pegol or the comparator treatment (adalimumab, etanercept or infliximab), patients may have received the following sequence of conventional DMARD as follow-up therapy: sulfasalazine, leflunomide, gold sodium thiomalate, hydroxychloroquine, azathioprine, cyclosporine, and penicillamine. Upon discontinuation of the last treatment in the sequence, patients received palliative therapy.
Structure of the Markov model. a Follow-up treatment states: duplicated for each follow-up treatment. Patients not responding in the first 6 months of follow-up treatment move to the next treatment in the sequence. b Reason for discontinuation (lack of efficacy or adverse events) determined by the probabilities after leaving treatment health state
After the first 12 months, cycle duration was six months to reflect monitoring frequency recommendations by the Spanish Rheumatology Society [7], the National Institute for Health and Clinical Excellence (NICE) and the British Society of Rheumatology (BSR) [29–31].
Incremental cost-effectiveness ratios (ICERs) are presented, representing the incremental costs necessary to achieve an additional QALY with certolizumab pegol versus the comparator selected.
Clinical effectiveness estimates and model assumptions
The natural history of the disease and the data on use of resources were derived from various sources, including previous economic evaluation [32]. Treatment duration was obtained from a study with over 2300 patients treated with a TNFα inhibitor over 9 years, which showed that the median treatment duration for TNFα inhibitors was 37 months (3.08 years) [33]. The duration of treatment with small-molecule DMARDs was taken from Chen et al. [32], and all-cause mortality rates for the general population were obtained from published age- and gender-specific mortality rates in Spain [34], adjusted according to Health Assessment Questionnaire-Disability Index (HAQ-DI). The starting mortality rate in cycle 1 is adjusted to the age and gender distribution of the model population and adjustment is made in each model cycle to represent the increased risk of death as patients become older. The base case estimate of relative risk of death of 1.330 per HAQ unit (95 % CI 1.099 to 1.610) is taken from a 35 year cohort study of 3501 RA patients in Canada [35].
Since there were no head-to-head studies directly comparing certolizumab pegol efficacy to that of the other anti-TNF agents, the relative efficacy of the comparators was estimated via an indirect analysis of data from certolizumab pegol studies (RAPID 1 [16, 17], RAPID 2 [36] and FAST4WARD [14]) and from published literature identified through a systematic review [32] included studies of all biological DMARDs published up to April 2009. Medline, Embase and the Cochrane Library (NHSEED) were searched to identify studies of certolizumab pegol, adalimumab, etanercept and infliximab in patients with rheumatoid arthritis. Other forms of arthritis were excluded as were non-English language studies. Studies of the following design were included: economic evaluations piggy-backed on to a clinical trial; cost-consequence, cost-benefit, cost-effectiveness, cost-utility and cost-minimisation analyses; studies in which the comparator was a biological DMARD or a conventional DMARD; and studies that reported quality of life and cost estimates, cost estimates or cost-effectiveness.
Abstracts were first screened by two independent reviewers and any disagreements resolved by a third reviewer. Duplicate citations and those which did not match the eligibility criteria were excluded. Full-text copies of all included and ambiguous studies were obtained. Data from all included studies were extracted independently by two reviewers, and any disagreements when extracted data were compared resolved by a third reviewer. Most data came from trials of biological DMARDs versus methotrexate or placebo. Two direct trials comparing etanercept with infliximab as combination therapies with methotrexate were excluded from the indirect analysis due to their small patient numbers.
RAPID 1 (52-weeks) and RAPID 2 (24-weeks) were both phase III, multicentre, double-blind, randomised placebo-controlled trials evaluating certolizumab pegol 400 mg at weeks 0, 2 and 4 followed by 200 mg or 400 mg plus methotrexate every 2 weeks, or placebo plus methotrexate, in patients with active RA (n = 982 and 619, respectively) [16, 17, 36]. Similarly, FAST4WARD was a 24-week, multicentre, double-blind, placebo-controlled phase III study, evaluating certolizumab pegol 400 mg monotherapy every 4 weeks or placebo (n = 220) [14]. ACR20 response rate at week 24 was the primary endpoint in all three studies (and co-primary in RAPID 1 study).
In addition to the RAPID 1 and RAPID 2 trials, data for the analysis of combination therapies, with respect to ACR responses at 3 months, were provided by 4 studies. These included one study of adalimumab [37], two of etanercept [38, 39] and one of infliximab [40]. Data on ACR response rates at 6 months were derived from 6 studies, including three studies of adalimumab [9, 37, 41], one of etanercept [39] and two of infliximab [42, 43]. All studies were randomised, double-blind, placebo-controlled studies, except for the study by Westhovens et al. [43], in which patients in the placebo group crossed over to receive active treatment between weeks 22–46.
Data for the analysis of monotherapies were provided by the FAST4WARD study and 3 additional studies each for ACR response rates at 3 and 6 months. The studies from which 3-month ACR response rates were derived included two studies of adalimumab [44, 45] and one study of etanercept [46]; and 6-month ACR response rates were derived from two studies of adalimumab [44, 47] and one of etanercept [46]. All studies were randomised, double-blind, placebo-controlled studies. Estimated ACR response rates, i.e. probabilities of transition of the model, for all agents following the indirect comparison are shown in Table 1.
Data regarding the safety of biological DMARDs varied in the published literature, in terms of the way it is reported and analysed, making it difficult to make indirect comparison between different treatments. However, since safety profiles for all TNF inhibitors, including certolizumab pegol, appear to be similar, costs and results associated with adverse events were not explicitly included in the model.
Resource use data were taken from published literature and unit costs (drug acquisition and administration, monitoring and resources) were taken from official Spanish sources or published references [48, 49]. Treatment unit costs are shown in Table 2 and resource unit costs are shown in Table 3. The cost of methotrexate was assumed to be zero and indirect costs were not included in the model. Costs per HAQ category were taken from a cohort study in Sweden and the United Kingdom [50].
Health effects were measured using the EuroQol Group 5 Dimension self-report questionnaire (EQ-5D). Upon entry into the model, the patient population was assigned a mean pre-treatment utility score of 0.38, derived from the EQ-5D data collected in the certolizumab pegol RAPID 1 and 2 trials [17, 36]. Over the first 6 months of initial treatment, patients were assigned an average change in the derived EQ-5D utilities which was dependent on their response category (Fig. 2). The magnitude of the change in EQ-5D utilities was estimated from the patient-level data of the certolizumab pegol trials by ANACOVA regression analysis and the effect is assumed to be the same for all comparators. The regression models were fitted with age, gender, baseline EQ-5D utilities, disease duration, number of previous conventional DMARDs and anti-CCP antibody status as covariates. Default estimates are adjusted for the selected analysis population and are varied in probabilistic sensitivity analyses. Regression coefficients were then used to calculate the change in utility. The base-case analysis also assumes that 80 % of the change over the first 6 months is achieved by week 4. Patients continuing treatment continue to improve over the year following the initial 6 months of treatment, but at a much smaller rate, as the majority of health benefit has been gained by this point (Fig. 2). This assumption was validated by expert clinicians who agreed that it was likely that patients would continue to improve beyond the first round of direct clinical improvements as quality of life impact then becomes more important. Again, it is assumed that certolizumab pegol and all comparators would perform similarly. Changes in HAQ-DI scores with time on treatment are estimated directly from the certolizumab pegol trials using repeated measures analyses and then mapped to EQ-5D utility benefit in the model through the Bansback conversion factor (ΔEQ-5D utility = −0.2102 ΔHAQ) [51]. Patients discontinuing treatment were assigned a decrease in utility equal to that applied for the initial response to treatment and an increase in utility as for the initial response to the first-line treatment, to account for the benefit of the treatment to which the patient moves. Thus the model does not favour interventions from which there is low discontinuation, since the benefit of initial treatment is replaced by a benefit of follow-up treatments. For the follow-on alternative treatments (conventional DMARDs or palliation) the model assumes a decline in the health state over time (Fig. 2).
Base-case analysis
The base-case analysis was conducted from the perspective of the Spanish National Health System (Sistema Nacional de Salud) and included the following direct healthcare costs: treatment (unit cost, administration, monitoring); hospital costs (outpatient and inpatient); costs of primary-health and specialist medical appointments. The base case analysis assumed a clinical response at 6 months and was conducted over a lifetime horizon (set at 45 years). In this model, for drugs for which the dose is adjusted for patient weight (abatacept, infliximab, azathioprine and cyclosporine), the weight distribution of the RA population in certolizumab pegol trials was used to calculate the percentages of patients receiving a specified number of vials; a patient-fixed average weight of 81.4 kg was assumed. An annual discount rate of 3.5 % was applied for costs and outcomes [52–54]. The analysis assumed a cost per unit approach (i.e. assumes unused drug was wasted). The cost year was 2009, with an inflation rate of 3 % for 2009 onwards. Annual inflation rates for the period 1997 to 2009 were taken from the official statistics published by the Instituto Nacional de Estadística (the Spanish National Statistics Institute) [55].
ICERs were evaluated against a €30,000/QALY willingness-to-pay (WTP) threshold [56–58].
Sensitivity analyses
Univariate sensitivity analyses were conducted by varying different model parameters including ACR response time of 3 months, costing method (per mg), HAQ-DI measurements for improvements in quality of life instead of EQ-5D, time horizons, discount rate for health costs and outcomes, an assumed association of zero between mortality and HAQ-DI, variation (between 0 and 100 %) in the patient's rate of deterioration on the HAQ-DI scale after discontinuing treatment, baseline HAQ scale score, the annual progression on the HAQ scale for the first-line treatment or continuation treatment.
Probabilistic sensitivity analyses were conducted for each probabilistic sensitivity analysis, 1000 simulations were generated using base case assumptions and parameter variability summarised in Table 4. The variables altered were: clinical effectiveness, mean age, baseline mean HAQ-DI score, number of previous DMARDs, disease duration and antibody status (modelled using a normal distribution), gender (using a beta distribution) and patient weight (using a certolizumab pegol-related cumulative distribution function). All other parameters were held constant. From the results of these simulations, cost-effectiveness planes and cost-effectiveness acceptability curves were drawn.
Results
Base-case analysis
Combination therapies
Certolizumab pegol plus methotrexate was the most cost-effective therapy when compared with other biologic TNF inhibitors plus methotrexate, at €30,000/QALY willingness-to-pay (WTP) threshold [56–58]. Certolizumab pegol + methotrexate dominated (most efficient as assessed through QALYs and less expensive) adalimumab (weekly), etanercept, infliximab 5 mg/kg, combination therapies. Combination certolizumab pegol plus methotrexate was cost-effective versus adalimumab (every 2 weeks) and infliximab 3 mg/kg in combination with methotrexate, with estimated ICERs of €12,346/QALY and €15,414/QALY, respectively (see Table 5).
Monotherapies
The analysis indicated that certolizumab pegol monotherapy was the most effective (as measured through QALYs) and less expensive compared with adalimumab (weekly or every 2 weeks). Certolizumab pegol monotherapy was also less expensive, but had comparable health gains when compared with etanercept monotherapy (6.416 QALYs vs 6.492), that lead to an ICER of €8788/QALY (etanercept vs certolizumab pegol). See Table 5.
Sensitivity analyses
Univariate sensitivity analysis
The results of the analysis of sensibility are show in the Tables 6 and 7. The sensitivity analyses indicated that the base case results were robust, with certolizumab pegol remaining the cost-effective treatment at the €30.000/QALY WTP threshold, when changes were applied to the discount rate, economic perspective of the analysis, the drug costing method, the choice of quality of life instrument and the association between HAQ score and mortality. The ICERs were sensitive to changes in the time horizon, timepoint of ACR response, baseline HAQ score, and the rate of deterioration in HAQ-DI scale after discontinuing the treatment.
Probabilistic sensitivity analyses
Cost-effectiveness planes for the PSA of certolizumab pegol vs. other therapies with the ACR six month definition of response are shown in Figs. 3 and 4. The probabilistic sensitivity analysis indicated that at €30.000/QALY WTP, certolizumab pegol plus methotrexate has the highest probability of being cost-effective against adalimumab (weekly) and infliximab (5 mg/kg), in 91 % and 78 % of the cases, respectively. When compared with adalimumab every 2 weeks, etanercept and infliximab (3 mg/kg), and certolizumab pegol had almost equal probability of being cost-effective (42 to 48 % at a €30.000/QALY WTP threshold).
Discussion
The cost utility of certolizumab pegol compared with other TNF inhibitors available in Spain in 2009 and administered alone or in combination with methotrexate in patients with moderate-to-severe RA who have had an inadequate response to methotrexate alone has been estimated. Evaluated against a €30,000/QALY WTP threshold and over a lifetime horizon, certolizumab pegol administered in combination with methotrexate was a dominant therapy compared with other TNF inhibitor combination therapies (weekly adalimumab, etanercept, and infliximab 5 mg/kg), and cost-effective versus 2-weekly adalimumab and infliximab (3 mg/kg) combination therapies.
Certolizumab pegol monotherapy was dominant versus weekly or 2-weekly adalimumab monotherapy and was also less expensive, but had comparable health gains when compared with etanercept monotherapy. Probabilistic sensitivity analyses confirmed the cost-effectiveness of certolizumab pegol + methotrexate combination therapy. Univariate sensitivity analyses showed that ICERs for certolizumab pegol administered with or without methotrexate were robust to changes in the majority of variables analysed, and that ICERs were sensitive to changing the time horizon, timepoint of ACR response, baseline HAQ score, and the rate of deterioration in HAQ-DI scale after discontinuing the treatment.
Comparison with other studies is difficult since most of the studies published to date support the cost-effectiveness of TNF inhibitors as a second-line strategy in patients who fail to respond to non-biological DMARDs, but relative evaluation of TNF inhibitors using indirect cost-effectiveness analyses are lacking. Only one study, evaluating the relative cost-effectiveness of the five current FDA-approved TNFα inhibitors in combination with methotrexate for the treatment of patients with moderate-to-severe active RA and with moderate or no response to methotrexate monotherapy from a US health payer perspective, has been recently published [59]. The study used Bayesian methods to determine the relative probabilities of achieving an ACR50 clinical response for each TNF inhibitor and Markov modelling, in which patients who achieved ACR50 criteria continued to receive combination therapy but those who did not switched to tocilizumab. Results of the study showed certolizumab pegol + methotrexate to be the second most cost-effective TNF inhibitor after etanercept + methotrexate, dominating infliximab, adalimumab and golimumab combination therapies at a WTP threshold of US$139,143/QALY.
Whilst estimation of relative effectiveness of the various biological DMARDs using ACR response data from only randomised, double-blind trials with methotrexate and/or placebo as controls helped to ensure the quality of these data, the lack of head-to-head studies, necessitating indirect analysis of ACR response data, is acknowledged as a limitation of our study. Also, although similar incidences of adverse events between the available biological DMARDs may be generally considered to be a reasonable assumption, exclusion of the costs associated with adverse events from the model is another acknowledged limitation of our study. Other limitations include the lack of data sources and cost-utility data (in Spain and worldwide) with which to compare our data, and the limited effectiveness of applying effectiveness information from 6 months, which is >1 treatment cycle.
The implications of our study findings for the public payer in Spain suggest that the Spanish NHS would be adopting the most cost-effective treatment if certolizumab pegol were offered to RA patients in combination with methotrexate instead of alternative TNF inhibitors currently recommended in Spain. Furthermore a recent study, in that equality of effectiveness is supposed, indicated that the addition of certolizumab pegol on the NHS in Spain, would generate large net savings of €10.3 million for the period 2013 to 2017 [60].
Conclusions
In our study, in terms of QALYs gained, certolizumab pegol was the most effective therapy in combination with methotrexate at the €30,000/QALY willingness-to-pay (WTP) threshold compared with other TNF inhibitors recommended in Spain in 2009 (adalimumab, etanercept and infliximab) for the treatment of patients with active RA who did not respond adequately to DMARDs. In an analysis of monotherapies, certolizumab pegol was more cost-effective versus adalimumab and less expensive with similar health gains versus etanercept (mean QALY 6.416 vs 6.492). Probabilistic sensitivity analyses indicated advantages in efficacy for certolizumab pegol over the other TNF inhibitors. These results indicate that moderate-to-severe RA treatment with certolizumab pegol is an efficient and economically valuable alternative for the Spanish NHS.
Abbreviations
- ACR:
-
American College of Rheumatology
- ACR20:
-
American College of Rheumatology 20 % response
- ACR50:
-
American College of Rheumatology 50 % response
- ACR70:
-
American College of Rheumatology 70 % response
- DAS28:
-
Disease Activity Score 28
- DMARDs:
-
disease-modifying antirheumatic drugs
- EQ-5D:
-
EuroQol Group 5 Dimension
- HAQ:
-
Health Assessment Questionnaire
- HAQ-DI:
-
Health Assessment Questionnaire-Disability Index
- ICERs:
-
incremental cost-effectiveness ratios
- LYG:
-
life year gained
- QALY:
-
quality-adjusted life year
- RA:
-
rheumatoid arthritis
- TNF:
-
tumour necrosis factor
- WTP:
-
willingness-to-pay
References
Disease Management Project: Rheumatoid Arthritis. [http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/rheumatology/rheumatoid-arthritis/]
Carmona L, Ballina J, Gabriel R, Laffon A. The burden of musculoskeletal diseases in the general population of Spain: results from a national survey. Ann Rheum Dis. 2001;60:1040–5.
Carbonell J, Cobo T, Balsa A, Descalzo MA, Carmona L, Group SS. The incidence of rheumatoid arthritis in Spain: results from a nationwide primary care registry. Rheumatology. 2008;47:1088–92.
A survey of barriers to treatment access in rheumatoid arthritis. Country Annex Report: Spain [http://www.comparatorreports.se/RA%20Barrier%20Report_FINAL_050110.pdf]
Scott DL, Kingsley GH. Tumor necrosis factor inhibitors for rheumatoid arthritis. N Engl J Med. 2006;355:704–12.
Rodriguez-Valverde V, Caliz Caliz R, Álvaro-Gracia Álvaro JM, Marenco de la Fuente JL, Mulero Mendoza J, Tornero Molina J, Andreu Sanchez JL, Ballina Garcia FJ, Batlle Gualda E, Canete Crespillo JD, et al. 3rd update on the Spanish Rheumatology Society consensus on biological therapy in rheumatoid arthritis. 2006.
Tornero Molina J, Sanmarti Sala R, Rodriguez Valverde V, Martin Mola E, Marenco de la Fuente JL, Gonzalez Alvaro I, et al. [Update of the consensus statement of the spanish society of rheumatology on the management of biologic therapies in rheumatoid arthritis]. Reumatologia clinica. 2010;6:23–36.
Genovese MC, Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, et al. Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Arthritis Rheum. 2002;46:1443–50.
Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50:1400–11.
Klinkhoff A. Biological agents for rheumatoid arthritis: targeting both physical function and structural damage. Drugs. 2004;64:1267–83.
Voll RE, Kalden JR. Do we need new treatment that goes beyond tumor necrosis factor blockers for rheumatoid arthritis? Ann N Y Acad Sci. 2005;1051:799–810.
Cimzia, certolizumab pegol: European Public Assessment Report (EPAR) summary for the public [http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/001037/WC500069733.pdf]
Cimzia 200 mg solution for injection: Summary of Product Characteristics [http://www.medicines.org.uk/emc/medicine/22323/SPC]
Fleischmann R, Vencovsky J, van Vollenhoven RF, Borenstein D, Box J, Coteur G, et al. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD study. Ann Rheum Dis. 2009;68:805–11.
Kavanaugh A, Smolen JS, Emery P, Purcaru O, Keystone E, Richard L, et al. Effect of certolizumab pegol with methotrexate on home and work place productivity and social activities in patients with active rheumatoid arthritis.[Erratum appears in Arthritis Rheum. 2010 Oct;62(10):1514]. Arthritis Rheum. 2009;61:1592–600.
Keystone E, van der Heijde D, Mason Jr D, Landewe R, Vollenhoven RV, Combe B, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study.[Erratum appears in Arthritis Rheum. 2009 May;60(5):1249]. Arthritis Rheum. 2008;58:3319–29.
Strand V, Mease P, Burmester GR, Nikai E, Coteur G, van Vollenhoven R, et al. Rapid and sustained improvements in health-related quality of life, fatigue, and other patient-reported outcomes in rheumatoid arthritis patients treated with certolizumab pegol plus methotrexate over 1 year: results from the RAPID 1 randomized controlled trial. Arthritis Res Ther. 2009;11:R170.
Allaart CF, Breedveld FC, Dijkmans BAC. Treatment of recent-onset rheumatoid arthritis: lessons from the BeSt study. J Rheumatol. 2007;80:25–33.
Barbieri M, Wong JB, Drummond M. The cost effectiveness of infliximab for severe treatment-resistant rheumatoid arthritis in the UK. Pharmacoeconomics. 2005;23:607–18.
Brennan A, Bansback N, Nixon R, Madan J, Harrison M, Watson K, et al. Modelling the cost effectiveness of TNF-alpha antagonists in the management of rheumatoid arthritis: results from the British Society for Rheumatology Biologics Registry. Rheumatology. 2007;46:1345–54.
Davies A, Cifaldi MA, Segurado OG, Weisman MH. Cost-effectiveness of sequential therapy with tumor necrosis factor antagonists in early rheumatoid arthritis. J Rheumatol. 2009;36:16–26.
Farahani P, Levine M, Goeree R. A comparison between integrating clinical practice setting and randomized controlled trial setting into economic evaluation models of therapeutics. J Eval Clin Pract. 2006;12:463–70.
Rubio-Terres C, Ordovas Baines JP, Pla Poblador R, Martinez Nieto C, Sanchez Garre MJ, Rosado Souviron MA, et al. [Use and cost of biological disease-modifying anti-rheumatic drugs in Spain (PRAXIS study)]. Farm Hosp. 2007;31:78–92.
Soini EJ, Hallinen TA, Puolakka K, Vihervaara V, Kauppi MJ. Cost-effectiveness of adalimumab, etanercept, and tocilizumab as first-line treatments for moderate-to-severe rheumatoid arthritis. J Med Econ. 2012;15:340–51.
Spalding JR, Hay J. Cost effectiveness of tumour necrosis factor-alpha inhibitors as first-line agents in rheumatoid arthritis. Pharmacoeconomics. 2006;24:1221–32.
Tanno M, Nakamura I, Ito K, Tanaka H, Ohta H, Kobayashi M, et al. Modeling and cost-effectiveness analysis of etanercept in adults with rheumatoid arthritis in Japan: a preliminary analysis. Mod Rheumatol. 2006;16:77–84.
Purcaru O, Taylor P, Emery P, Palmer S. Cost-effectiveness of certolizumab pegol plus methotrexate or as monotherapy for the treatment of active rheumatoid arthritis in the United Kingdom [abstract]. Ann Rheum Dis. 2010;69:718.
Fries JF. Aging, natural death, and the compression of morbidity. 1980. Bull World Health Organ. 2002;80:245–50.
Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics. 1998;13:397–409.
Luqmani R, Hennell S, Estrach C, Birrell F, Bosworth A, Davenport G, et al. British Society for Rheumatology and british health professionals in Rheumatology guideline for the management of rheumatoid arthritis (the first two years). Rheumatology. 2006;45:1167–9.
Costing template for infliximab for the treatment of adults with psoriasis (Guidance TA134) [http://guidance.nice.org.uk/TA134/CostingTemplate]
Chen YF, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, Fry-Smith A, Burls A. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess (Winchester, England). 2006; 10:iii-iv, xi-xiii, 1–229.
Du Pan SM, Dehler S, Ciurea A, Ziswiler HR, Gabay C, Finckh A. Comparison of drug retention rates and causes of drug discontinuation between anti-tumor necrosis factor agents in rheumatoid arthritis. Arthritis Rheum. 2009;61:560–8.
Mortality Tables for the Spanish Population 1991–2007: Mortality tables for the Spanish population by year, age, gender and functions [http://www.ine.es/jaxi/tabla.do?path=/t20/p319a/1991-2007/l0/&file=01001.px&type=pcaxis&L=0]
Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37:481–94.
Smolen J, Landewe RB, Mease P, Brzezicki J, Mason D, Luijtens K, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis. 2009;68:797–804.
Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial.[Erratum appears in Arthritis Rheum. 2003 Mar;48(3):855]. Arthritis Rheum. 2003;48:35–45.
Lan JL, Chou SJ, Chen DY, Chen YH, Hsieh TY, Young Jr M. A comparative study of etanercept plus methotrexate and methotrexate alone in Taiwanese patients with active rheumatoid arthritis: a 12-week, double-blind, randomized, placebo-controlled study. J Formos Med Assoc. 2004;103:618–23.
Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253–9.
Abe T, Takeuchi T, Miyasaka N, Hashimoto H, Kondo H, Ichikawa Y, et al. A multicenter, double-blind, randomized, placebo controlled trial of infliximab combined with low dose methotrexate in Japanese patients with rheumatoid arthritis. J Rheumatol. 2006;33:37–44.
Kim H. A randomized, double-blind, placebo-controlled, phase III study of the human anti-tumor necrosis factor antibody adalimumab administered as subcutaneous injections in Korean rheumatoid arthritis patients treated with methotrexate. APLAR J Rheumatol. 2007;10:9–16.
Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis. 2008;67:1096–103.
Westhovens R, Yocum D, Han J, Berman A, Strusberg I, Geusens P, et al. The safety of infliximab, combined with background treatments, among patients with rheumatoid arthritis and various comorbidities: a large, randomized, placebo-controlled trial.[Erratum appears in Arthritis Rheum. 2007 May;56(5):1675 Note: Dosage error in article text]. Arthritis Rheum. 2006;54:1075–86.
Miyasaka N, Investigators CS. Clinical investigation in highly disease-affected rheumatoid arthritis patients in Japan with adalimumab applying standard and general evaluation: the CHANGE study. Mod Rheumatol. 2008;18:252–62.
van de Putte LBA, Rau R, Breedveld FC, Kalden JR, Malaise MG, van Riel PLCM, et al. Efficacy and safety of the fully human anti-tumour necrosis factor alpha monoclonal antibody adalimumab (D2E7) in DMARD refractory patients with rheumatoid arthritis: a 12 week, phase II study. Ann Rheum Dis. 2003;62:1168–77.
Moreland LW, Schiff MH, Baumgartner SW, Tindall EA, Fleischmann RM, Bulpitt KJ, et al. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med. 1999;130:478–86.
van de Putte LB, Atkins C, Malaise M, Sany J, Russell AS, van Riel PL, et al. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis. 2004;63:508–16.
BOT Medicines Database [http://www.portalfarma.com/Home.nsf/Home?OpenForm]
Information on products included in the Spanish Health System pharmaceutical list (can be dispensed by community pharmacists): Invoicing List for December/2009 [http://www.msssi.gob.es/profesionales/nomenclator.do]
Kobelt G, Jonsson L, Lindgren P, Young A, Eberhardt K. Modeling the progression of rheumatoid arthritis: a two-country model to estimate costs and consequences of rheumatoid arthritis. Arthritis Rheum. 2002;46:2310–9.
Modelling the cost effectiveness of TNF-α inhibitors in the management of rheumatoid arthritis: Results from the British Society for Rheumatology Biologics Registry [http://www.shef.ac.uk/polopoly_fs/1.43358!/file/HEDS-DP-06-12.pdf]
Puig-Junoy J, Oliva Moreno J, Trapero Beltrán M, Abellan Perpinan JM, Brosa Riestra M. Guía y recomendaciones para la realización y presentación de evaluaciones económicas y análisis de impacto presupuestario de medicamentos en el ámbito del CatSalut. Barcelona: Servei Català de la Salut (CatSalut); 2014.
Pinto JL, Sánchez Martínez FI. Métodos para la evaluación económica de nuevas prestaciones. Madrid, 2007. Madrid: Centre de Recerca en Economia i Salut y Ministerio de Sanidad y Consumo; 2007.
López Bastida J, Oliva J, Antoñanzas F, García-Altés A, Gisbert R, Mar J, et al. Propuesta de guía para la evaluación económica aplicada a las tecnologías sanitarias. Gac Sanit. 2010;24:154–70.
Standard of living, quality of life and living conditions: consumer price index [http://www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736176802&menu=ultiDatos&idp=1254735976607]
Guide to the methods of technology appraisal [http://www.nice.org.uk/article/pmg9/chapter/Foreword]
Rodriguez Barrios JM, Perez Alcantara F, Crespo Palomo C, Gonzalez Garcia P, Anton De Las Heras E, Brosa Riestra M. The use of cost per life year gained as a measurement of cost-effectiveness in Spain: a systematic review of recent publications. Eur J Health Econ. 2012;13:723–40.
Sacristan JA, Oliva J, Del Llano J, Prieto L, Pinto JL. [What is an efficient health technology in Spain?]. Gaceta sanitaria/SESPAS. 2002;16:334–43.
Nguyen CM, Bounthavong M, Mendes MA, Christopher ML, Tran JN, Kazerooni R, et al. Cost utility of tumour necrosis factor-alpha inhibitors for rheumatoid arthritis: an application of Bayesian methods for evidence synthesis in a Markov model. Pharmacoeconomics. 2012;30:575–93.
Hidalgo A, Villoro R, Ivanova A, Morell A, Talavera P, Ferro B. Tratamiento biológico de la Artritis reumatoide en España. Análisis de impacto presupuestario de la utilización de certolizumab pegol. Pharmacoecon Span Res Artic. 2014;11:97–107.
Maini RN, Taylor PC, Szechinski J, Pavelka K, Broll J, Balint G, et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006;54:2817–29.
Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–97.
Strand V, Balbir-Gurman A, Pavelka K, Emery P, Li N, Yin M, et al. Sustained benefit in rheumatoid arthritis following one course of rituximab: improvements in physical function over 2 years. Rheumatology. 2006;45:1505–13.
eSalud - Financial information on the healthcare sector [http://oblikue.com/bddcostes/]
Acknowledgments
UCB Pharma, Brussels was responsible for the design and conduct of the cost-utility analysis; UCB Pharma, S.A. Spain was responsible for adapting the model to Spanish data (Spanish cost, population, etc.) in collaboration with Castilla-La Mancha University and Max Weber Institute (with funding from UCB Pharma S.A., Spain) and with Health Technology Assessment in Spain (without funding or payments from UCB Pharma S.A., Spain).
Medical writing assistance was provided by Andrea Bothwell of in Science Communications, Springer Healthcare, with funding from UCB Pharma, S.A., Spain.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Álvaro Hidalgo is a professor at University of Castilla-La Mancha which received consulting fees for its contribution to this study.
Renata Villoro is a researcher at Max Weber Institute which received consulting fees for its contribution to this study.
Pablo Talavera and Belén Ferro are employees of UCB Pharma Spain.
Oana Purcaru is an employee of UCB Pharma Belgium.
Juan Antonio Blasco has no competing interests.
Authors’ contributions
OP was responsible for the design and conduct of the cost-utility analysis; PT and BF were responsible for adapting the model to Spanish data (Spanish cost, population, etc.) in collaboration with AH, RV and JAB. All authors read and approved the final manuscript.
Renata Villoro, Juan Antonio Blasco, Pablo Talavera, Belén Ferro and Oana Purcaru contributed equally to this work.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hidalgo-Vega, Á., Villoro, R., Blasco, J.A. et al. Cost-utility analysis of certolizumab pegol versus alternative tumour necrosis factor inhibitors available for the treatment of moderate-to-severe active rheumatoid arthritis in Spain. Cost Eff Resour Alloc 13, 11 (2015). https://doi.org/10.1186/s12962-015-0037-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12962-015-0037-9