Abstract
Background
Detrimental exposures during pregnancy have been implicated in programming offspring to develop permanent changes in physiology and metabolism, increasing the risk for developing diseases in adulthood such as hypertension, diabetes, heart disease and obesity. This study investigated the effects of protein restriction on the metabolism of amino acids within the oocyte, liver, and whole organism in a rat model as well as effects on mitochondrial ultrastructure and function in the cumulus oocyte complex.
Methods
Wistar outbred female rats 8–11 weeks of age (n = 24) were assigned to three isocaloric dietary groups, including control (C), low protein (LP) and low protein supplemented with folate (LPF). Animals were superovulated and 48 h later underwent central catheterization. Isotopic tracers of 1-13C-5C2H3-methionine, 2H2-cysteine, U-13C3-cysteine and U-13C3-serine were administered by a 4 h prime-constant rate infusion. After sacrifice, oocytes were denuded of cumulus cells and liver specimens were obtained.
Results
Oocytes demonstrated reduced serine flux in LP vs. LPF (p < 0.05), reduced cysteine flux in LP and LPF vs. C (p < 0.05), and a trend toward reduced transsulfuration in LP vs. C and LPF. Folic acid supplementation reversed observed effects on serine flux and transsulfuration. Preovulatory protein restriction increased whole-body methionine transmethylation, methionine transsulfuration and the flux of serine in LP and LPF vs. C (p = 0.003, p = 0.002, p = 0.005). The concentration of glutathione was increased in erythrocytes and liver in LP and LPF vs. C (p = 0.003 and p = 0.0003). Oocyte mitochondrial ultrastructure in LP and LPF had increased proportions of abnormal mitochondria vs. C (p < 0.01 and p < 0.05). Cumulus cell mitochondrial ultrastructure in LP and LPF groups had increased proportions of abnormal mitochondria vs. C (p < 0.001 and p < 0.05). Preovulatory protein restriction altered oocyte expression of Drp1, Opa-1, Mfn1/2, Parl and Ndufb6 (p < 0.05) and Hk2 (p < 0.01), which are genes involved in mitochondrial fission (division) and fusion, mitochondrial apoptotic mechanisms, respiratory electron transport and glucose metabolism.
Conclusions
Preovulatory protein restriction resulted in altered amino acid metabolism, abnormal cumulus oocyte complex mitochondrial ultrastructure and differential oocyte expression of genes related to mitochondrial biogenesis.
Similar content being viewed by others
Background
Prenatal exposure to aberrant conditions such as protein restriction in the rat, high fat diet, maternal diabetes, maternal obesity, and variable stress in the mouse, and famine in humans, have been implicated in programming mammalian offspring to develop permanent changes in physiology and metabolism, increasing the risk for developing diseases in adulthood such as hypertension, diabetes, heart disease and obesity [1,2,3,4,5,6,7,8,9,10]. The increased susceptibility is the result of fetal adaptations in utero in response to altered nutrient availability [11,12,13]. This investigation explores the ovary as a postulated target vulnerable to malnutrition, representing a window of time prior to conception when adaptations to altered nutrient availability may impact future pregnancies [14,15,16]. In this model the oocyte must adapt to maternal exposures, potentially predisposing future embryos, and thus offspring, to developmental abnormalities.
Maternal dietary protein restriction has been shown to induce significant changes in embryos and offspring in animal models [5, 17, 18]. Given 3–4 weeks prior to pregnancy, a low-protein (LP) diet results in altered mitochondrial metabolism in 2-cell embryos [19]. Given during the preimplantation period, a LP diet decreases the rate of blastocyst cellular proliferation, reduces maternal serum levels of essential amino acids, and induces intrauterine growth restriction and hypertension in pups [20]. Given during pregnancy, a LP diet results in insulin resistance in adult male offspring as well as epigenetic modifications in hepatic gene expression [21]. These hepatic epigenetic changes are prevented with folate supplementation [22, 23]. Epigenetic modifications include DNA methylation at CpG sites and modification of chromatin. CpG sites are DNA regions consisting of cytosine and guanine nucleotides joined by a phosphate group whereby epigenetic modification occurs when the cytosine is methylated changing the expression of a gene. The mechanism by which folic acid reverses these observed epigenetic changes is via its action as a single carbon donor. The metabolic pathway responsible for the production of single carbon groups is closely linked to folic acid metabolism. Hence, folic acid modulates the expression of DNA methyltransferases which in turn has been shown to ameliorate altered DNA methylation due to maternal undernutrition. It is recommended that women of reproductive age consume at least 400 mcg of folic acid prior to and during pregnancy.
A gestational LP diet causes insulin resistance in adult male offspring via an inefficient insulin-induced insulin receptor, as well as impaired GLUT-4 translocation [24]. GLUT-4 is the insulin-regulated glucose transporter that is located in adipose, skeletal and cardiac tissues. It has therefore been proposed that one-carbon metabolism is central to the mechanism of this altered phenotype [25,26,27]. These experiments investigated folate supplementation in the reproductive aged female rat as a potential means to ameliorate potential adverse effects on the oocyte due to dietary protein deficiency [28].
The oocyte requires an enormous supply of energy both during maturation and after fertilization to support critical events such as the resumption of meiosis, spindle formation and cellular division [29]. Mitochondria are maternally-inherited organelles within the oocyte responsible for the production of the chemical energy precursor adenosine triphosphate (ATP) via oxidative phosphorylation [30]. Reactive oxygen species (ROS) can damage mitochondria if not appropriately neutralized by antioxidants such as glutathione (GSH). The formation of GSH is dependent upon the availability of precursors such as folate, serine and cysteine. In this study oocyte kinetics of serine and cysteine were evaluated to evaluate the process of transsulfuration, which results in the production of cysteine (rate-limiting precursor to the formation of GSH) (Table 3). This study also sought to assess the effect of protein restriction on the concentration of amino acids within cumulus cells (Table 3). Mitochondria also have key roles in maintaining cellular homeostasis and regulating apoptosis [31]. Importantly, embryonic mitochondrial replication does not occur until after blastocyst hatching and implantation, and the existing pool of mitochondria available to each cell of the developing embryo diminishes with each successive cellular division [32, 33]. Sufficient ATP production by mitochondria is therefore required for successful nuclear and cytoplasmic maturation of the oocyte [34].
Oocyte mitochondria from mice on a high fat diet or from mice with diabetes demonstrate disarrayed cristae, inner mitochondrial membrane swelling, and increased vacuoles within the organelle [15, 35]. Additionally, under physiologic conditions, mitochondrial morphology is in part maintained by a balance of fusion and fission [36, 37]. Mitochondrial fusion and fission are actions that fuse and divide, respectively, the two lipid bilayers that encompass mitochondria [36, 37]. DRP-1 is a molecule that regulates mitochondrial fission [38]. Cellular starvation causes phosphorylation of DRP1, decreasing its recruitment to mitochondria and therefore inhibiting fission [39]. Similarly, mouse embryonic fibroblasts exposed to various cellular stressors such as ultraviolet radiation or actinomycin D causes stress-induced mitochondrial hyperfusion (SIMH) [40]. Mitochondrial elongation can increase the ATP synthesis capacity of each mitochondrion [41].
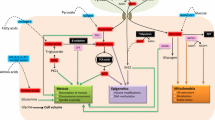
Dietary protein restriction may reduce the intracellular production of antioxidants capable of detoxifying local ROS produced by mitochondria via altered amino acid metabolism (Fig. 1). The primary intracellular antioxidant is glutathione, which is capable of preventing cellular damage by serving as an electron donor via the reduction of disulfide bonds. Glutathione is a reducing agent, and once an electron has been donated it is converted to its oxidized form, glutathione disulfide (GSSG). GSSG can be reduced back to GSH via glutathione reductase. Figure 1 illustrates the transmethylation and transsulfuration pathways. Integral to these pathways is the regulation of the transfer of methyl groups for the synthesis of metabolic intermediates and the methylation of DNA, RNA and proteins. Serine and glycine are nutritionally non-essential amino acids but are critical for proper cell function. Serine provides methyl groups to tetrahydrofolate (THF) to form 5-methyl-THF, which is an intermediate in the conversion of homocysteine to methionine. Therefore, the availability of methionine is in part a reflection of resynthesis from homocysteine. The methylation of homocysteine to methionine requires an adequate supply of folic acid (which is twice reduced to THF) [42].
A low-protein diet may alter the kinetics of whole-body and tissue-specific amino acid metabolism. In addition to resynthesis from homocysteine, methionine, a nutritionally essential amino acid in humans, is obtained through dietary intake and released from protein breakdown [43, 44]. Methionine is a critical intermediary in the production of glutathione, the primary intracellular antioxidant, and improving cellular access to methionine may improve alterations in metabolism that occur as a result of protein deficiency. Therefore, folic acid supplementation may ameliorate decreased methionine availability by improving the efficiency of RM of homocysteine to methionine, as folate acts as a methyl donor to support the remethylation of homocysteine to methionine [45]. Methionine is the sulfur donor for cysteine synthesis, where cysteine is the rate-limiting precursor for the synthesis of glutathione, the primary intracellular antioxidant [44].
The liver is a digestive organ that is essential for the synthesis of proteins, regulation of glycogen storage, and detoxification of metabolites, among other important metabolic roles [46]. Normal hepatic function necessitates proper amino acid metabolism, and in humans approximately 50% of methionine is metabolized to S-adenosylmethionine (SAMe) by the liver. In turn, SAMe is utilized for the methylation of substrates and for protein synthesis [47]. Therefore, studying amino acid metabolism in metabolically-active tissues (including the liver and the oocyte) in a low protein environment presents a more complete picture of the whole body metabolic effects. Thus a low-protein diet, when given before conception, may reduce oocyte amino acid concentrations through altered amino acid kinetics, restricting the availability of substrates needed for the protection, optimal growth, and fertilization of oocytes. Additionally, Our hypothesis is that preovulatory exposure to a protein-restricted diet alters the metabolism of amino acids and augments mitochondrial ultrastructure and function in the rat cumulus oocyte complex.
Methods
Baylor College of Medicine Institutional Review Board (IRB) and the Institutional Animal Care and Use Committee (IACUC) approved this protocol. The isocaloric diets used in this study were customized through Teklad Laboratory Animal Diets (Envigo), and included a control group with a normal amount of protein (20% casein), a low protein group (6% casein), and a low protein plus supplemental folate group (6% casein plus 5 mg/kg folate) [5]. The recommended daily allowance (RDA) for rats is approximately 60 kcal with 20% protein and 1 mg/kg folic acid. The RDA for protein and folic acid were utilized for development of the control diet. The caloric requirement during pregnancy in the rat is 10 to 30% greater than that of the mature, nonpregnant female rat. [48] The protein-derived calories that were reduced in the low protein groups were replaced by carbohydrate sources. 8–9 week old virgin female Wistar rats (200 g) were randomized to three groups (n = 8 each) for a 40-day isocaloric dietary intervention. The length of dietary intervention was chosen because the length of the estrous cycle in a rat is approximately 4 to 5 days, and 40 days represents approximately 8 estrous cycles in the rat. In the human, primordial follicles leave the resting state 26 weeks prior to ovulation, and the most extensive follicular growth (from primary to late tertiary) occurs from around 14 weeks preovulation [27]. During this time the oocyte grows 60–70 fold in size and undergoes rapid cellular biosynthesis. Therefore, 40 days in the rat represents the length of time needed to expose primordial follicles throughout their growth and maturation until they become preovulatory follicles.
Amino acid kinetics
At the conclusion of the dietary intervention the animals were injected with 15 U of PMSG (pregnant mare serum gonadotropin) in preparation for the amino acid stable isotope infusion. Animals were fasted for 12 h prior to carotid and jugular catheter implantation on the morning of the infusion experiment. Central catheterization was performed as previously described [49]. In order to provide an understanding of methionine homeostasis in a low protein environment, the metabolism of methionine and homocysteine were investigated via a stable isotope infusion protocol using the tracer dilution method. Kinetic parameters of the methionine (one-carbon) cycle were measured including transmethylation (TM), remethylation (RM), and transsulfuration (TS) [44, 50,51,52].
Stable isotope-labeled amino acids (1-13C-5C2H3-methionine, 2H2-cysteine, U-13C3-cysteine and U-13C3-serine), were administered as a primed bolus and then as a continuous infusion, through jugular vein catheters for 4 h. A prime dose of U-13C3-cysteine was also given to prime the secondary pool of cysteine with labels derived from U-13C3-serine. Timed blood samples were taken during the infusion followed by harvesting of tissues and oocytes, which allowed for the calculation of the metabolic flux, or rate of turnover of amino acids in the whole body and within the oocyte. The plasma or tissue isotope enrichments of methionine, homocysteine, cysteine, serine and glutathione were measured by liquid chromatography tandem mass spectrometry (LC-MS/MS) as previously described by us [44, 51]. The fluxes of methionine, cysteine, serine and methionine TM, TS and RM rates were calculated using standard steady state equations as previously described [44, 50,51,52].
In order to understand GSH homeostasis in a low protein environment, both with and without folate supplementation, GSH kinetics and concentration were measured in vivo. GSH synthesis rate was measured using the rate of incorporation of 2H2-cysteine and the precursor/product equation. To measure the isotopic enrichment of erythrocyte and liver tissue glutathione, liver tissue and RBC-free extract was first reduced by adding a 0.5 M dithiothreitol (DTT) solution, and then was analyzed by LC-MS/MS. Erythrocyte and liver tissue glutathione concentrations were measured by an isotope dilution method. Briefly, a known amount of sample was spiked with a known quantity of labeled glutathione-[glycine-13C2,15N] (Cambridge Isotope Laboratories, Tewksbury, MA) as an internal standard. The samples were then analyzed by LC/MS and concentrations calculated as previously described [44].
Cumulus oocyte complex mitochondrial ultrastructure
Twenty-four ovarian specimens were processed (n = 8 for each of the three experimental groups) for transmission electron microscopy (TEM) as previously described. Once an antral follicle with an intact cumulus oocyte complex (COC) was identified at 40x magnification, the ovarian tissue block was cut into thin (50 nm) sections by the ultramicrotome. These thin sections were placed on a copper grid and stained with heavy metals. This produced mounted tissue specimens that were available for imaging under the electron beam of the TEM.
One antral follicle was imaged from each of 12 animals, with four animals in each of the three experimental groups. The COCs were too large to fit wholly within one grid box. Therefore, each antral follicle was analyzed as follows in a systematic and semi-quantitative manner. Three random fields of each oocyte were imaged at 2500x magnification, and three random fields of cumulus cells from each COC were imaged at 2500x magnification. The number of mitochondria that were visualized in each of the fields at 2500x was tallied.
One thousand three hundred forty oocyte mitochondria and 1708 cumulus cell mitochondria were imaged and analyzed in a qualitative fashion. Cumulus and oocyte mitochondria were stratified by their qualitative appearance as normal or abnormal. Normal mitochondria in germinal vesicle stage oocytes appear spherical or oval in shape with a dense matrix, well-organized cristae, and clearly delineated inner and outer mitochondrial membranes. Normal mitochondria are not elongated, do not have vacuoles or inclusions, and do not have swollen inner mitochondrial membrane cristae. Cumulus oocyte mitochondria were imaged at 2500x magnification and categorized by their subjective appearance regarding the aforementioned criteria that represent normal and abnormal mitochondria.
Oocyte mRNA expression
The Qiagen REPLI-g WTA (whole transcriptome amplification) kit was used for whole transcriptome amplification of total RNA from small cohorts of denuded oocytes that were used for the creation of a cDNA library. The REPLI-g amplified cDNA library was stored at − 20 °C for the downstream application of real-time qPCR. The cDNA library was diluted to 1:100, at a volume of 2–3 μl diluted DNA for each 20 μl real-time PCR reaction volume. The Quantiscript RT mix, ligation mix, and REPLI-g SensiPhi amplification mix were always prepared fresh. All buffers and reagents were vortexed before use to ensure thorough mixing. The Quantiscript RT Enzyme Mix, Ligase Mix, and REPLI-g SensiPhi DNA Polymerase were thawed on ice. All other components were thawed at room temperature. All incubation steps of the protocol were preprogrammed on a thermal cycler.
The resulting cDNA was amplified by real-time qPCR using SYBR Green (Bio-Rad Laboratories) as the fluorophore in a CFX96 real-time thermal cycler (Bio-Rad Laboratories). Specific pairs of primers (Integrated Device Technology) were used for each gene amplification. PCR conditions used were 10 min at 95 °C for 1 cycle, 15 s at 95 °C, 30 s at 60 °C, and 15 s at 72 °C for 40 cycles, followed by a melt curve analysis (0.5 °C every 5 s from 65 to 95 °C). Results were calculated using the 2-ΔΔCT method and expressed as fold changes of expression of genes of interest. All reactions were performed in duplicate, and GAPDH was used as an internal control.
Data analysis
The animal weights and dietary intake data in Tables 1-3 are presented as means ± SD. Kinetic data are presented as means ± SEM. ANOVA followed by post-hoc comparisons were performed for kinetic data. Two- sided P values of 0.05 indicated significance for tests of interactions and main effects. The data were analyzed using GraphPad Prism version 6.0 (GraphPad software, San Diego, CA, USA).
Results
Amino acid kinetics
The animals were allowed unlimited access to food and water across all experimental dietary groups. The daily average feed consumed and the daily average pup weights were not statistically different among the three experimental groups (Figs. 2a-b). Each of the three isocaloric dietary formulations (control, LP, LPF) contained 3.8 Kcal/g of feed. Since the amount of feed intake was not significantly different among the three groups, the energy consumed was also not significantly different between the three dietary groups throughout the 40 day dietary intervention. In the LP dietary group, 6.5% of Kcal consumed were derived from protein. Next, components of TM and TS were investigated, including metabolic flux, which describes the rate of turnover of molecules within the metabolic pathway. Plasma methionine TM to S-adenosyl homocysteine (SAMe) was increased in the LP and LPF groups. Plasma methionine TS was also increased in the LP and LPF groups, indicating that the methionine cycle was turning over more rapidly as both the transfer of methyl groups to acceptors and sulfur groups to cysteine were occurring at a faster rate (Table 1). The flux of serine was also increased in the LP and LPF groups, indicating that the transfer of methyl groups from homocysteine to cystathionine, a critical step in the production of glutathione, was increased (Table 1).
There was a significantly higher plasma cysteine concentration in the LPF group compared to the control group which might ultimately support a more rapid synthesis of glutathione via TS with supplemental folate. This is further supported by a higher concentration of glutathione both in red blood cells and in the liver of LP and LPF animals (Table 2). The absolute synthesis rate of glutathione in both red blood cells and in the liver also trended higher toward significance in the LP group when compared to the control and LPF groups (Table 2).
Amino acid kinetics was then examined specifically within the cumulus oocyte complex. Within the oocyte proper, the flux of serine was lower in the LP group compared to the LPF group (Table 3). The flux of cysteine was decreased in the LP and LPF groups compared to the control group (Table 3). The de novo synthesis of cysteine from the TS of methionine combining with serine demonstrated a decrease that trended toward significance in the LP group compared to the control and LPF groups (Table 3). There were no significant differences detected in the concentrations of amino acids among the groups in pooled groups of cumulus cells.
The cumulus cells were denuded from around the oocyte, purified, and analyzed separately. The concentration of methionine in the LPF group was significantly higher than in the control group, suggesting an increased availability of this amino acid in the folic acid supplemented group (Table 3). The concentrations of homocysteine and serine trended higher in both the LP and LPF groups when compared to the control group. Conversely, the concentration of glycine trended lower in the LP and LPF groups when compared to the control group. Together, these patterns in the cumulus cells suggest that protein restriction causes altered amino acid metabolism, with improved methionine availability in the LPF group when compared to both the control and low protein groups.
In summary, with regard to amino acid kinetics within the rat, preovulatory dietary protein restriction increased transmethylation, transsulfuration and the flux of serine within the plasma, reflecting altered whole body metabolism. Within the oocyte proper, serine flux was decreased in the LP compared to the LPF group, and cysteine flux was decreased in the LP and LPF groups compared to the control group, suggesting a reduced availability of serine to provide methyl groups to tetrahydrofolate (THF) to form 5-methyl-THF, an intermediate in the conversion of homocysteine to methionine. Additionally, protein restriction increased the concentration of glutathione in both red blood cells and in the liver in LP and LPF groups compared to the control group (p = 0.003 and p = 0.0003). Serine is necessary for the provision of methyl groups for the formation of metabolic intermediates as well as the methylation of DNA, RNA and protein, and a reduced flux in the oocyte when exposed to protein restriction may affect reproductive competency of the cell.
Cumulus oocyte complex mitochondrial ultrastructure
Here the mitochondria of oocytes and cumulus cells in antral follicles were characterized in a semi-quantitative manner by assessing the prevalence of ultrastructural features that have previously been correlated with abnormal mitochondrial function. Antral follicles were identified under light microscopy (Fig. 3a). Mitochondrial ultrastructure in oocytes and cumulus cells was observed utilizing transmission electron microscopy.
Oocytes in the LP and LPF groups demonstrated an increased prevalence of abnormal mitochondria (p < 0.01 and p < 0.05, respectively) compared to oocytes in the control group (control = 14.6%, LP = 52.7%, LPF = 47.1%, Figs. 3b-d). Cumulus cells in the LP and LPF groups also demonstrated an increased prevalence of abnormal mitochondria compared to the control group (p < 0.001 and p < 0.05, respectively). The proportion of abnormal mitochondria in the cumulus cells was 12.7% in the control group, 52.4% in the LP group and 38.9% in the LPF group.
Oocyte mRNA expression
The transcriptional control of mitochondrial biogenesis is complex and involves the activation of nuclear transcription factors. Regulation of mitochondrial fission and fusion results in appropriate mitochondrial network organization. The mRNA expression of 18 genes involved in mitochondrial biogenesis and metabolism were quantified in pooled oocytes from control, LP and LPF groups of rats, and 9 of these genes were differentially regulated in the protein restricted groups, including Bmp15, Drp1, Hk2, Mfn1, Mfn2, Ndufb6, Opa1, Parl and Tfam (Fig. 4). Gapdh was used as the housekeeping gene for all PCR experiments.
Bmp15, Parl, and Tfam mRNA expression were increased in the LP group compared to the control group. Drp1, Hk2, Mfn1 and Ndufb6 mRNA expression were decreased in the LP group compared to the control group. Opa1 mRNA expression was increased in the LPF group, and there was a trend toward increased expression in the LP group, compared to the control group. Mfn2 expression was decreased in the LPF group, and there was a trend toward decreased expression in the LP group, compared to the control group (Figs. 5a-i).
Discussion
It is remarkable that the oocyte, as a precursor to human life, can thrive in an arguably fragile environment representing a watershed of nutrient access during maturation and early embryonic growth. Maternal dietary intake critically supports the oocyte through maturation, fertilization and until embryo genome activation. With high-energy demands, a limited pool of mitochondria and a reduced capability of protecting itself from oxidative damage, the oocyte must adapt to its host environment to survive – potentially at significant future costs to offspring. The oocyte, embryo and fetus alike may be exposed to metabolic stressors during development. Extensive data studying the effects of the Dutch famine that occurred during the winter of 1944–45 indicate that gestational exposure to maternal undernutrition can have lasting effects on offspring health. Specifically, glucose intolerance, obesity, coronary heart disease, and altered lipid profiles were seen in 2414 people born during or after the Dutch famine [53]. Adverse health effects were more severe in offspring the earlier the exposure during development. Other observations from offspring conceived during the Dutch famine suggest increased risk of schizophrenia and depression with worse cognitive task performance, possibly due to altered aging mechanisms [54]. Interestingly, observations from the Chinese famine of 1959–62 indicate a sex-specific association with metabolic syndrome, with fetal and childhood-exposed women having a higher prevalence of metabolic syndrome compared to non-exposed [55].
The maternal-zygotic transition is a sensitive stage in human development that is poorly understood. Maternal dietary intake is the only source of sustenance for the oocyte and early embryo [7]. Nutrient availability prior to ovulation must support fertilization, unpacking of the paternal genome and early cleavage of the zygote until embryo genome activation begins. Protein synthesis in the human increases 38-fold during the growth phase of oocytes [56], and growing follicles are located in the well-vascularized corticomedullary border, facilitating nutrient exchange in the growing oocyte [57]. Gap junctions between the oocyte and granulosa cells allow free transit of small molecules [58]. Amino acids within the ovary and early embryo serve as substrates necessary for DNA and organelle synthesis. Production of the antioxidant glutathione helps prevent cellular damage, especially in mitochondria, and accumulates during oocyte growth and maturation.
Integral to the optimization of intracellular processes is the regulation of the transfer of methyl groups for the synthesis of metabolic intermediates and the methylation of DNA, RNA and proteins. Serine and glycine are nutritionally non-essential amino acids that are critical for proper cell function. Serine provides the carbon skeleton for the synthesis of cysteine from methionine, and is additionally a precursor for glycine synthesis, one of the amino acids that forms glutathione (Fig. 1). Serine provides methyl groups to tetrahydrofolate (THF) to form 5-methyl-THF, which is an intermediate in the conversion of homocysteine to methionine. Therefore, the availability of methionine is in part a reflection of resynthesis from homocysteine. The methylation of homocysteine to methionine requires an adequate supply of folic acid. Through the TS pathway, serine combines with homocysteine to produce cystathionine, and ultimately cysteine, which is the rate-limiting substrate for the production of the major intracellular antioxidant glutathione. Single carbon transfers are critical for normal cellular function and require the availability of necessary precursors such as the amino acids serine, glycine, and methionine. Serine and glycine are nutritionally non-essential for humans, but methionine is nutritionally essential. Folic acid derivatives are critical for the remethylation of homocysteine to methionine, and in the setting of protein restriction (limiting access to methionine), folic acid may provide the cell a mechanism for compensation.
Preovulatory protein restriction in this model resulted in significant changes in amino acid metabolism (within the plasma and also within specific tissues such as the liver and oocyte), in cumulus oocyte complex mitochondrial ultrastructure and in the mRNA expression of mitochondrial biogenesis and metabolism related genes in the rat. Preovulatory dietary protein restriction, in plasma, increased methionine transmethylation, transsulfuration, and serine flux. This suggests that the rate of amino acid turnover is increased when the absolute availability of these precursors is decreased. Protein restriction also increased the concentration of glutathione in erythrocytes and in liver. Together, it is hypothesized that the decreased availability of GSH precursors during protein restriction leads to metabolic compensation; that is, increased methionine TM, TS, and serine flux. This allows for an increased concentration of GSH during periods of perceived increased ROS exposure. This may protect the organism from ROS-induced damage during dietary protein restriction. The effects of folic acid supplementation had differential effects in the plasma when compared to the oocyte. In plasma, supplemental folic acid in the setting of protein restriction did not appear to ameliorate the observed metabolic effects.
Preovulatory protein restriction in the oocyte demonstrated differential metabolic effects. The flux of serine was suppressed in the LP group compared to the LPF group, and transsulfuration trended lower in the LP group compared to the LPF and C groups, suggesting that the oocyte is unable to increase antioxidant precursor production in a state of protein starvation. Cysteine flux was also suppressed in the LP and LPF groups compared to the control group. Importantly, oocyte serine flux and transsulfuration demonstrated reversal of LP changes when supplemented with folic acid. This suggests that folic acid supplementation allowed for partial normalization of the observed effects within the oocyte. Future studies will focus on embryonic effects of low protein exposure during the preovulatory period. This will be done via mating of protein-restricted female rats prior to and during ovulation with control male rats to assess embryonic and fetal development.
It may be inferred that oocytes exposed to reduced protein availability are vulnerable to intracellular oxidative stress as they cannot increase glutathione production. ROS are formed locally by mitochondria as a byproduct of the formation of ATP, capable of inducing oxidative damage to mitochondrial DNA (mtDNA) [59]. Compared to nuclear DNA, mtDNA is more susceptible to mutation due both to its proximity to ROS as well as a reduced capacity for DNA repair [32]. Increased mtDNA mutations may limit normal mitochondrial function, leading to decreased energy production and a subsequent inability to support increasing intracellular demands. Structural mitochondrial abnormalities have been documented after exposure to starvation and other models of cellular stress and nutritional deficiency [15, 35]. Observed metabolic aberrations in the oocyte exposed to protein restriction, including reduced serine flux and trend toward reduced transsulfuration, may indicate an inability within the oocyte to increase glutathione precursors and may contribute to the ultrastructural changes that were seen in cumulus oocyte mitochondria. Abnormal mitochondria that are inherited by offspring may precipitate adverse health effects in the future, potentially related to metabolic syndrome, aging, fertility, and cardiovascular disease. Folic acid did not rescue the observed adverse effects in mitochondrial ultrastructure. Cumulus oocyte complexes have high cellular energy demands and structurally abnormal mitochondria may affect the reproductive potential of oocytes. Further, dietary protein restriction during follicular development altered mRNA expression patterns of many genes related to mitochondrial biogenesis and metabolism within the rat oocyte. Supplemental folate did not appear to reliably ameliorate these changes in gene transcription.
Genes that were differentially expressed in the protein-restricted groups included Bmp15, Drp1, Hk2, Mfn1, Mfn2, Ndufb6, Opa1, Parl and Tfam. Expression of Drp1 in pooled oocytes was significantly lower in the LP and LPF groups compared to the control group and this potentially reflects altered fission of the outer mitochondrial membrane. Trends in Mfn1 and Mfn2 expression, that were decreased in the LP and LPF groups, respectively, suggest potentially inhibited downstream mitochondrial fusion mechanisms. TFAM activates transcription and replication of the mitochondrial genome and oocyte mRNA expression of Tfam was significantly increased in the LP group compared to the control group, suggesting increased mtDNA replication in a protein restricted environment. Bmp15 is involved in oocyte maturation and follicular development via paracrine signaling with cumulus cells that is mediated by SMAD activation and downstream gene target activation [60]. SMAD proteins are responsible for the signal transduction of receptors of the transforming growth factor beta superfamily. Defects in Bmp15 are implicated in cases of ovarian dysgenesis. The mRNA expression of Bmp15 was significantly increased in the LP group compared to the control group, suggesting that ovarian function may be altered with protein restriction. These altered mRNA expression patterns suggest that protein restriction may alter not only mitochondrial ultrastructure in cumulus oocyte complexes but also, ultimately, function.
Future studies will focus on the impact of preovulatory protein restriction and folate supplementation on embryonic development. Specifically, we will study in vitro early embryonic morphokinetic parameters utilizing EmbryoScope time lapse microscopy in embryos up to the hatching blastocyst stage. Subsequent studies will focus on the impact of dietary protein restriction on late gestational and postnatal offspring development.
Conclusions
This series of experiments provides metabolic, ultrastructural and transcriptional evidence that protein restriction during follicular development adversely affects the growth and development of cumulus oocyte complexes in a rat model. In the whole body, protein restriction preferentially shunted nutrients toward the production of antioxidant precursors. Within the oocyte proper, protein restriction preferentially shunted nutrients toward the remethylation of methionine, away from the production of antioxidant precursors. Folic acid may be able to rescue some of the observed metabolic effects within the oocyte. With high energy demands, a limited pool of mitochondria and a reduced capability of protecting itself from oxidative damage, the oocyte must adapt to its host environment – potentially at significant future costs to offspring.
References
Barker DJ, Martyn CN. The maternal and fetal origins of cardiovascular disease. J Epidemiol Community Health. 1992;46(1):8–11.
Burdge GC, Lillycrop KA. Environment-physiology, diet quality and energy balance: the influence of early life nutrition on future energy balance. Physiol Behav. 2014;134:119–22.
Chang AS, Dale AN, Moley KH. Maternal diabetes adversely affects preovulatory oocyte maturation, development, and granulosa cell apoptosis. Endocrinology. 2005;146(5):2445–53.
Fleming TP, Velazquez MA, Eckert JJ. Embryos, DOHaD and David Barker. J Dev Orig Health Dis. 2015;6(5):377–83.
Sathishkumar K, Elkins R, Yallampalli U, Yallampalli C. Protein restriction during pregnancy induces hypertension and impairs endothelium-dependent vascular function in adult female offspring. J Vasc Res. 2009;46(3):229–39.
Jang H, Serra C. Nutrition, Epigenetics, and Diseases. Clin Nutri Res. 2014;3(1):1–8.
Leese HJ. Metabolism of the preimplantation embryo: 40 years on. Reproduction. 2012;143(4):417–27.
Sun C, Denisenko O, Sheth B, Cox A, Lucas ES, Smyth NR, Fleming TP. Epigenetic regulation of histone modifications and Gata6 gene expression induced by maternal diet in mouse embryoid bodies in a model of developmental programming. BMC Dev Biol. 2015;15:3.
Tamashiro KL, Terrillion CE, Hyun J, Koenig JI, Moran TH. Prenatal stress or high-fat diet increases susceptibility to diet-induced obesity in rat offspring. Diabetes. 2009;58(5):1116–25.
Wyman A, Pinto AB, Sheridan R, Moley KH. One-cell zygote transfer from diabetic to nondiabetic mouse results in congenital malformations and growth retardation in offspring. Endocrinology. 2008;149(2):466–9.
Aubert R, Suquet JP, Lemonnier D. Long-term morphological and metabolic effects of early under- and over-nutrition in mice. J Nutr. 1980;110(4):649–61.
Lillycrop KA, Burdge GC. Environmental challenge, epigenetic plasticity and the induction of altered phenotypes in mammals. Epigenomics. 2014;6(6):623–36.
Lillycrop KA, Burdge GC. Maternal diet as a modifier of offspring epigenetics. J Dev Orig Health Dis. 2015;6(2):88–95.
Gu L, Liu H, Gu X, Boots C, Moley KH, Wang Q. Metabolic control of oocyte development: linking maternal nutrition and reproductive outcomes. Cell Mol Life Sci. 2015;72(2):251–71.
Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, Schedl T, Moley KH. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS One. 2012;7(11):e49217.
Moley KH, Vaughn WK, DeCherney AH, Diamond MP. Effect of diabetes mellitus on mouse pre-implantation embryo development. J Reprod Fertil. 1991;93(2):325–32.
Fleming TP, Watkins AJ, Sun C, Velazquez MA, Smyth NR, Eckert JJ. Do little embryos make big decisions? How maternal dietary protein restriction can permanently change an embryo. Reprod Fertil Dev. 2015.
Watkins AJ, Wilkins A, Cunningham C, Perry VH, Seet MJ, Osmond C, Eckert JJ, Torrens C, Cagampang FR, Cleal J, Gray WP, Hanson MA, Fleming TP. Low protein diet fed exclusively during mouse oocyte maturation leads to behavioural and cardiovascular abnormalities in offspring. J Physiol. 2008;586(8):2231–44.
Eckert JJ, Porter R, Watkins AJ, Burt E, Brooks S, Leese HJ, Humpherson PG, Cameron IT, Fleming TP. Metabolic induction and early responses of mouse blastocyst developmental programming following maternal low protein diet affecting life-long health. PLoS One. 2012;7(12):e52791.
Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127(19):4195–202.
Lillycrop KA, Slater-Jefferies JL, Hanson MA, Godfrey KM, Jackson AA, Burdge GC. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br J Nutr. 2007;97(6):1064–73.
Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135(6):1382–6.
Altobelli G, Bogdarina IG, Stupka E, Clark AJ, Langley-Evans S. Genome-wide methylation and gene expression changes in newborn rats following maternal protein restriction and reversal by folic acid. PLoS One. 2013;8(12):e82989.
Blesson CS, Sathishkumar K, Chinnathambi V, Yallampalli C. Gestational protein restriction impairs insulin-regulated glucose transport mechanisms in gastrocnemius muscles of adult male offspring. Endocrinology. 2014;155(8):3036–46.
Anckaert E, Romero S, Adriaenssens T, Smitz J. Effects of low methyl donor levels in culture medium during mouse follicle culture on oocyte imprinting establishment. Biol Reprod. 2010;83(3):377–86.
Burdge GC, Lillycrop KA. Folic acid supplementation in pregnancy: are there devils in the detail? Br J Nutr. 2012;108(11):1924–30.
Steegers-Theunissen RP, Twigt J, Pestinger V, Sinclair KD. The periconceptional period, reproduction and long-term health of offspring: the importance of one-carbon metabolism. Hum Reprod Update. 2013;19(6):640–55.
Kwong WY, Adamiak SJ, Gwynn A, Singh R, Sinclair KD. Endogenous folates and single-carbon metabolism in the ovarian follicle, oocyte and pre-implantation embryo. Reproduction. 2010;139(4):705–15.
Grindler NM, Moley KH. Maternal obesity, infertility and mitochondrial dysfunction: potential mechanisms emerging from mouse model systems. Mol Hum Reprod. 2013;19(8):486–94.
Seidler EA, Moley KH. Metabolic determinants of mitochondrial function in oocytes. Semin Reprod Med. 2015.
Perez GI, Trbovich AM, Gosden RG, Tilly JL. Mitochondria and the death of oocytes. Nature. 2000;403(6769):500–1.
Chappel S. The role of mitochondria from mature oocyte to viable blastocyst. Obstet Gynecol Int. 2013;2013:183024.
Piko L, Matsumoto L. Number of mitochondria and some properties of mitochondrial DNA in the mouse egg. Dev Biol. 1976;49(1):1–10.
Ben-Meir A, Burstein E, Borrego-Alvarez A, Chong J, Wong E, Yavorska T, Naranian T, Chi M, Wang Y, Bentov Y, Alexis J, Meriano J, Sung HK, Gasser DL, Moley KH, Hekimi S, Casper RF, Jurisicova A. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015;14(5):887–95.
Wang Q, Ratchford AM, Chi MM, Schoeller E, Frolova A, Schedl T, Moley KH. Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol Endocrinol. 2009;23(10):1603–12.
Blackstone C, Chang CR. Mitochondria unite to survive. Nat Cell Biol. 2011;13(5):521–2.
Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11(12):872–84.
Chang CR, Blackstone C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann N Y Acad Sci. 2010;1201:34–9.
Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8(10):939–44.
Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, Herzig S, Da Cruz S, Clerc P, Raschke I, Merkwirth C, Ehses S, Krause F, Chan DC, Alexander C, Bauer C, Youle R, Langer T, Martinou JC. SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J. 2009;28(11):1589–600.
Gomes LC, Di Benedetto G, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol. 2011;13(5):589–98.
Kalhan SC, Hanson RW. Resurgence of serine: an often neglected but indispensable amino acid. J Biol Chem. 2012;287(24):19786–91.
Sekhar RV, Patel SG, Guthikonda AP, Reid M, Balasubramanyam A, Taffet GE, Jahoor F. Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation. Am J Clin Nutr. 2011;94(3):847–53.
Jahoor F, Badaloo A, Reid M, Forrester T. Sulfur amino acid metabolism in children with severe childhood undernutrition: methionine kinetics. Am J Clin Nutr. 2006;84(6):1400–5.
Kurpad AV, Anand P, Dwarkanath P, Hsu JW, Thomas T, Devi S, Thomas A, Mhaskar R, Jahoor F. Whole body methionine kinetics, transmethylation, transulfuration and remethylation during pregnancy. Clin Nutr. 2014;33(1):122–9.
Stanger BZ. Cellular homeostasis and repair in the mammalian liver. Annu Rev Physiol. 2015;77:179–200.
Mato JM, Martínez-Chantar ML, Lu SC. S-adenosylmethionine metabolism and liver disease. Ann Hepatol. 2013;12(2):183–9.
Nutrition NRCUSoLA. Nutrient Requirements of the Laboratory Rat. 1995.
Feng J, Fitz Y, Li Y, Fernandez M, Cortes Puch I, Wang D, Pazniokas S, Bucher B, Cui X, Solomon SB. Catheterization of the carotid artery and jugular vein to perform hemodynamic measures, infusions and blood sampling in a conscious rat model. J Vis Exp. 2015;(95).
Badaloo A, Hsu JW, Taylor-Bryan C, Green C, Reid M, Forrester T, Jahoor F. Dietary cysteine is used more efficiently by children with severe acute malnutrition with edema compared with those without edema. Am J Clin Nutr. 2012;95(1):84–90.
Jahoor F, Badaloo A, Reid M, Forrester T. Sulfur amino acid metabolism in children with severe childhood undernutrition: cysteine kinetics. Am J Clin Nutr. 2006;84(6):1393–9.
MacCoss MJ, Fukagawa NK, Matthews DE. Measurement of intracellular sulfur amino acid metabolism in humans. Am J Physiol Endocrinol Metab. 2001;280(6):E947–55.
Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum Dev. 2006;82(8):485–91.
Roseboom TJ, Painter RC, van Abeelen AF, Veenendaal MV, de Rooij SR. Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas. 2011;70(2):141–5.
Wang N, Wang X, Li Q, Han B, Chen Y, Zhu C, Lin D, Wang B, Jensen MD, Lu Y. The famine exposure in early life and metabolic syndrome in adulthood. Clin Nutr. 2017;36(1):253–9.
Griffin J, Emery BR, Huang I, Peterson CM, Carrell DT. Comparative analysis of follicle morphology and oocyte diameter in four mammalian species (mouse, hamster, pig, and human). J Exp Clin Assist Reprod. 2006;3:2.
Wang Q, Chi MM, Schedl T, Moley KH. An intercellular pathway for glucose transport into mouse oocytes. Am J Physiol Endocrinol Metab. 2012;302(12):E1511–8.
Sturmey RG, Brison DR, Leese HJ. Symposium: innovative techniques in human embryo viability assessment. Assessing embryo viability by measurement of amino acid turnover. Reprod BioMed Online. 2008;17(4):486–96.
Zhang L, Han L, Ma R, Hou X, Yu Y, Sun S, Xu Y, Schedl T, Moley KH, Wang Q. Sirt3 prevents maternal obesity-associated oxidative stress and meiotic defects in mouse oocytes. Cell Cycle. 2015;14(18):2959–68.
Thompson JG, Lane M, Gilchrist RB. Metabolism of the bovine cumulus-oocyte complex and influence on subsequent developmental competence. Soc Reprod Fertil Suppl. 2007;64:179–90.
Acknowledgements
Thank you to Baylor College of Medicine, Texas Children’s Hospital and the Children’s Nutrition Research Center for their collaborative support.
Funding
This work was supported by National Institutes of Health grants for C.Y. (HL102866 and HL58144). Supported by federal funds from the USDA, Agricultural Research Service, under Cooperative Agreement 58–6250-6001.
Availability of data and materials
All data generated or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
AS and CB were integral in all aspects of experimental design, execution, analysis, interpretation and writing of the manuscript. JH analyzed and interpreted the data regarding amino acid metabolism and was a major contributor in writing the manuscript. FJ and CY were integral in experimental design, infusion methodology, data analysis and interpretation. CT and WG were integral in experimental design and were major contributors in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Author’s information
AS: Qualifications include MD, MSCI (Master of Science in Clinical Investigation). Assistant Professor of Reproductive Endocrinology and Infertility in the Department of Obstetrics and Gynecology at the Baylor College of Medicine in Houston, Texas. Completed thesis work for MSCI in the laboratory of CY studying developmental programming.
CB: Qualifications include PhD. Reproductive Biologist and Clinical Embryologist at the Baylor College of Medicine. Specializes in reproductive endocrinology and metabolism with extensive experience investigating the developmental origins of disease and its role in reproduction and metabolism.
JH: Qualifications include PhD. Staff Scientist at the Baylor College of Medicine working in the lab of FJ studying intermediary metabolism in different pathological states.
CV: Qualifications include MD. Associate Professor of Reproductive Endocrinology and Infertility in the Department of Obstetrics and Gynecology at the Baylor College of Medicine in Houston, Texas. Experience with translational metabolic research.
WEG: Qualifications include MD. Professor, Division Director of Reproductive Endocrinology and Infertility in the Department of Obstetrics and Gynecology at the Baylor College of Medicine in Houston, Texas.
FJ: Qualifications include PhD. Professor of Pediatrics and Nutrition at the Baylor College of Medicine with international expertise in intermediary metabolism in different pathological states. Extensive experience with infusion-based amino acid metabolism experimental designs.
CY: Qualifications include DVM, PhD. The Yallampalli lab conducts basic science and perinatology research with a focus on fetal programming of adult health and diseases. The Yallampalli lab utilizes the low-protein diet model to study its effects before and during pregnancy and assesses the consequences in gametes and offspring.
Ethics approval and consent to participate
Baylor College of Medicine Institutional Review Board (IRB) and the Institutional Animal Care and Use Committee (IACUC) approved this protocol.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Schutt, A.K., Blesson, C.S., Hsu, J.W. et al. Preovulatory exposure to a protein-restricted diet disrupts amino acid kinetics and alters mitochondrial structure and function in the rat oocyte and is partially rescued by folic acid. Reprod Biol Endocrinol 17, 12 (2019). https://doi.org/10.1186/s12958-019-0458-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12958-019-0458-y