Abstract
Background
Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis (SJS/TEN) is the most Serious Cutaneous Adverse Reaction (SCAR) often with a fatal outcome. Coronavirus Disease (COVID-19) is caused by Severe Acute Respiratory Syndrome–Coronavirus—2 (SARS-COV2) and is an emergent pandemic for which no cure exist at the moment. Several drugs have been tried often with scant clinical evidence and safety.
Case presentation
Here we report the case of 78-years-old woman with cardiometabolic syndrome and COVID-19. A multidrug regimen including others hydroxychloroquine, antibiotics, dexamethasone and paracetamol, low-molecular-weight-heparin and potassium canrenoate was started. After almost 3 weeks, the patient started to display a violaceous rash initially involving the flexural folds atypical targetoid lesions and showing a very fast extension, blister formation and skin detachments of approximately 70% of the total body surface area and mucous membranes involvement consistent with toxic epidermal necrolysis (TEN). The ALDEN algorithm was calculated inserting all drugs given to the patient in the 28 days preceding the onset of the skin manifestations. The highest score retrieved was for hydroxychloroquine. Other less suspicious drugs were piperacillin/tazobactam, ceftriaxone and levofloxacin.
Conclusions
To our knowledge, this is the first case of TEN in a patient suffering from COVID-19 probably associated with hydroxychloroquine. Given the activation of the immune system syndrome induced by the virus and the widespread off-label use of this drug, we suggest a careful monitoring of skin and mucous membranes in all COVID-19 positive patients treated with hydroxychloroquine in order to early detect early signs of toxicities.
Similar content being viewed by others
Background
Toxic Epidermal Necrolysis (TEN) is a rare Serious Cutaneous Adverse Reaction (SCAR). It displays an acute onset and is characterized by erythematous or violaceous patches, atypical targetoid lesions, bullae, erosions and skin detachment. It differs from Stevens-Johnson Syndrome (SJS) only in the percentage of skin involvement, which in TEN is greater than 30% of the body surface [1]. Etiopathogenetically, it results from the combination of drug and host genetic factors (such as drug metabolism and T cell clonotypes) resulting in a delayed-type hypersensitivity reaction, where drug or drug-peptide complexes are recognized by T cell receptors [2].
Coronavirus Disease (COVID-19) is caused by Severe Acute Respiratory Syndrome–Coronavirus—2 (SARS-CoV-2) and can be potentially fatal disease [3]. The most common symptoms at onset of COVID-19 illness are fever, cough, and fatigue, followed by dyspnea and diarrhea [4]. Lymphopenia, elevation of creatinine kinase (CK), lactate dehydrogenase (LDH) are also frequently observed. The lung involvement is frequently that of an interstitial pneumonia, COVID-19 later shows an over exuberant inflammatory response with a no correlation between viral load and the worsening of symptoms [5].
At the moment there is no effective cure for COVID-19, several drugs often in combination have been used, often with scant clinical evidence or conflicting results, with the aim of targeting the virus replication and/or the inflammatory process, such as anti-retrovirals, macrolides, hydroxychloroquine and monoclonal antibodies targeted on inflammatory cytokines [6,7,8,9]. There is limited data establishing their safety profile during SARS-CoV-2 infection.
Case presentation
Here we report the case of 78-year old woman with several comorbidities (hypertension, obesity, unstable angina, type 2 diabetes) admitted to our hospital for respiratory insufficiency with fever requiring non-invasive ventilation and a diagnosis of COVID-19 lung infection with bacterial superinfection was formulated given the radiological evidence of a bilateral interstitial pneumonia, the positivity of nasopharyngeal swab for SARS-CoV-2 and (Fig. 1). Lymphopenia (0.55 103/µL [1.00–4.00]), elevation of CK (252 U/L [24–170]) and LDH (407 U/L [30–250]) were also observed.
A multidrug regimen with hydroxychloroquine 200 mg twice a day, sodium enoxaparin and dexamethasone were started. Besides, an antibiotic treatment with ceftriaxone for 1 day, followed by piperacillin/tazobactam for 4 days was given. The patient was initially treated in a sub-intensive care unit and after the favorable clinical evolution was transferred to our general medicine division, 18 days after the admission. At the arrival in our unit, the patient started to display a violaceous erythematous rash mainly involving the flexural folds. The patient was still taking hydroxychloroquine, along with levofloxacin (started the day before); other drugs were reported in Table 1.
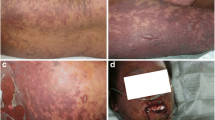
The rash presented in the course of 3 days a rapid extension with the involvement of the whole trunk and buttocks, reaching approximately 70% of the total body surface area, with the appearance of atypical targetoid lesions and the formation of blisters, with subsequent skin detachment (Figs. 2, 3 and 4). Nikolsky’s sign was present. A severe desquamation of the buccal and nasal mucosa was also observed. The patient referred severe skin pain requiring morphine. Blood tests did not show eosinophilia or alterations of liver and renal function tests.
A clinical diagnosis of toxic epidermal necrolysis (TEN) also confirmed by the dermatologist was formulated. Methylprednisone 1 mg/kg, as an intravenous bolus regiment was administred along with intravenous immunoglobulin (IVIG) 1 mg/kg for 3 days. Then oral prednisone 1 mg/kg daily was given and subsequently tapered in 1 month.
To identify the culprit drug, the ALDEN algorithm was calculated (Table 1) [10] (Additional file 1). The highest score retrieved was for hydroxychloroquine, with a possible correlation (+4 score). Other less suspicious drugs were piperacillin/tazobactam, ceftriaxone and levofloxacin. All suspected drugs according to the ALDEN algorithm were promptly stopped (Additional file 2).
The patient was cared for by the wound care service of the hospital with topical therapy, a marked improvement in the skin conditions was observed progressively over a period of 6 weeks, until the complete resolution (Figs. 2 and 3). Paracetamol, pantoprazole, enoxaparin were tolerated after the reaction, ruling out their correlation with the skin reaction.
Due to the COVID-19 epidemic, no skin biopsies were technically feasible for logistical problems. However, the clinical manifestations were highly compelling for SJS/TEN. Other differential diagnosis included Drug-Reaction with Eosinophilia and Systemic Symptoms (DRESS), which was excluded given the absence of eosinophilia and internal organ involvement, Staphylococcal Scalded Skin Syndrome (SSSS), which is a pediatric form, and bullous dermatoses that were ruled out in our case given the minor mucous involvement and intense skin inflammation.
Discussion
SJS/TEN is the most severe SCAR being associated with a high mortality rate [1]. It is a delayed reaction occurring after 4–28 days from drug exposure, therefore it is of paramount importance to acquire precise retrospective pharmacological information for a long period of time preceding the onset of skin manifestations.
STS/TEN has an approximate incidence of 1 or 2 cases/1,000,000 annually. It results from the clonal expansion of CD8 + cytotoxic T lymphocytes (CTLs) and Natural Killer cell (NK),which are major histo-compatibility complex (MHC)-restricted and induce epidermal apoptosis [1, 11, 12]. Recently, it has been shown that low molecular weight drugs can directly bind the T cell receptor (TCR) of T cells or the Human Leukocyte Antigen (HLA) of antigen-presenting cells [13,14,15,16].
Usually implicated drugs are allopurinol, anticonvulsant drugs nonsteroidal anti-inflammatory drugs (NSAIDs), antibiotics such as cephalosporins, aminopenicillins and rarely macrolides, as shown in the EuroSCAR study, together with the REACT project and the regiSCAR project [17,18,19,20,21].
The ALDEN algorithm represents the gold standard tool in SJS/TEN to identify the culprit drug and to discriminate it from the “innocent” drugs which can be safely administered. It gives to each drug taken by patient a score, ranging from −12 to +10, which corresponds to the probability of having caused the reaction, ranging from very unlikely to very probable [10]. Besides, from a recent analysis a significant statistic for agreement (kappa 0.571) was found between ALDEN score for related-drugs (ALDEN ≥ 4) and lymphocyte transformation test (LTT) results performed after recovery [22].
According to the ALDEN algorithm results, the most likely implicated drug in our case is hydroxychloroquine.
Hydroxychloroquine is an 4-aminoquinoline with a low molecular weight (333,9 Da) [23,24,25,26]. It has been used for decades to treat rheumatologic conditions (with a dose of 6 mg/kg) with a general good safety, even though in last years cases of skin reactions, also severe, have increasingly been reported [27].
We searched the Eudravigilance database and found 30 cases of TENs related to the use of hydroxychloroquine [28].
Hydroxychloroquine is being profusely used to treat SARS-CoV-2 infection with an unconventionally high dosage (from 200 mg twice a day to 200 mg three times a day, which is independent of body weight sometimes with a loading dose, due to its high volume of distribution).
Interestingly, some new severe skin manifestation to hydroxychloroquine have also been reported during the treatment of SARS-CoV-2 positive patient [29,30,31].
In this instance, it is not to be discounted that a rare side effect, such as TEN, occurring with a not commonly associated drug, hydroxychloroquine, could have been favored by the particular immune stimulation induced by the virus SARS-CoV-2 [3]. SJS/TEN, and SCAR more generally, have been classically demonstrated to be associated with viral replication [11, 32,33,34].
To our knowledge, this is the first case of TEN in a COVID-19 positive patient.
Another salient aspect of the case is the favorable evolution of the patient given that this type of SCAR is typically associated with a bad prognosis [35,36,37], even more so because the patient displayed all the negative typical prognostic factors also for COVID-19 [38,39,40,41], indeed the calculation of the severity-of-illness score for toxic epidermal necrolysis (SCORTEN) in our patient led to an estimated mortality rate of 58.3% (CI 36,6 –77,59) (Table 2) [42, 43].
It is possible that the prompt diagnosis of TEN, the suspension of all the suspected drugs (in particularly hydroxychloroquine) and the administration of steroid and IVIG, both targeting the inflammatory syndrome initially triggered by the virus, may have had contributed to a better prognosis, [1, 5].
Further studies are needed to assess the safety profile of hydroxychloroquine in patients with COVID-19.
Conclusion
To our knowledge, this is the first case of TEN associated with hydroxychloroquine in patient suffering from COVID-19. Given the widespread off-label use of this drug, the high degree of immune activation induced by the virus and the recent increase of hydroxychloroquine-related SCARs, we believe that it is necessary to adequately monitor the skin in all COVID-19 positive patients treated with hydroxychloroquine in order to early detect early signs of toxicities.
Availability of data and materials
Not applicable.
Abbreviations
- CK:
-
Creatinine Kinase
- COVID-19:
-
COronaVIrus Disease 19
- CTLs:
-
Cytotoxic T Lymphocytes
- DRESS:
-
Drug Reaction with Eosinophilia and Systemic Symptoms
- HLA:
-
Human Leukocyte Antigen
- IVIG:
-
IntraVenous Immunoglobulin
- LDH:
-
Lactate Dehydrogenase
- LTT:
-
Lymphocyte Transformation Test
- MHC:
-
Major Histocompatibility Complex
- NK:
-
Natural Killer
- NSAIDs:
-
NonSteroidal Anti-Inflammatory Drugs
- SARS-CoV-2:
-
Severe Acute Respiratory Syndrome– - Coronavirus—2
- SCAR:
-
Severe Cutaneous Adverse Reaction
- SCORTEN:
-
Severity-of-illness Score for Toxic Epidermal Necrolysis
- SSSS:
-
Staphylococcal Scalded Skin Syndrome
- SJS:
-
Stevens Johnson Syndrome
- TCR:
-
T-Cell Receptor
- TEN:
-
Toxic Epidermal Necrolysis
References
Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010. https://doi.org/10.1186/1750-1172-5-39.
Su SC, Chung WH. Cytotoxic proteins and therapeutic targets in severe cutaneous adverse reactions. Toxins. 2013. https://doi.org/10.3390/toxins6010194.
Rothan HA, Byrareddy SN. The epidemeology and pathogensis of coronavirus (Covid-19) outbreak. J Autoimmun. 2020. https://doi.org/10.1016/j.jaut.2020.102433.
Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, Cina. Lancet. 2019. https://doi.org/10.1016/S0140-6736(20)30183-5.
Stebbing J, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet. 2020. https://doi.org/10.1016/S1473-3099(20)30132-8.
Costanzo M, De Giglio MAR, Roviello GN. SARS CoV-2: recent Reports on antiviral therapies based on lopinavir/ritonavir, darunavir/umifenovir, hydroxychloroquine, remdesivir, favipiravir and other drugs for the treatment of the new coronavirus. Curr Med Chem. 2020. https://doi.org/10.2174/0929867327666200416131117.
Yao X, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2(SARS-CoV-2). Clin Infect Dis. 2020;2:1–25.
Cao B, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020. https://doi.org/10.1056/NEJMoa2001282.
van Kraaij T, et al. Tocilizumab in severe COVID-19 pneumonia and concomitant cytokine release syndrome. Eur J Case Reports Intern Med. 2017. https://doi.org/10.12890/2020_001675.
Sassolas B, et al. ALDEN, an algorithm for assessment of drug causality in stevens-johnson syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther. 2010. https://doi.org/10.1038/clpt.2009.252.
Honma M, et al. Toxic epidermal necrolysis with prominent facial pustules: a case with reactivation of human herpesvirus 7. Dermatology. 2010. https://doi.org/10.1159/000319756.
Abe R, Shimizu T, Shibaki A, Nakamura H, Watanabe H, Shimizu H. Toxic epidermal necrolysis and Stevens-Johnson syndrome are induced by soluble fas ligand. Am J Pathol. 2003. https://doi.org/10.1016/S0002-9440(10)64284-8.
Pichler WJ. Immune pathomechanism and classification of drug hypersensitivity. Allergy Eur. J. Allergy Clin Immunol. 2019. https://doi.org/10.1111/all.13765.
Pichler WJ, Hausmann O. Classification of drug hypersensitivity into allergic, p-i, and pseudo-allergic forms. Int Arch Allergy Immunol. 2017. https://doi.org/10.1159/000453265.
Correia O. Cutaneous T-cell recruitment in toxic epidermal necrolysis. Arch Dermatol. 1993. https://doi.org/10.1001/archderm.1993.01680250078010.
Dodiuk-Gad RP, Chung WH, Valeyrie-Allanore L, Shear NH. Stevens-johnson syndrome and toxic epidermal necrolysis: an update. Am J Clin Dermatol. 2015. https://doi.org/10.1007/s40257-015-0158-0.
De Luca F, et al. Tolerated drugs in subjects with severe cutaneous adverse reactions (SCARs) induced by anticonvulsants and review of the literature. Clin Mol Allergy. 2017. https://doi.org/10.1186/s12948-017-0072-5.
Techasatian L, Panombualert S, Uppala R, Jetsrisuparb C. Drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in children: 20 years study in a tertiary care hospital. World J Pediatr. 2017. https://doi.org/10.1007/s12519-016-0057-3.
Frey N, Bodmer M, Bircher A, Jick SS, Meier CR, Spoendlin J. Stevens-johnson syndrome and toxic epidermal necrolysis in association with commonly prescribed drugs in outpatient care other than anti-epileptic drugs and antibiotics: a population-based case-control study. Drug Saf. 2019. https://doi.org/10.1007/s40264-018-0711-x.
Diphoorn J, et al. Incidence, causative factors and mortality rates of stevens-johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) in Northern Italy: data From the REACT Registry. Pharmacoepidemiol Drug Saf. 2016. https://doi.org/10.1002/pds.
Mockenhaupt M. “RegiSCAR project,” Last, 2007. http://www.regiscar.org/ita/Project.html.
Bellón T, et al. Assessment of drug causality in Stevens-Johnson syndrome/toxic epidermal necrolysis: concordance between lymphocyte transformation test and ALDEN. Allergy Eur J Allergy Clin Immunol. 2020. https://doi.org/10.1111/all.14062.
Lim HS, et al. Pharmacokinetics of hydroxychloroquine and its clinical implications in chemoprophylaxis against malaria caused by plasmodium vivax. Antimicrob Agents Chemother. 2009. https://doi.org/10.1128/AAC.00339-08.
Furst DE. Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases. Lupus. 1996;5(Suppl 1):S11–5.
F. D. A. Food and Drug Administration.FDA Approved Drug Products: Hydroxychloroquine Oral Tablets.2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/009768s037s045s047lbl.pdf. Accessed 15 May 2020.
Torigoe M, Sakata K, Ishii A, Iwata S, Nakayamada S, Tanaka Y. Hydroxychloroquine efficiently suppresses inflammatory responses of human class-switched memory B cells via Toll-like receptor 9 inhibition. Clin Immunol. 2018. https://doi.org/10.1016/j.clim.2018.07.003.
Sharma AN, Mesinkovska NA, Paravar T. Characterizing the adverse dermatologic effects of hydroxychloroquine: a systematic review. J Am Acad Dermatol. 2020. https://doi.org/10.1016/j.jaad.2020.04.024.
E. M. A. European Medicines Agency. EudraVigilance Oped Data ADR hydroxychloroquine. 2020. https://bi.ema.europa.eu/analyticsSOAP/saw.dll?PortalPages.
Grandolfo M, et al. Drug reaction with eosinophilia and systemic symptoms syndrome to hydroxychloroquine, an old drug in the spotlight. Dermatol Ther. 2020. https://doi.org/10.1111/dth.13499.
Schwartz RA, Janniger CK. Generalized pustular figurate erythema: a newly delineated severe cutaneous drug reaction linked with hydroxychloroquine. Dermatol Ther. 2020. https://doi.org/10.1111/dth.13380.
Whitworth AL, Mann NH, Larkum AWD. Cutaneous side-effects of the potential COVID-19 drugs. Ultrasound Obs Gynecol. 2006. https://doi.org/10.1111/1462-2920.12735.
Peter J, Choshi P, Lehloenya RJ. Drug hypersensitivity in HIV infection. Curr Opin Allergy Clin Immunol. 2019. https://doi.org/10.1097/ACI.0000000000000545.
Yang CW, Cho YT, Hsieh YC, Hsu SH, Chen KL, Chu CY. The interferon-γ-induced protein 10/CXCR3 axis is associated with human herpesvirus-6 reactivation and the development of sequelae in drug reaction with eosinophilia and systemic symptoms. Br J Dermatol. 2020. https://doi.org/10.1111/bjd.18942.
Tagajdid MR, Doblali T, Elannaz H, Hammi S, Belfequih B, Mrani S. Reactivation of cytomegalovirus in a patient with Stevens-Johnson syndrome-toxic epidermal necrolysis. Iran J Med Sci. 2013;38(Suppl 2):195–7.
Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: Part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013. https://doi.org/10.1016/j.jaad.2013.05.002.
Papp A, et al. Treatment of toxic epidermal necrolysis by a multidisciplinary team A review of literature and treatment results. Burns. 2018. https://doi.org/10.1016/j.burns.2017.10.022.
Miliszewski MA, Kirchhof MG, Sikora S, Papp A, Dutz JP. Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: an Analysis of Triggers and Implications for Improving Prevention. Am J Med. 2016. https://doi.org/10.1016/j.amjmed.2016.03.022.
Di Stadio A, Ricci G, Greco A, de Vincentis M, Ralli M. Mortality rate and gender differences in COVID-19 patients dying in italy a comparison with other countries. Eur Rewiew Med Pharmacol Sci. 2020. https://doi.org/10.1056/nejmoa2004500.
I. S. S. Istituto Superiore di Sanità and I. S. T. A. T. Istituto Nazionale di Statistica. Impatto dell’epidemia covid-19 sulla mortalità totale della popolazione residente primo trimestre 2020. https://www.epicentro.iss.it/coronavirus/pdf/Rapporto_Istat_ISS.pdf. Accessed 15 May 2020.
D. P. C. DipartimentoProtezione Civile. COVID-19: Monitoraggio della situazione. 2020. http://opendatadpc.maps.arcgis.com/apps/opsdashboard/index.html#/b0c68bce2cce478eaac82fe38d4138b1. Accessed 15 May 2020.
Guan WJ, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2019. https://doi.org/10.1056/NEJMoa2002032.
Guégan S, Bastuji-Garin S, Poszepczynska-Guigné E, Roujeau JC, Revuz J. Performance of the SCORTEN during the first five days of hospitalization to predict the prognosis of epidermal necrolysis. J Invest Dermatol. 2006. https://doi.org/10.1038/sj.jid.5700068.
Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. Scorten: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000. https://doi.org/10.1046/j.1523-1747.2000.00061.x.
Acknowledgements
Not applicable.
Funding
The authors declare that they have not received any funding.
Author information
Authors and Affiliations
Contributions
CMR and FNB collected data. CMR and FNB wrote the draft paper and analyzed the data. CMR, GT and SM followed clinical course of the patient. DZ revised the draft paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Informed consent to clinical data collection and publication was obtained from the patient.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1:
Details regarding the calculation of the ALDEN score [10].
Additional file 2:
Timing of the drugs administered to the patients regarding the calculation of the ALDEN score [10].
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rossi, C.M., Beretta, F.N., Traverso, G. et al. A case report of toxic epidermal necrolysis (TEN) in a patient with COVID-19 treated with hydroxychloroquine: are these two partners in crime?. Clin Mol Allergy 18, 19 (2020). https://doi.org/10.1186/s12948-020-00133-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12948-020-00133-6