Abstract
Background
The association of a Classical left bundle branch block (LBBB) contraction pattern and better outcome after cardiac resynchronization therapy (CRT) has only been studied using vendor-specific software for echocardiographic speckle-tracked longitudinal strain analysis. The purpose of this study was to assess whether a Classical LBBB contraction pattern on longitudinal strain analysis using vendor-independent software is associated with clinical outcome in CRT recipients with LBBB.
Methods
This was a retrospective cohort study including CRT recipients with LBBB, heart failure, and left ventricular (LV) ejection fraction ≤35%. Speckle-tracked echocardiographic longitudinal strain analysis was performed retrospectively on echocardiograms using vendor-independent software. The presence of a Classical LBBB contraction pattern was determined by consensus of two readers. The primary end point was a composite of time to death, heart transplantation or LV assist device implantation. Secondary outcome was ≥15% reduction in LV end-systolic volume. Intra- and inter-reader agreement of the longitudinal strain contraction pattern was assessed by calculating Cohen’s κ.
Results
Of 283 included patients, 113 (40%) were women, mean age was 66 ± 11 years, and 136 (48%) had ischemic heart disease. A Classical LBBB contraction pattern was present in 196 (69%). The unadjusted hazard ratio for reaching the primary end point was 1.93 (95% confidence interval, 1.36–2.76, p < 0.001) when comparing patients without to patients with a Classical LBBB contraction pattern. Adjusted for ischemic heart disease and QRS duration < 150 milliseconds the hazard ratio was 1.65 (95% confidence interval, 1.12–2.43, p = 0.01). Of the 123 (43%) patients with a follow-up echocardiogram, 64 of 85 (75%) of patients with a Classical LBBB contraction pattern compared to 13 of 38 (34%) without, had ≥15% reduction in LV end-systolic volume (p < 0.001). Cohen’s κ were 0.86 (95% confidence interval, 0.71–1.00) and 0.42 (95% confidence interval, 0.30–0.54) for intra- and inter-reader agreement, respectively.
Conclusion
Using vendor-independent strain software, a Classical LBBB contraction pattern is associated with better outcome in CRT recipients with LBBB, but inter-reader agreement for the classification of contraction pattern is only moderate.
Similar content being viewed by others
Introduction
Cardiac resynchronization therapy (CRT) is an effective treatment for patients with heart failure with reduced ejection fraction and left bundle branch block (LBBB). [1, 2] Unfortunately, a substantial proportion of patients receiving CRT do not improve their functional or echocardiographic status. [3, 4] In recent years, a Classical (also called Typical) LBBB contraction pattern on two-dimensional speckle-tracked echocardiographic longitudinal strain analysis has been associated with improved echocardiographic function and survival free of heart transplantation or left ventricular (LV) assist device implantation in CRT recipients independent of QRS duration and ischemic etiology. [5, 6] However, these studies were performed using vendor-specific software for analysis of prospectively acquired high frequency speckle-tracked strain images, and the results have not been replicated using vendor-independent software analysis of images acquired using standard acquisition protocols. While vendor-independent software correlates well with different vendor-specific software for assessment of global longitudinal strain, [7, 8] assessment of specific contraction patterns may not be equally feasible. The use of vendor-independent software can be required in certain situations, e.g. in health centers with multiple vendors of echocardiography systems, or if retrospective analysis on images saved in Digital Imaging and Communications in Medicine (DICOM) format is needed.
The aim of this study was to assess whether the presence of a Classical LBBB contraction pattern on speckle-tracked longitudinal strain analysis using vendor-independent strain software was associated with better survival and echocardiographic response in CRT recipients with LBBB.
Methods
This was a retrospective cohort study performed at Duke University Hospital, a high-volume tertiary medical center.
Study population
All patients who received a CRT with defibrillator from April 2006 to September 2015 were identified using an institutional dataset prepared for the National Cardiovascular Data Registry. Patients were eligible for inclusion if they had symptomatic heart failure with LV ejection fraction ≤35%, an adequate baseline echocardiogram within 365 days and an available electrocardiogram (ECG) within 180 days before CRT implantation, QRS duration ≥120 milliseconds, and LBBB morphology. Patients were excluded if they had a prior CRT device, failed LV lead implantation, 2nd or 3rd degree atrioventricular block at baseline, or cardiac surgery or percutaneous coronary intervention between the baseline echocardiogram and CRT implantation. A subset of patients with a follow-up echocardiogram (ordered for clinical reasons) performed within 60–365 days after CRT implantation were included in secondary analyses.
Clinical data
Clinical data were obtained from the institutional dataset and through chart review. Patients were categorized as having a history of atrial fibrillation if they had a previous diagnosis of atrial fibrillation or they had atrial fibrillation on the baseline 12-lead ECG. Two authors (D.F. and K.E.), who were blinded to outcomes, reviewed all baseline ECGs and designated QRS morphologies. LBBB morphology was further classified as either strict or non-strict LBBB. [9]
Echocardiographic analyses
The most recent echocardiogram prior to CRT implantation was analyzed. Measurements of LV end-diastolic and -systolic volumes and two-dimensional speckle-tracked longitudinal strain analyses were performed in the vendor-independent software system ImageArena version 4.6 (TomTec Imaging Systems, Unterschleissheim, Germany) using apical 2-, 3-, and 4-chamber views stored in DICOM format. LV volumes were calculated using a modified Simpson’s triplane method included in the software. The apical 4-chamber view and at least one of the two other views were required for analysis.
For the analysis of longitudinal strain contraction pattern, QRS onset was set as the starting point when possible; when QRS onset was not available in the beginning of the loop, the earliest point available in the cardiac cycle was used. Endocardial borders were traced manually at end-systole, and tracking was assessed visually. If the tracking was considered inadequate, retracing was performed until tracking was deemed correct. The software itself did not provide any information about its assessment of tracking quality.
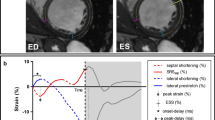
Longitudinal strain contraction patterns were classified as a “Classical LBBB contraction pattern” when the septal peak shortening occurred within the initial 70% of the ejection phase, and the lateral wall was initially stretched and had peak shortening after aortic valve closure (Fig. 1) as described by Risum et al. [5] Time from QRS onset to aortic valve opening and closure were measured on continuous or pulsed wave spectral Doppler images and manually set accordingly in the strain analysis. All longitudinal strain contraction patterns were read independently by two readers (P.S. and K.E.) blinded to outcome and clinical characteristics. In case of disagreement, the two readers studied the strain images in unison and classified the contraction pattern by consensus. This was done blinded to outcome, clinical characteristics and the initial classifications of each of the readers. The initial reads were used for assessment of inter-reader agreement. A small subgroup of patients had echocardiograms available for analysis of longitudinal strain contraction pattern using vendor-specific software (EchoPAC version 112, GE Healthcare, Chicago, IL, USA), and these were used for assessing agreement between vendor-independent and vendor-specific software.
Example of Classical LBBB contraction pattern. The features of a Classical LBBB contraction pattern are the following: 1) Peak shortening of the mid- and/or basal septum (light and dark red lines) within the initial 70% of the ejection phase (red arrow), 2) Initial stretch (blue arrow) of the mid- and/or basal lateral wall (light and dark green lines), and 3) late peak shortening after aortic valve closure (AVC - dotted line) of the mid- and/or basal lateral wall (yellow arrow). The apical segments are usually disregarded, when assessing the Classical LBBB contraction pattern, and therefore they have been omitted from this figure. The dots on each line mark the peak shortening of each segment
Outcomes and analyses
The primary end point was time from CRT implantation to the first event of either death of all causes, heart transplantation or LV assist device implantation. End points were assessed on May 24, 2017 through a query of Duke Enterprise Data Unified Content Explorer (DEDUCE) by incorporating data from hospital billing claims, hospital records, and the United States Social Security Death Index. [10]
Secondary analyses on the subgroup of patients with an eligible follow-up echocardiogram included echocardiographic response defined as a reduction in LV end-systolic volume ≥ 15%, along with relative changes in LV end-diastolic and end-systolic volumes and absolute changes in LV ejection fraction and global longitudinal strain from the baseline to the follow-up echocardiogram.
Statistical analyses
Normally distributed continuous variables are presented as mean ± standard deviation and differences were tested using the Student t test. Non-normally distributed continuous variables are presented as median (25th–75th percentile) and differences were tested using the Wilcoxon rank-sum test. Categorical variables are presented as n (%) and differences were tested using Fisher’s exact test.
Survival free from heart transplantation or LV assist device implantation are presented using Kaplan-Meier curves and differences were tested using the log-rank test. Cox proportional hazards regression was used to estimate hazard ratios in uni- and multivariable analysis of the primary end point. The primary multivariable model included the prespecified covariates QRS duration < 150 milliseconds and ischemic heart disease in accordance with previous literature. [6] A secondary, expanded multivariable model including age, gender, ischemic heart disease, QRS duration < 150 milliseconds, history of atrial fibrillation/flutter, New York Heart Association functional class, creatinine > 1.2 mg/dL, end-systolic global longitudinal strain and use of angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker was also performed to adjust for further potential confounding. Proportional hazards assumptions were checked visually by plotting Schoenfeld’s residuals against time since CRT implantation. No significant violations of the proportional hazards assumptions were observed.
Sensitivity and specificity of a Classical LBBB contraction pattern for echocardiographic response were calculated for the secondary analyses. Intra-reader agreement on longitudinal strain contraction pattern was assessed for one reader (K.E.) by reanalysis of 50 randomly selected patients at least 90 days after the initial read. Inter-reader agreement was assessed using all included patients. For intra- and inter-reader variability and agreement between vendor-independent and vendor-specific software, overall agreement and Cohen’s κ was calculated.
Sensitivity analyses excluding patients without sinus rhythm on the baseline echocardiogram and excluding patients < 25 frames per cardiac cycle were performed.
All statistical analyses were performed in RStudio version 1.1.453 (RStudio, Inc., Boston, MA, USA) running R version 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria). The R package “survival” was used for Cox proportional hazards models and to create Kaplan-Meier curves. [11] A two-sided p-value < 0.05 was considered statistically significant.
Results
A total of 1001 patients received a CRT device at Duke University Hospital during the period April 2006–September 2015, and 302 of the 1001 met the inclusion criteria. Of these, 283 did not meet any exclusion criteria and were thus included in the study cohort (Fig. 2). The mean age of included patients was 66 ± 11 years, 113 (40%) were women and 136 (48%) had ischemic heart disease. All but 10 (4%) patients had all three apical views available; 9 (3%) were missing the apical 3-chamber view, while 1 (< 1%) was missing the apical 2-chamber view. The median frame rate on analyzed images was 30 frames/second (30–46 frames/second) and the median number of frames per cardiac cycle was 27 (24–37).
Classical LBBB contraction pattern
Of the 283 included patients, 196 (69%) were classified as having a Classical LBBB contraction pattern. Patients without a classical LBBB contraction pattern were more likely to be male, have ischemic heart disease, have diabetes mellitus, had shorter QRS duration and higher creatinine level (Table 1). Patients with a Classical LBBB contraction pattern had larger LV end-systolic volume, lower LV ejection fraction, and lower end-systolic global longitudinal strain values (Table 2).
Primary outcome
A total of 131 (46%) patients reached the composite end point over a median follow-up of 2.8 years (1.8–5.3 years), with death being the most common cause for reaching the end point (n = 106, 37%). In patients without a Classical LBBB contraction pattern, 51 of 87 (59%) reached the end point compared to 80 of 196 (41%) of patients with a Classical LBBB contraction pattern (Fig. 3). In univariable Cox regression, the hazard ratio was 1.93 (95% confidence interval, 1.36–2.76, p < 0.001) for patients without compared to patients with a Classical LBBB contraction pattern. Adjusted for ischemic heart disease and QRS duration < 150 milliseconds, the hazard ratio was 1.65 (95% confidence interval, 1.12–2.43, p = 0.01). Additional adjustment for age, gender, history of atrial fibrillation, New York Heart Association functional class, creatinine > 1.2 mg/dL, end-systolic global longitudinal strain and angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use yielded a hazard ratio of 1.54 (95% confidence interval, 1.01–2.35, p = 0.046).
Kaplan-Meier plots for patients with and without a Classical LBBB contraction pattern. Survival free of heart transplantation or LVAD implantation for patients with and without a Classical LBBB contraction pattern
CRT = cardiac resynchronization therapy; LBBB = left bundle branch block; LVAD = left ventricular assist device.
Sensitivity analysis excluding patients without sinus rhythm on the baseline echocardiogram (n = 41, 14%) yielded an adjusted hazard ratio of 1.80 (95% confidence interval, 1.18–2.77). Excluding patients with < 25 frames per cardiac cycle (n = 88, 31%) resulted in an adjusted hazard ratio of 1.87 (95% confidence interval, 1.16–3.01). Excluding both patients without sinus rhythm and with < 25 frames per cardiac cycle on the baseline echocardiogram yielded an adjusted hazard ratio of 1.91 (95% confidence interval, 1.14–3.19).
Echocardiographic outcomes
A follow-up echocardiogram between 60 and 365 days after CRT implantation was available for 123 (43%) of the patients. Median time from CRT implantation to the follow up echocardiogram was 182 days (122–250 days) in patients with a Classical LBBB contraction pattern and 164 days (101–245 days) in patients without (p = 0.55). Relative reductions in LV end-diastolic and -systolic volumes, and improvements in LV ejection fraction and end-systolic global longitudinal strain were larger in patients with than in patients without a Classical LBBB contraction pattern (Table 3). Of the 123 patients with a follow-up echocardiogram, 77 (63%) had a relative decrease in LV end-systolic volume ≥ 15% and thus were classified as echocardiographic responders, and 64 of 85 (75%) of patients with compared to 13 of 38 (34%) of patients without a Classical LBBB contraction pattern were echocardiographic responders (p < 0.001). Sensitivity and specificity of a Classical LBBB contraction pattern to predict echocardiographic response were 83 and 54%, respectively.
Sensitivity analyses excluding patients without sinus rhythm or < 25 frames per cardiac cycle on the baseline echocardiogram yielded similar results (data not shown).
Intra- and inter-reader agreement and agreement between vendor-independent and vendor-specific software
In the randomly selected 50 patients, the intra-reader agreement was 94%, with a Cohen’s κ of 0.86 (95% confidence interval, 0.71–1.00). The inter-reader agreement for the entire cohort was 75% with a Cohen’s κ of 0.42 (95% confidence interval, 0.30–0.54). Thirty-three (12%) patients had an echocardiogram available for analysis of longitudinal strain contraction pattern by vendor-specific software. Of these, 24 (73%) had a Classical LBBB contraction pattern both by vendor-independent and vendor-specific strain analysis. Overall agreement between vendor-independent and vendor-specific software was 88%, with a Cohen’s κ of 0.69 (95% confidence interval, 0.41–0.98).
Discussion
Using vendor-independent software analysis of standard echocardiographic images, it was found that the presence of a Classical LBBB contraction pattern was associated with better long-term outcome and echocardiographic response after CRT. Furthermore, intra-reader agreement was very good, whereas inter-reader agreement was only moderate. [12]
Differences between vendor-specific and -independent software
In this study, the association between a Classical LBBB contraction pattern and outcome after CRT was not as strong as in prior studies using vendor-specific software. [5, 6] Furthermore, inter-reader agreement for the Classical LBBB contraction pattern was 75% with a Cohen’s κ of 0.42 in this study compared to inter-reader agreements of 96 and 93% with a Cohen’s κ of 0.87 in the studies using vendor-specific software. [5, 6] Finally, in this study, there was to some extent disagreement between vendor-specific and vendor-independent software. There are several possible explanations for this. The differences may be caused by the different methods used by the software algorithms. Another possible explanation is related to the different image formats used by the different software. Vendor-specific software has the possibility of analyzing raw data, whereas vendor-independent software (at least in this study) analyzed images in DICOM format. More than half of these were temporally compressed to 30 frames/second as well as spatially compressed, whereas strain analysis using vendor-specific software is often performed at a frame rate > 60 frames/second. [5, 6, 13]
A recent study found that at least 25–30 frames per cardiac cycle is necessary for accurate measurement of peak global longitudinal strain. [14] It would be reasonable to assume that at least the same number of frames per cardiac cycle are necessary to accurately determine regional strain values and curves. The temporal compression of the DICOM images to 30 frames/second means that 25 frames per cardiac cycle will not be available in patients with a heart rate > 72 /min, resulting in an underestimation of the regional strain values and a blunting of the regional strain curves. Ultimately, this could cause rapid changes in the cardiac contraction to be missed, for instance the early peak contraction of the septum, and thus affect the overall interpretation of the longitudinal strain contraction pattern. It could also lead to difficulties in determining the actual 70% cutoff point for the early septal peak contraction. The performed sensitivity analysis excluding patients with < 25 frames per cardiac cycle yielded higher hazard ratios, which supports that the compressed frame rate explains part of the discrepancy between the current and prior studies.
The spatial compression of DICOM images means that fewer data points are available for the software, decreasing the ability to discriminate and increasing the susceptibility to noise. The combination of blunted strain curves and a higher susceptibility to noise can produce contraction patterns that are difficult to interpret and may explain the lower inter-reader agreement in this study compared to studies using vendor-specific software. [5, 6]
Activation delay and outcome after cardiac resynchronization therapy
Evidence suggests that a substantial proportion of CRT recipients do not improve their clinical or echocardiographic status. [3, 4] The reasons for lack of improvement after CRT are multiple and complex. However, studies suggest that not all patients thought to have LBBB have a LV activation delay, [15, 16] and this may explain why some CRT recipients do not improve. Therefore, there has been a massive interest in identification of LV activation delay. The Classical LBBB contraction pattern is one such approach. It identifies the mechanical consequences of LBBB with opposing movements of the LV septum and free wall, and studies suggest that patients exhibiting the Classical LBBB contraction pattern benefit the most from CRT. [5, 6] This is further supported by studies finding similar associations between variations of the Classical LBBB contraction pattern with outcome in CRT recipients. [17,18,19,20] The results in the current study are in parallel with these studies, albeit the association is not as strong despite comparable sample sizes. This study performed strain analyses retrospectively on echocardiograms obtained for clinical indications and rarely optimized for strain analysis, whereas the other studies used prospectively collected data with echocardiograms optimized for strain analysis. [5, 6, 13, 17] Based on the current results, retrospective application of longitudinal strain contraction pattern using vendor-independent software on echocardiograms stored in compressed DICOM format cannot be recommended for clinical decision making in potential CRT candidates.
Standardization of speckle-tracked strain
It is recognized that differences between the software used for speckle-tracked strain, is a major limitation of the clinical use of strain analyses, and therefore a task force was made in a cooperation between the European Association of Cardiovascular Imaging, American Society of Echocardiography, and industry representatives with the mission of standardizing speckle-tracked deformation analysis. [21] So far, their work has mainly focused on the quantitative aspects of strain measurements, and there has been less focus on qualitative aspects like contraction patterns. [8, 22, 23] A recent study of CRT recipients found that there were significant differences between vendor-specific and vendor-independent software for both qualitative and quantitative measures of mechanical dyssynchrony. [24] Furthermore, dyssynchrony parameters derived from vendor-independent software had a weaker association with echocardiographic response than parameters derived from vendor-specific software. [24] These results support the notion that qualitative assessment of LBBB contraction pattern may be less accurately determined with vendor-independent software.
Limitations
This was a retrospective cohort study with no control group of patients that did not receive CRT. Thus, it is not possible to assess the effect of CRT in patients with or without a Classical LBBB contraction pattern. Furthermore, the retrospective design limited the study to include only patients who had a baseline echocardiogram performed at Duke University Hospital. Selection bias in this subgroup of CRT recipients is very probable, however it is unlikely to affect the associations between contraction pattern and outcome after CRT. In addition, echocardiographic follow-up data were only available for those who had an echocardiogram ordered for clinical reasons, and the time from CRT implantation to the follow-up echocardiogram was therefore inconsistent. This means that echocardiographic response rate and magnitudes of volume reductions may not be generalizable. Furthermore, the analyses were performed retrospectively on the available echocardiographic images, which were not always optimized for speckle-tracked strain analysis, and it is not possible to determine if the modest performance of vendor-independent strain software analysis is due to image compression, image acquisition, or the software itself. Finally, a comparison between the performance of vendor-independent and vendor-specific software regarding the Classical LBBB contraction pattern and its association with CRT outcome was not possible, due to the limited number of patients with a baseline echocardiogram allowing for strain analysis using vendor-specific software.
Conclusion
A classical LBBB contraction pattern derived from two-dimensional speckle-tracked longitudinal strain by vendor-independent software is associated with improved survival free from heart transplantation and LV assist device implantation and with higher probability of echocardiographic response. However, inter-reader agreement was only moderate, which diminishes the clinical utility of vendor-independent software for decision making in potential CRT candidates.
Abbreviations
- CRT:
-
cardiac resynchronization therapy
- DICOM:
-
Digital Imaging and Communications in Medicine
- ECG:
-
electrocardiogram
- LBBB:
-
left bundle branch block
- LV:
-
left ventricle/ventricular
References
Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50.
Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–38.
Khan FZ, Virdee MS, Palmer CR, Pugh PJ, O’Halloran D, Elsik M, et al. Targeted left ventricular lead placement to guide cardiac resynchronization therapy. the TARGET study: a randomized, controlled trial J Am Coll Cardiol. 2012;59:1509–18.
Sommer A, Kronborg MB, Norgaard BL, Poulsen SH, Bouchelouche K, Bottcher M, et al. Multimodality imaging-guided left ventricular lead placement in cardiac resynchronization therapy: a randomized controlled trial. Eur J Heart Fail. 2016;18:1365–74.
Risum N, Jons C, Olsen NT, Fritz-Hansen T, Bruun NE, Hojgaard MV, et al. Simple regional strain pattern analysis to predict response to cardiac resynchronization therapy: rationale, initial results, and advantages. Am Heart J. 2012;163:697–704.
Risum N, Tayal B, Hansen TF, Bruun NE, Jensen MT, Lauridsen TK, et al. Identification of typical left bundle branch block contraction by strain echocardiography is additive to electrocardiography in prediction of long-term outcome after cardiac resynchronization therapy. J Am Coll Cardiol. 2015;66:631–41.
Risum N, Ali S, Olsen NT, Jons C, Khouri MG, Lauridsen TK, et al. Variability of global left ventricular deformation analysis using vendor dependent and independent two-dimensional speckle-tracking software in adults. J Am Soc Echocardiogr. 2012;25:1195–203.
Farsalinos KE, Daraban AM, Unl€ S€, Thomas JD, Badano LP, Voigt J-U. Head-to-head comparison of global longitudinal strain measurements among nine different vendors. The EACVI/ASE Inter-Vendor Comparison Study J Am Soc Echocardiogr. 2015;28:1171–1181.e2.
Strauss DG, Selvester RH, Wagner GS. Defining left bundle branch block in the era of cardiac resynchronization therapy. Am J Cardiol. 2011;107:927–34.
Horvath MM, Winfield S, Evans S, Slopek S, Shang H, Ferranti J. The DEDUCE guided query tool: providing simplified Acces to clinical data for research and quality improvement. J Biomed Inform. 2011;44:266–76.
Therneau TM. Package “survival”: survival analysis [internet]; 2018. Accessed 11 May 2018.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74.
Maréchaux S, Guiot A, Castel AL, Guyomar Y, Semichon M, Delelis F, et al. Relationship between two-dimensional speckle-tracking septal strain and response to cardiac resynchronization therapy in patients with left ventricular dysfunction and left bundle branch block: a prospective pilot study. J Am Soc Echocardiogr. 2014;27:501–11.
Rösner A, Barbosa D, Aarsæther E, Kjønås D, Schirmer H, D’Hooge J. The influence of frame rate on two-dimensional speckle-tracking strain measurements: a study on silico-simulated models and images recorded in patients. Eur Heart J Cardiovasc Imaging. 2015;16:1137–47.
Vassallo JA, Cassidy DM, Marchlinski FE, Buxton AE, Waxman HL, Doherty JU, et al. Endocardial activation of left bundle branch block. Circulation. 1984;69:914–23.
Auricchio A, Fantoni C, Regoli F, Carbucicchio C, Goette A, Geller C, et al. Characterization of left ventricular activation in patients with heart failure and left bundle-branch block. Circulation. 2004;109:1133–9.
Menet A, Bernard A, Tribouilloy C, Leclercq C, Gevaert C, Guyomar Y, et al. Clinical significance of septal deformation patterns in heart failure patients receiving cardiac resynchronization therapy. Eur Heart J Cardiovasc Imaging. 2017;18:1388–97.
Szulik M, Tillekaerts M, Vangeel V, Ganame J, Willems R, Lenarczyk R, et al. Assessment of apical rocking: a new, integrative approach for selection of candidates for cardiac resynchronization therapy. Eur J Echocardiogr. 2010;11:863–9.
Ghani A, Delnoy PPHM, Ottervanger JP, Ramdat Misier AR, Smit JJJ, Adiyaman A, et al. Association of apical rocking with long-term major adverse cardiac events in patients undergoing cardiac resynchronization therapy. Eur Heart J Cardiovasc Imaging. 2016;17:146–53.
Stankovic I, Prinz C, Ciarka A, Daraban AM, Kotrc M, Aarones M, et al. Relationship of visually assessed apical rocking and septal flash to response and long-term survival following cardiac resynchronization therapy (PREDICT-CRT). Eur Heart J Cardiovasc Imaging. 2016;17:262–9.
Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2015;16:1–11.
Mirea O, Pagourelias ED, Duchenne J, Bogaert J, Thomas JD, Badano LP, et al. Variability and reproducibility of segmental longitudinal strain measurement: a report from the EACVI-ASE strain standardization task force. JACC Cardiovasc Imaging. 2018;11:15–24.
Mirea O, Pagourelias ED, Duchenne J, Bogaert J, Thomas JD, Badano LP, et al. Intervendor differences in the accuracy of detecting regional functional abnormalities: a report from the EACVI-ASE strain standardization task force. JACC Cardiovasc Imaging. 2018;11:25–34.
van Everdingen WM, Maass AH, Vernooy K, Meine M, Allaart CP, De Lange FJ, et al. Comparison of strain parameters in dyssynchronous heart failure between speckle tracking echocardiography vendor systems. Cardiovasc Ultrasound. 2017;15:25.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the Augustinus Foundation (KE), the Knud Højgaard Foundation (KE), Gerda & Hans Hansen’s Foundation (KE), SEB Pension (KE), Danish Society of Cardiology (KE), and National Institutes of Health [T32 HL069749] (DJF). None of the funding sources had any influence on the design or conduct of the study; in the collection, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to institutional policies but are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
KE, JK and PS were primarily responsible for the conception and design of the study with inputs from DJF, JML, NR, AMT and BDA. KE performed all echocardiographic analyses. KE and DJF reviewed all electrocardiograms using customized software made by PLS and CG. KE and PS reviewed all longitudinal strain contraction patterns with inputs from JK. DJF acquired all clinical data from relevant databases. DJF and SMH united all collected data in one database and performed relevant data management, KE performed the statistical analyses. All authors contributed to the interpretation of the data. KE drafted the paper, and the remaining authors all revised it critically. KE finalized and submitted the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved and the need for consent was retrospectively waived by the Duke University Institutional Review Board.
Consent for publication
Not applicable.
Competing interests
DJF has received educational grants from Boston Scientific, Abott, and Medtronic; research grants from the National Cardiovascular Data Registry and Biosense Webster; and is supported by the Joseph C. Greenfield, Jr., M.D. Scholar in Cardiology Award. SMH has received research grants from the Danish Heart Foundation, the Laerdal Foundation and TrygFonden; and speaker’s honoraria from AstraZeneca. BDA has received research grants from Boston Scientific and Abbott, consultation fees from Abbott, Medtronic, Biotronik, and Boston Scientific; and is a member of the Speakers Bureau for Medtronic. JK has received speaker’s honoraria from Phillips Medical and GE Healthcare. PS has received speaker’s honoraria from GE Healthcare; is an advisory board member for Novartis Pharmaceuticals Corp., Astra Zeneca Pharmaceuticals, and Biotronik; and has received research grants from Wics, Bayer, and GE Healthcare. The remaining authors have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Emerek, K., Friedman, D.J., Sørensen, P.L. et al. The Association of a classical left bundle Branch Block Contraction Pattern by vendor-independent strain echocardiography and outcome after cardiac resynchronization therapy. Cardiovasc Ultrasound 17, 10 (2019). https://doi.org/10.1186/s12947-019-0160-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12947-019-0160-4