Abstract
Background
Triglyceride glucose (TyG) index is a reliable marker of insulin resistance, which is linked to obstructive sleep apnea (OSA). However, the relationship between TyG index and OSA has not been adequately assessed. This study aimed to evaluate the association between TyG index and OSA.
Methods
TyG index was assessed in 180 (mean age: 48.6 ± 13.8 years; 73.9% male) consecutive Korean adults with suspected OSA admitted to the sleep clinic at St. Paul’s Hospital between 2010 and 2012. The occurrence of more than 5 apnea-hypopnea index (AHI) events/h was used to define OSA. TyG index was calculated using the following equation: In [fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2]. All participants were grouped according to TyG index tertiles. Multivariate logistic regression analysis was used to determine factors associated with increased OSA risk.
Results
The overall prevalence of OSA in study participants was determined to be 83.9%. The prevalence of OSA increased (I [lowest]: 71.6%; II: 88.7%; III [highest]: 91.4%), and lowest peripheral oxygen saturation (SpO2) levels decreased (I: 83.3 ± 8.5%; II: 79.9 ± 8.7%; III: 79.0 ± 8.3%), as TyG index tertile increased (P < 0.05). TyG index was correlated with AHI (r = 0.179) and lowest SpO2 (r = − 0.188) (P < 0.05, respectively). Univariate linear regression analysis revealed an association between TyG and AHI (β = 10.084; P = 0.016). Multivariate logistic regression analysis showed that TyG index (odds ratio [OR]: 3.348; 95% confidence interval [CI]: 1.081–10.372), age ≥ 55 years (OR: 5.426; 95% CI: 1.642–17.935), and obesity (OR: 3.801; 95% CI: 1.468–9.842) were associated with increased OSA risk (all P < 0.05). The optimal TyG index cut-off value for predicting OSA was 8.83 (sensitivity: 61.6%; specificity: 69.0%; area under the curve: 0.688; P = 0.001). The predictive value of the OSA cut-off value improved when age ≥ 55 years and obesity were considered.
Conclusion
Increased TyG index was independently associated with increased OSA risk.
Similar content being viewed by others
Background
Sleep-disordered breathing is a major health problem, and obstructive sleep apnea (OSA), in particular, is an important medical condition. The prevalence of OSA in the general population is estimated to be 9 to 38%, and OSA is more common in males, the elderly, and obese individuals relative to the general population [1]. Obstructed breathing contributes to arousal, sympathetic activation, and oxygen desaturation in OSA. Therefore, OSA increases risk of cardiovascular (CV) morbidity and mortality [2, 3].
Patients with OSA often display clinical features similar to those of metabolic syndrome, which frequently involves insulin resistance (IR) [4, 5]. A number of previous studies reported that IR adversely affects clinical outcomes [6,7,8]. Recently, triglyceride glucose (TyG) index has been suggested as a reliable marker for IR [9,10,11]. However, data analyzing an association between TyG index and OSA remain insufficient. In the present study, we aimed to evaluate the association between TyG index and OSA in adults with suspected OSA.
Methods
Study design and subjects
We retrospectively analyzed clinical characteristics of 180 consecutive patients referred to the sleep clinic for polysomnography examinations at St. Paul’s Hospital between January 2010 and October 2012. All patients displayed at least one symptom suggesting OSA, including snoring, excessive daytime sleepiness, witnessed apneic incidents, and nocturnal choking. Overnight polysomnographic examination using a Somnostar Pro 7-3a system (Cardinal Health, Inc., Dublin, OH, USA) was performed on all study participants. Electroencephalographic, electrocardiographic, electrooculographic, and electromyographic surface electrodes were used to record patient data. Oral and nasal airflow, tracheal sounds, and abdominal and thoracic movement were also simultaneously recorded. Transcutaneous peripheral oxygen saturation (SpO2) was continuously monitored using a pulse oximeter. Positional changes during sleep were also recorded. After data were collected using a computerized polysomnographic system, a manual scoring was performed. Sleep status was defined using Rechtschaffen and Kales criteria [12]. The criteria of the American Academy of Sleep Medicine were applied for respiratory events [13]. Airflow reduction of ≥ 90% of baseline values for ≥ 10 s was defined as apnea. Events involving ≥ 30% airflow reduction for ≥ 10 s accompanied by a ≥ 3% drop in oxygen saturation or arousal were defined as hypopnea. Apnea-hypopnea index (AHI) was defined as the number of apnea and hypopnea events per hour that occurred during sleep. The occurrence of ≥ 5 AHI events/h was used to determine OSA. Among patients with OSA, the severity was defined as mild (5–15 AHI events), moderate (16–30 AHI events), and severe (> 30 AHI events) based on the number of events per night of sleep [14]. All blood samples were obtained after at least 8 h fasting. TyG index was determined using the following equation: ln [fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2]. Body mass index (BMI) was defined as the patient’s weight (kg)/height2 (m2). Participants with a BMI of ≥ 25 kg/m2 were considered obese as specified by cut-off values for Asian individuals. Hypertension was defined as systolic/diastolic blood pressure (BP) ≥ 140/90 mmHg, anti-hypertensive medication use, or a previous hypertension diagnosis. Diabetes was defined as fasting glucose ≥ 126 mg/dL, anti-diabetic medication use, or a previous diabetes diagnosis. Dyslipidemia was defined as total cholesterol ≥ 240 mg/dL, triglyceride ≥ 150 mg/dL, high-density lipoprotein cholesterol (HDL-C) ≤ 40 mg/dL, low-density lipoprotein cholesterol (LDL-C) ≥ 130 mg/dL, or lipid lowering medication use. Excessive daytime sleepiness was defined as Epworth sleepiness score (ESS) values > 10 [15]. The institutional review board approved the protocol used in the study (approval number: PC14OISI0059), and all participants provided written informed consent.
Statistical analysis
Categorical and continuous variables are presented as number (percentage) and mean ± standard deviation, respectively. One-way analysis of variance was used to compare continuous variables. The χ2- test or Fisher’s exact test was used to compare categorical variables. Pearson’s correlation test was used to evaluate the correlation between variables. Univariate linear regression analysis was used to assess the association between clinical variables and AHI. Univariate and multivariate logistic regression analyses were used to evaluate independent risk factors for OSA. In logistic regression models, variables with P values < 0.05 in univariate regression analysis were regarded as confounding variables and were entered into multivariate regression analysis. To determine an optimal cut-off value of TyG index for predicting OSA, receiver operating characteristic (ROC) curve analysis with Youden index was used. Then, we compared the significance of this cut-off value to predict OSA considering other independent risk factors together. SAS version 9.1.3 (SAS Institute Inc., Cary, NC, USA) software was used in all statistical analyses. P values < 0.05 were considered significant.
Results
Baseline characteristics of all study participants are included in Table 1. The mean age of the participants (133 males, 73.9%) was 48.6 ± 13.8 y. Mean BMI was 26.4 ± 4.1 kg/m2, and the prevalence of hypertension, diabetes, dyslipidemia, and obesity in the study participants was 78.3, 27.8, 72.8, and 59.4%, respectively. The prevalence of alcohol consumption and the percentage of patients with a history of smoking were 61.1 and 37.2%, respectively. The mean AHI value determined for all study participants was 31.6 ± 28.3/h. The overall prevalence of OSA (n = 151) was 83.9%, and the prevalences of mild (n = 42), moderate (n = 36), and severe (n = 73) OSA were 23.3, 20.0, and 40.6%, respectively.
All participants were divided into three groups based on TyG index tertiles. Patients with TyG indexes of 7.60–8.72, 8.73–9.06, and 9.07–10.49 were included in groups I (lowest), II, and III (highest), respectively. Mean TyG indexes of 8.39 ± 0.23, 8.89 ± 0.11, and 9.47 ± 0.35 were determined for groups I, II, and III, respectively. The prevalence of OSA significantly increased as TyG index tertile increased (group I [lowest]: 71.6% vs. group II: 88.7% vs. group III [highest]: 91.4%; P = 0.006), and no significant difference was observed between group II and group III (Fig. 1a). In contrast, the SpO2 nadir significantly decreased as TyG index tertile increased (group I: 83.3 ± 8.5% vs. group II: 79.9 ± 8.7% vs. group III: 79.0 ± 8.3%; P = 0.016), and no significant difference was observed between group II and group III (Fig. 1b). The occurrence and severity of OSA associated with all TyG index tertiles are shown in Fig. 2.
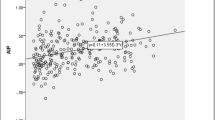
Univariate linear regression analysis revealed that alcohol consumption, smoking, BMI, and TyG index were significantly associated with AHI (Table 2). TyG index correlated with increased AHI (r = 0.179; P = 0.016) and decreased SpO2 (r = − 0.188; P = 0.011) in all participants (Fig. 3a and b).
Univariate logistic regression analysis showed that male sex (odds ratio [OR]: 2.796; 95% confidence interval [CI]: 1.225–6.383; P = 0.015), age ≥ 55 years (OR: 3.790; 95% CI: 1.255–11.449; P = 0.018), obesity (OR: 4.998; 95% CI: 2.071–12.058; P < 0.001), history of smoking (OR: 3.344; 95% CI: 1.210–9.242; P = 0.020), and elevated TyG index (OR: 5.130; 95% CI: 1.922–13.691; P = 0.001) were associated with OSA. Further, multivariate logistic regression analysis was used to show that age ≥ 55 years (OR: 5.426; 95% CI: 1.642–17.935; P = 0.006), obesity (OR: 3.801; 95% CI: 1.468–9.842; P = 0.006), and elevated TyG index (OR: 3.348; 95% CI: 1.081–10.372; P = 0.036) were independently associated with OSA (Table 3).
ROC curve analysis showed that the optimal TyG index cut-off value used for predicting the presence of OSA, as determined via Youden index, was 8.83 (sensitivity: 61.6%; specificity: 69.0%; area under the curve [AUC]: 0.688; 95% CI: 0.582–0.794; P = 0.001) (Fig. 4). The usefulness of TyG indexes greater than 8.83 for predicting OSA significantly improved after considering whether patients were aged ≥ 55 years (TyG ≥ 8.83 vs. TyG ≥ 8.83 mg/dL for patients aged ≥ 55 years; AUC: 0.618 vs. 0.703; P = 0.002) and whether patients were aged ≥ 55 years and obese (TyG ≥ 8.83 vs. TyG ≥ 8.83 in patients aged ≥ 55 years and obese; AUC: 0.618 vs. 0.772; P < 0.001) (Table 4).
Discussion
To the best of our knowledge, this is the first study to evaluate the association between TyG index and OSA risk in adults with suspected OSA. In the present study, elevated TyG index was independently associated with an increased OSA risk. We identified the optimal cut-off value that indicates the limits of its usefulness for predicting OSA in the population assessed. The predictive value of the cut-off was significantly improved after considering other independent risk factors of OSA.
IR has been implicated in metabolic syndrome and CV disease pathogenesis [6,7,8]. Also, previous studies have revealed a close relationship between IR and sleep-disordered breathing. Punjabi et al. [16] reported that sleep-disordered breathing was associated with impaired glucose tolerance and IR, independent of obesity. Ip et al. [17] also reported that obesity was the major determinant of IR, and despite adjusting for obesity and other confounding factors associated with IR, minimum oxygen saturation and AHI were determined to be significant determinants of fasting insulin level and homeostatic model assessment of insulin resistance (HOMA-IR) index. The HOMA-IR has been used as a marker of IR, but insulin level must be measured to determine HOMA-IR [18]. Recent data have shown the close relationship between HOMA-IR and TyG index [10, 11, 19, 20]. However, little is known regarding the significance of TyG index in the risk of OSA despite the close relationship between IR and OSA.
Drager et al. [21] reported that OSA was independently associated with factors important for diagnosing metabolic syndrome, including triglyceride (OR: 3.26; 95% CI: 1.47–7.21) and glucose (OR: 2.31; 95% CI: 1.12–4.80) levels. Moreover, Meszaros et al. [22] recently reported that the co-occurrence of OSA and hypertriglyceridemia is affected by genetics, and heritable factors might play a crucial role in dyslipidemia pathogenesis in OSA. These results indicated that there may be a close relationship between TyG index and OSA. In the present study, despite the relatively small sample size, a significant positive correlation between TyG index and AHI was observed, although the individual lipid profile did not significantly correlate with AHI (Supplementary table 1). In addition, TyG index was negatively correlated with decreased SpO2. Similarly, TyG index also had an inverse association with mean SpO2 value (Supplementary figure 1). After controlling for confounding factors, TyG index, together with age (≥ 55 years) and obesity, was determined to be associated with OSA risk.
Park et al. [23] recently reported the predictive significance of TyG index for subclinical coronary artery disease in patients with no traditional CV risk factors; the mean TyG index was 8.31 ± 0.46. In the present study, the mean TyG index was higher than that reported by Park et al. This suggests that patients with suspected OSA have increased IR. Although the predictive significance of the optimal cut-off value of TyG index for OSA was poor, this was moderately improved after considering other independent risk factors. Its predictive power might be acceptable in populations with specific clinical conditions. Considering that data on the relationship between TyG index and OSA risk has been limited in sleep medicine, the present study has the potential to provide important information applicable to the adult population at high risk for OSA.
Study strengths and limitations
The present study had some limitations. First, this study had an observational design. Thus, clinical factors not considered could influence the study results. Second, the population considered had an unbalanced sex distribution; more males than females were referred to sleep clinics within the study period. Third, we were unable to consider HOMA-IR, because there was a paucity of data regarding insulin level for the population studied. However, the close relationship between HOMA-IR and TyG index has previously been well established. Fourth, we could not eliminate the possible effects of underlying medications on OSA because of the observational design of this study. Finally, considering that only patients highly suspected of having OSA were included in the present study, these results should be applied with caution to the general population. Despite these limitations, these findings revealed that TyG index may be used to predict OSA in Korean adults with suspected OSA.
Conclusions
In this cross-sectional, observational study, TyG index was determined to be independently associated with risk of OSA in Korean adults admitted to the sleep clinic. Further, prospective, large-scale, and longitudinal studies will be needed to confirm the value of the TyG index for predicting OSA.
Availability of data and materials
The datasets used and analyzed during the current study will be provided by the corresponding author upon reasonable request.
Abbreviations
- AHI:
-
Apnea-hypopnea index
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- CV:
-
Cardiovascular
- CI:
-
Confidence interval
- ESS:
-
Epworth sleepiness score
- HDL-C:
-
High-density lipoprotein cholesterol
- HOMA-IR:
-
Homeostatic model assessment of insulin resistance
- IR:
-
Insulin resistance
- LDL-C:
-
Low-density lipoprotein cholesterol
- OR:
-
Odds ratio
- OSA:
-
Obstructive sleep apnea
- ROC:
-
Receiver operating characteristic
- SpO2 :
-
Peripheral oxygen saturation
- TyG:
-
Triglyceride glucose
References
Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81.
Loo G, Tan AY, Koo CY, Tai BC, Richards M, Lee CH. Prognostic implication of obstructive sleep apnea diagnosed by post-discharge sleep study in patients presenting with acute coronary syndrome. Sleep Med. 2014;15:631–6.
Eeão S, Conde B, Fontes P, Calvo T, Afonso A, Moreira I. Effect of obstructive sleep apnea in acute coronary syndrome. Am J Cardiol. 2016;117:1084–7.
NCEP. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285:2486–97.
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American heart association/national heart, lung, and blood institute scientific statement. Circulation. 2005;112:2735–52.
Reaven G. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607.
Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, et al. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in caucasian subjects from the general population: the Bruneck study. Diabetes Care. 2007;30:318–24.
Eddy D, Schlessinger L, Kahn R, Peskin B, Schiebinger R. Relationship of insulin resistance and related metabolic variables to coronary artery disease: a mathematical analysis. Diabetes Care. 2009;32:361–6.
Simental-Mend’ıa LE, Rodr’ıguez-Mor’an M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304.
Vasques AC, Novaes FS, de Oliveira MS, Souza JR, Yamanaka A, Pareja JC, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93:e98–e100.
Guerrero-Romero F, Villalobos-Molina R, Jim’enez-Flores JR, Simental-Mendia LE, Méndez-Cruz R, Murguía-Romero M, et al. Fasting triglycerides, and glucose index as a diagnostic test for insulin resistance in young adults. Arch Med Res. 2016;47:382–7.
Rechtschaffen A, Kales A. Editors. A manual of standardized terminology, techniques and scoring system for sleep stages in human subjects. Brain Information Service, Brain Research Institute: Los Angeles; 1968.
Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus CL, Vaughn BV, et al. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, version 2.0. American Academy of Sleep Medicine: Darien (IL); 2012.
McNicholas WT. Diagnosis of obstructive sleep apnea in adults. Proc Am Thorac Soc. 2008;5:154–60.
Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5.
Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165:677–82.
Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–6.
Wallace TM, Matthews DR. The assessment of insulin resistance in man. Diabet Med. 2002;19:527–34.
Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun YC, et al. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS One. 2016;11:e0149731.
Lee SH, Kwon HS, Park YM, Ha HS, Jeong SH, Yang HK, et al. Predicting the development of diabetes using the product of triglycerides and glucose: the Chungju metabolic disease cohort (CMC) study. PLoS One. 2014;9:e90430.
Drager LF, Lopes HF, Maki-Nunes C, Trombetta IC, Toschi-Dias E, Alves MJ, et al. The impact of obstructive sleep apnea on metabolic and inflammatory markers in consecutive patients with metabolic syndrome. PLoS One. 2010;5:e12065.
Meszaros M, Tarnoki AD, Tarnoki DL, Kovacs DT, Forgo B, Lee J, et al. Obstructive sleep apnea and hypertriglyceridaemia share common genetic background: Results of a twin study. J Sleep Res. 2020;6:e12979.
Park GM, Cho YR, Won KB, Yang YJ, Park S, Ann SH, et al. Triglyceride glucose index is a useful marker for predicting subclinical coronary artery disease in the absence of traditional risk factors. Lipids Health Dis. 2020;19:7.
Acknowledgements
We acknowledge the Medical Information Center of Ulsan University Hospital for their assistance with statistical analyses.
Funding
None.
Author information
Authors and Affiliations
Contributions
HHK, SWK, and SHL contributed to the conception and design of the work. HHK and SHL contributed to the acquisition, analysis, or interpretation of data. HHK drafted the manuscript. SHL critically revised the manuscript. All authors gave final approval and are accountable for all aspects of the work and its integrity and accuracy.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the local Institutional Review Board of St. Paul’s Hospital, The Catholic University of Korea, and informed consent for involvement in the study was obtained from each participant.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
Association of individual lipid profile with AHI. Figure S1. Association between TyG index and mean SpO2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kang, H.H., Kim, S.W. & Lee, S.H. Association between triglyceride glucose index and obstructive sleep apnea risk in Korean adults: a cross-sectional cohort study. Lipids Health Dis 19, 182 (2020). https://doi.org/10.1186/s12944-020-01358-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-020-01358-9