Abstract
Background
Angiopoietin-like 4 (ANGPTL4) inhibits lipoprotein lipase, whereas phospholipid transfer protein (PLTP) enhances hepatic triglyceride secretion. Both factors may be upregulated by inflammatory pathways. Since the extent to which these circulating factors are interrelated is unknown, we determined the relationship between plasma ANGPTL4 and PLTP activity, and assessed whether such a relationship could be explained by high sensitivity C-reactive protein (hsCRP) levels as a marker of low-grade chronic inflammation.
Methods
Fasting plasma ANGPTL4, PLTP activity (liposome-vesicle high density lipoprotein system) and hsCRP were measured in 41 type 2 diabetic (T2DM) subjects and 36 non-diabetic subjects.
Results
Plasma ANGPTL4 and PLTP activity were increased in T2DM (p < 0.001 for each), coinciding with elevated hsCRP, triglycerides and non-esterified fatty acids (NEFA) (p = 0.031 to 0.001). In univariate analysis, ANGTLP4 was correlated with PLTP activity (Rs = 0.309, p = 0.006), whereas both factors were related to hsCRP and NEFA levels (Rs = 0.304 to 0.411, p < 0.01 to < 0.001). In multivariable linear regression analysis adjusting for age, sex, glucose, total cholesterol, triglycerides and NEFA, ANGPTL4 and PLTP activity each remained positively associated with hsCRP (β = 0.315, p = 0.003 and β = 0.299, p = 0.034, respectively). Plasma ANGPTL4 remained positively associated with PLTP activity when taking account of age, sex, glucose, total cholesterol, triglycerides and NEFA (β = 0.315, p = 0.003). Notably, this association disappeared after further adjustment for hsCRP (β = 0.131, p = 0.25).
Conclusions
In conclusion, plasma ANGPTL4 and PLTP activity are interrelated, which may at least in part be explained by low-grade chronic inflammation. A pro-inflammatory state could affect triglyceride metabolism via concerted effects on ANGPTL4 and PLTP.
Similar content being viewed by others
Background
During the past few years, the key role of angiopoietin like proteins (ANGPTL) in lipid metabolism and energy balance is increasingly appreciated [1,2,3]. Among this family of proteins, ANGPTL4 is a secreted glycoprotein that is highly expressed in liver and adipose tissue [1,2,3]. Evidence abounds that ANGPTL4 functions as an inhibitor of lipoprotein lipase, thereby attenuating the uptake of triglyceride-rich lipoprotein-derived fatty acids in several cell types including adipocytes [2]. Importantly, non-esterified fatty acids (NEFA) serve as potent activators of ANGPTL4 expression in several tissues, which is mediated by peroxisome proliferator-activated receptors (PPARs) [2, 4,5,6,7]. Consistent with these data, circulating ANGPTL4 concentrations have been reported to correlate positively with plasma NEFA, although this relationship has not been consistently observed, while a relationship with fasting triglycerides appears to be modest or even absent [8,9,10].
The metabolism of triglycerides is also governed by phospholipid transfer protein (PLTP), a lipid transfer protein that is produced by a variety of cell systems, including hepatocytes, adipocytes and macrophages [11,12,13]. PLTP transfers phospholipids between lipoproteins, and converts high density lipoproteins (HDL) to smaller and larger particles [11,12,13]. Of note, studies in transgenic mice have shown that PLTP deficiency attenuates the secretion of very low density lipoproteins (VLDL) by the liver, whereas PLTP overexpression has the opposite effect [14, 15]. Moreover, studies in humans have shown that plasma PLTP activity is increased after a prolonged exposure to an intravenously administered triglyceride emulsion, while a decrease has been observed when plasma NEFA availability is attenuated by Acipimox administration [16, 17].
Interestingly, recent evidence has linked ANGPTL4 regulation to inflammatory pathways. Specifically, treatment of mice with lipopolysaccharide or zymosan was shown to increase ANGPTL4 expression in several tissues [18]. Furthermore, Angptl4 mRNA and protein levels are upregulated by various inflammatory stimuli in human THP1 macrophages in vitro [10]. Clinically, plasma ANGPTL4 correlates positively with high sensitivity C-reactive protein (hsCRP), an established marker of enhanced low-grade inflammation [8, 10, 19]. Accordingly, the plasma ANGPTL4 concentration is likely to be elevated in subjects with pro-inflammatory conditions such as the metabolic syndrome and Type 2 diabetes mellitus (T2DM) [9, 10, 20]. Notably, plasma PLTP activity is associated with enhanced low-grade chronic inflammation as well, as evidenced by its positive relationship with hsCRP [21,22,23]. In keeping with a role of inflammation in the regulation of this lipid transfer protein, plasma PLTP activity is elevated during the acute phase of sepsis in humans [24]. Taken together, these data [10, 21,22,23] support the hypothesis that enhanced low-grade inflammation, as reflected by plasma hsCRP, may result in upregulation of both circulating ANGPTL4 and PLTP activity. Pathophysiologically, such a relationship between ANGPTL4 and PLTP would conceivably link a pro-inflammatory state to alterations in triglyceride metabolism. Since diabetic dyslipidemia is featured by abnormalities in triglyceride metabolism [10, 11], it is relevant to study ANGPTL4 and PLTP activity together in T2DM.
In the absence of any data concerning the possible relationship between circulating ANGPTL4 levels and PLTP activity, we initiated the present study to determine i) the extent to which plasma ANGPTL4 relates to PLTP activity among subjects with and without T2DM, and ii) whether such a relationship may be explained at least in part by an association of low-grade chronic inflammation, using hsCRP as marker, with ANGPTL4 and PLTP.
Methods
Subjects
The study protocol was approved by the medical ethics committee of the University Medical Center Groningen, and written informed consent had been obtained from all participants. Subjects with and without T2DM were aged > 18 years, and were recruited by advertisement in local newspapers. T2DM had been diagnosed by primary care physicians applying guidelines from the Dutch College of General Practitioners (fasting plasma glucose ≥ 7.0 mmol/l; non-fasting plasma glucose ≥ 11.1 mmol/l). The use of metformin, sulfonylurea was allowed but insulin use was an exclusion criterion. Subjects taking antihypertensive medication were also allowed to participate. Subjects with a history of cardiovascular disease, chronic kidney disease (estimated glomerular filtration rate < 60 ml/min/1.73 m2 and/or proteinuria), abnormal liver function tests (transaminases > 3 times the upper reference limit) or thyroid dysfunction (thyroid stimulating hormone > 10 or < 0.40 mU/l or use of thyroid function influencing medication), as well as current smokers and subjects who used lipid lowering drugs were also excluded.
Blood pressure was measured after 15 min rest at the left arm in sitting position using a sphygmomanometer. Body mass index (BMI in kg/m2) was calculated as weight divided by height squared. The participants were studied after an overnight fast.
Laboratory analyses
Serum and EDTA-anticoagulated plasma samples were stored at − 80 °C until analysis. Plasma glucose was measured shortly after blood collection with an APEC glucose analyzer (APEC Inc., Danvers, MA, USA). Plasma NEFA were measured with a kit from Wako Diagnostics (HR Series NEFA-HR(2), Wako Chemicals GmbH, Neuss, Germany). Plasma total cholesterol and triglycerides were measured by routine enzymatic methods (Roche/Hitachi cat. Nos 11,875,540 and 1,187,602, respectively; Roche Diagnostics GmBH, Mannheim, Germany). HDL cholesterol was assayed by a homogeneous enzymatic colorimetric test (Roche/Hitachi, cat.no 04713214). Non-HDL cholesterol was calculated as the difference between total cholesterol and HDL cholesterol. Low density lipoprotein (LDL) cholesterol was calculated by the Friedewald formula in case of triglycerides < 4.5 mmol/l. Apolipoprotein A-I (apoA-I) and apolipoprotein B (apoB) were measured by immunoturbidimetry (Roche/Cobas Integra Tinaquant cat no. 03032566, Roche Diagnostics). hsCRP was assayed by nephelometry with a lower limit of 0.175 mg/l (BNII N; Dade Behring, Marburg, Germany). HbA1c was measured by high-performance liquid chromatography (Bio-Rad, Veenendaal, the Netherlands; normal range: 27–43 mmol/mol).
Plasma ANGPTL4 was assayed by enzyme-linked immunosorbent assay (ELISA) as described [5, 10]. In brief, 96-well plates were coated with anti-human ANGPLT4 polyclonal goat IgG antibody (AF3485, R&D systems Netherlands, Abingdon, Oxon, UK) and incubated overnight at 4 °C. After 4 washes with 300 μL phosphate buffered saline (PBS)-Tween20 0.1%, 300 μl of blocking solution (PBS containing 1% bovine serum albumin) was added per well and left for 1 h at room temperature under gentle agitation. 100 μl of 20-fold diluted human plasma was applied to each well, followed by 2 h incubation at room temperature under gentle agitation. A standard curve was prepared with recombinant human ANGPTL4 (3485-AN, R&D systems). This procedure was followed by adding 100 μL of diluted biotinylated anti-human ANGPTL4 polyclonal goat IGG antibody (BAF3485, R&D systems) for 2 h, followed by addition of streptavidin-conjugated horseradish peroxidase for 20 min, and tetramethyl benzidine substrate reagent for 6 min. The reaction was stopped by adding 50 μl of 10% H2SO4, and the absorbance was measured at 450 nm. The intra-assay coefficient of variation (CV) is 7%. Plasma PLTP activity was assayed with a phospholipid vesicles-HDL system, using [14C]-labeled dipalmitoyl phosphatidylcholine as described [25, 26]. The phospholipid transfer promoting properties of cholesteryl ester transfer protein do not interfere with this excess exogenous substrate assay. The method is specific for PLTP activity, and represents the level of active PLTP [25]. Plasma PLTP activity (expressed in arbitrary units (AU); 100 AU corresponds to 13.6 μmol phosphatidylcholine transferred per mL plasma per hour) was related to its activity in reference human pool plasma, and was measured in duplicate. The inter-assay CV amounts to 5%.
Statistical analysis
IBM SPSS software (SPSS, version 22.0, SPSS Inc. Chicago, IL, USA) was used for data analysis. Data are expressed in medians (interquartile ranges). Between group differences in variables were determined by Mann-Whitney U tests and Chi-square tests where appropriate. Univariate correlations were determined by Spearman correlation coefficients. Multivariable linear regression analyses were carried out to disclose the independent relationships of ANGPTL4 and PLTP activity separately with hsCRP taking account of glycemia, NEFA and lipid levels. Additional analysis were done to discern the influence of hsCRP on the relationship between ANGPTL4 and PLTP activity. In these analyses, ANGPTL4, triglycerides and hsCRP were loge transformed to achieve approximately normal distributions. Two-sided p-values < 0.05 were considered statistically significant.
Results
We studied 41 subjects with T2DM and 36 non-diabetic subjects (Table 1). Besides dietary advice which had been given to all diabetic subjects, metformin was taken by 5 individuals, and sulfonylurea by 6 individuals; 14 participants used both drugs. The diabetic subjects did not take other glucose lowering drugs. Additionally, anti-hypertensive drugs, particularly angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists and diuretics, alone or in combination were used by 19 T2DM subjects. None of the non-diabetic subjects used anti-hypertensive drugs. Three non-diabetic women were using estrogens. Other medications were not taken.
The T2DM subjects were older than the non-diabetic participants; the difference in sex distribution between T2DM and non-diabetic subjects was not significant (Table 1). Blood pressure, BMI, fasting glucose, HbA1c, hsCRP and fasting NEFA levels were higher in T2DM subjects (Table 1). Total cholesterol, non-HDL cholesterol, LDL cholesterol and apoB levels were not different between diabetic and non-diabetic subjects. Triglycerides were elevated in T2DM subjects, coinciding with lower HDL cholesterol and apoA-I. Plasma ANGPTL4 levels and PLTP activity were each higher in T2DM subjects than in non-diabetic subjects (Table 1). ANGPTL4 and PLTP activity were not different in men vs. women (ANGPTL4: 5.24 (4.13–6.01) μg/l in men and 4.35 (3.03–6.10) μg/l in women, p = 0.17; PLTP activity: 97.9 (92.4–103.7) AU in men and 100.2 (91.6–110.5) AU in women, p = 0.46).
Univariate correlation coefficients of ANGPTL4 and PLTP activity with various clinical variables in all subjects combined are shown in Table 2. Plasma ANGPTL4 was correlated positively with PLTP activity (Fig. 1). Both ANGPTL4 and PLTP activity were correlated positively with hsCRP (Fig. 2). Furthermore, plasma ANGPTL4 and PLTP activity were both correlated positively with systolic blood pressure, BMI, glucose, hsCRP and NEFA levels. Plasma PLTP activity was also correlated positively with diastolic blood pressure. Plasma ANGPTL4 was correlated inversely with total cholesterol and LDL cholesterol, whereas PLTP activity was related positively to triglycerides and apoB, and inversely to HDL cholesterol (Table 2). Plasma ANGPTL4 was not significantly correlated with triglycerides.
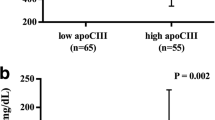
Relationship of plasma angiopoietin-like 4 (ANGPTL4) levels. a ANGPTL4 and phospholipid transfer protein (PLTP) activity (b) ANGPTL4 with high sensitivity C-reactive protein (hsCRP) in 77 subjects (41 subjects with Type 2 diabetes mellitus T2DM and 36 non-diabetic subjects. a Spearman correlation coefficient: 0.377, p = 0.001. b Spearman correlation coefficient: 0.373, p = 0.001
Using multivariable linear regression analysis, we next tested the extent to which the relationship of plasma ANGPTL4 and PLTP activity with hsCRP was independent of NEFA, lipids (total cholesterol and triglycerides) and glycemia. ANGPTL4 remained positively associated with hsCRP when taking account of age, sex, NEFA, total cholesterol, triglycerides and the presence of diabetes (Table 3, Model A1) or alternatively of fasting glucose (Table 3, Model A2). ANGPTL4 was also independently associated with hsCRP after further adjustment for the use of metformin, sulfonylurea and antihypertensives (Model A1: β = 0.320; p = 0.003; Model A2: β = 0.332, p = 0.001). Likewise, plasma PLTP activity was positively and independently associated with hsCRP taking account of either the presence of diabetes (Table 3, Model B1) or of fasting glucose (Table 3, Model B2). The relationship of PLTP activity with hsCRP was still significant after adjustment for the use of metformin, sulfonylurea and antihypertensives (Model A1: β = 0.224; p = 0.044; Model A2: β = 0.266, p = 0.016). In subsidiary analyses with BMI in addition to age, sex, the presence of diabetes, NEFA, total cholesterol and triglycerides as dependent variables, plasma PLTP activity but not ANGPTL4 was independently associated with BMI (β = 0.239, p = 0.05 and β = 0.137, p = 0.23, respectively; data not shown).
In view of the observation that plasma ANGPTL4 and PLTP activity were positively correlated with each other in univariate analysis, and that both ANGPTL4 and PLTP activity were independently associated with hsCRP, we next sought to determine whether the relationship between plasma ANGPTL4 and PLTP activity was independent of plasma glucose, total cholesterol and triglycerides, In age- and sex-adjusted multivariable linear regression analysis, plasma ANGPTL4 was independently associated with PLTP activity (Table 4, Model A). This relationship was essentially unaltered after further adjustement for the use of metformin, sulfonylurea and antihypertensives (β = 0.226, p = 0.058). Notably, when hsCRP was included in the analysis, the relationship between plasma ANGPTL4 and PLTP activity disappeared (Table 4, Model B). This finding is consistent with the possibility that the relationship of plasma ANGPTL4 with PLTP activity is at least in part explained by their associations with hsCRP.
Discussion
The main novel finding of the present study is that the plasma ANGPTL4 concentration is positively correlated with PLTP activity. This relationship remained present when taking account of age, sex, fasting glucose, NEFA and lipid levels. Notably, ANGPTL4 was no longer associated with PLTP activity after further adjustment for hsCRP, a marker of low-grade chronic inflammation. Since our study also shows that ANGPTL4 and PLTP activity are each independently associated with hsCRP, these findings are consistent with the hypothesis that enhanced low-grade inflammation may result in increased circulating levels of both ANGTPL4 and PLTP activity. This would explain at least in part the positive relationship between ANGTPL4 and PLTP. In addition, given the different mechanisms whereby ANGPTL4 and PLTP act on triglyceride metabolism [1,2,3, 11,12,13,14,15], it is plausible to postulate that ANGPTL4 and PLTP may act together to elevate circulating triglycerides under pro-inflammatory circumstances.
In agreement with several earlier reports [10, 20], plasma ANGPTL4 was elevated in T2DM subjects. However, neither the presence of T2DM nor the fasting glucose concentration predicted plasma ANGPTL4 in age- and sex-adjusted multivariable linear regression analysis in which we also accounted for hsCRP, NEFA, total cholesterol and triglycerides. hsCRP and NEFA levels were elevated in T2DM, and in view of their independent relationships with plasma ANGPTL4, it is likely that these variables jointly contributed to the higher ANGPTL4 levels as presently observed in T2DM. On the other hand in fully adjusted analysis, plasma PLTP activity remained associated with T2DM or alternatively with fasting glucose, in keeping with other data [11,12,13, 23, 27]. In addition, although both plasma ANGPTL4 and PLTP activity were expectedly correlated with obesity and NEFA, ANGPTL4 was inversely correlated with total cholesterol and LDL cholesterol, whereas PLTP activity was significantly correlated with triglycerides [8, 9, 11, 12]. Furthermore, the production of ANGTPL4 is stimulated by PPARγ activation in vitro [28], whereas plasma PLTP activity was found to change little in response to the PPARγ agonist pioglitazone in vivo in humans [29]. Thus, the interrelationship between plasma ANGPTL4 and PLTP activity, even after adjustment for dysglycemia, NEFA and lipid levels, is remarkable.
ANGPTL4 is upregulated by lipopolysaccharide (LPS) which likely involves the Toll-like receptor 4 (TRL 4) [10, 30]. Interestingly, PLTP shares homology with LPS [12, 31] but while PLTP increases in response to acute inflammation, it may decrease after LPS administration [24]. In addition, our unpublished data indicate that the level of Angptl4 mRNA in rat hepatocytes is markedly increased by interleukin-1β (IL-1β) and tumor necrosis factor α (TNFα). On the other hand, the ability of interleukin-6 (IL-6) to induce TNFα is abolished in PLTP knockout mice [32], raising the possibility that TNFα could be involved in interrelation between ANGPTL4 and PLTP. We used hsCRP as a marker of enhanced low-grade chronic inflammation, but it seems unlikely that CRP plays a major direct role in regulating ANGPTL4 and PLTP. Obviously however, given the complex and yet to be more precisely delineated processes by which inflammatory stimuli affect ANGTPL4 and PLTP regulation, the mechanisms responsible for the association of a pro-inflammatory state with both plasma ANGPTL4 and PLTP activity await further study.
Loss of function mutations in ANGPTL4 and genetic variations in PLTP that attenuate its plasma activity have been associated with an attenuated risk of coronary artery disease, emphasizing the relevance of ANGTP4- and PLTP-mediated processes on the development of atherosclerosis [33, 34]. Based in the present findings, we postulate that yet to be more precisely delineated inflammatory pathways may give rise to increased plasma levels of ANGPTL4 and PLTP activity, conceivably contributing to joint effects on triglyceride elevations. Thus, in the context of low-grade chronic inflammation, ANGPTL4 and PLTP gene-environment interactions could aggravate abnormalities in NEFA and triglyceride metabolism.
Several other methodological issues of the present study need consideration. The ANGPTL4 ELISA that we used measures the full length protein and the C-terminal truncated fragment but does not detect the N-terminal truncated fragment. The N-terminal but not the C-terminal fragment carries LPL inhibitory activity. Concerning PLTP, its activity as reported here reflects the level of active PLTP [25, 26]. We preferred to use this type of activity assay, which is independent of the endogenous lipoproteins present in the individual patient samples, because PLTP circulates in an active and inactive form. Consequently, PLTP activity and mass levels are not closely related [35,36,37]. Furthermore, we excluded diabetic subjects using insulin and lipid lowering drugs in order to obviate confounding due to effects on glucose and lipid metabolism. As a result, we mainly included T2DM subjects with rather mild hyperglycemia and dyslipidemia which would limit the generalizability of our findings. Also, in view of the cross-sectional design of the current study, it should be stressed that cause-effect relationships cannot be established with certainty. Indeed, PLTP is involved in the innate immune system, and has been implicated to causally modulate inflammatory processes [31, 32]. Finally, we regard the present findings as preliminary given the limited numbers of diabetic and non-diabetic participants.
Conclusion
In conclusion, plasma ANGPTL4 and PLTP activity are interrelated, which may in part be attributable to a stimulatory influence of low grade inflammation on these two protein fators. A pro-inflammatory state could affect triglyceride metabolism via concerted effects on ANGPTL4 and PLTP.
Abbreviations
- ANGPTL:
-
Angiopoietin like proteins
- apoA-I:
-
Apolipoprotein A-I
- apoB:
-
Apolipoprotein B
- BMI:
-
Body mass index
- HDL:
-
High density lipoproteins
- hsCRP:
-
High sensitivity C-reactive protein
- IL-1β:
-
Interleukin-1β
- IL-6:
-
Interleukin-6
- NEFA:
-
Non-esterified fatty acids
- PLTP:
-
Phospholipid transfer protein
- PPARs:
-
Peroxisome proliferator-activated receptors
- T2DM:
-
Type 2 diabetes mellitus
- TNFα:
-
Tumor necrosis factor α
- VLDL:
-
Very low density lipoproteins
References
Mattijssen F, Kersten S. Regulation of triglyceride metabolism by angiopoietin-like proteins. Biochim Biophys Acta. 2012;1821:782–9.
Dijk W, Kersten S. Regulation of lipoprotein lipase by Angptl4. Trends Endocrinol Metab. 2014;25:146–55.
Dijk W, Kersten S. Regulation of lipid metabolism by angiopoietin-like proteins. Curr Opin Lipidol. 2016;27:249–56.
Yoshida K, Shimizugawa T, Ono M, Furukawa H. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. J Lipid Res. 2002;43:1770–2.
Kersten S, Lichtenstein L, Steenbergen E, Mudde K, Hendriks HF, Hesselink MK, Schrauwen P, Müller M. Caloric restriction and exercise increase plasma ANGPTL4 levels in humans via elevated free fatty acids. Arterioscler Thromb Vasc Biol. 2009;29:969–74.
Georgiadi A, Lichtenstein L, Degenhardt T, Boekschoten MV, van Bilsen M, Desvergne B, Müller M, Kersten S. Induction of cardiac Angptl4 by dietary fatty acids is mediated by peroxisome proliferator-activated receptor beta/delta and protects against fatty acid-induced oxidative stress. Circ Res. 2010;106:1712–21.
Brands M, Sauerwein HP, Ackermans MT, Kersten S, Serlie MJ. Omega-3 long-chain fatty acids strongly induce angiopoietin-like 4 in humans. J Lipid Res. 2013;54:615–21.
Robciuc MR, Tahvanainen E, Jauhiainen M, Ehnholm C. Quantitation of serum angiopoietin-like proteins 3 and 4 in a Finnish population sample. J Lipid Res. 2010;51:824–31.
Mehta N, Qamar A, Qu L, Qasim AN, Mehta NN, Reilly MP, Rader DJ. Differential association of plasma angiopoietin-like proteins 3 and 4 with lipid and metabolic traits. Arterioscler Thromb Vasc Biol. 2014;34:1057–63.
Tjeerdema N, Georgiadi A, Jonker JT, van Glabbeek M, Alizadeh Dehnavi R, Tamsma JT, Smit JW, Kersten S, Rensen PC. Inflammation increases plasma angiopoietin-like protein 4 in patients with the metabolic syndrome and type 2 diabetes. BMJ Open Diabetes Res Care. 2014;2(1):e000034.
Dallinga-Thie GM, Dullaart RP, van Tol A. Concerted actions of cholesteryl ester transfer protein and phospholipid transfer protein in type 2 diabetes: effects of apolipoproteins. Curr Opin Lipidol. 2007;18:251–7.
Tzotzas T, Desrumaux C, Lagrost L. Plasma phospholipid transfer protein (PLTP): review of an emerging cardiometabolic risk factor. Obes Rev. 2009;10:403–11.
Dullaart RP, van Tol A, Dallinga-Thie GM. Phospholipid transfer protein, an emerging cardiometabolic risk marker: is it time to intervene? Atherosclerosis. 2013;228:38–41.
Jiang XC, Qin S, Qiao C, Kawano K, Lin M, Skold A, Xiao X, Tall A. Apolipoprotein B secretion and atherosclerosis are decreased in mice with phospholipid-transfer protein deficiency. Nat Med. 2001;7:847–52.
Lie J, de Crom R, van Gent T, van Haperen R, Scheek L, Lankhuizen I, van Tol A. Elevation of plasma phospholipid transfer protein in transgenic mice increases VLDL secretion. J Lipid Res. 2002;43:1875–80.
Riemens SC, van Tol A, Sluiter WJ, Dullaart RP. Plasma phospholipid transfer protein activity is lowered by 24-h insulin and acipimox administration: blunted response to insulin in type 2 diabetic patients. Diabetes. 1999;48:1631–7.
Riemens SC, Van Tol A, Sluiter WJ, Dullaart RP. Acute and chronic effects of a 24-hour intravenous triglyceride emulsion challenge on plasma lecithin:cholesterol acyltransferase, phospholipid transfer protein, and cholesteryl ester transfer protein activities. J Lipid Res. 1999;40:1459–66.
Lu B, Moser A, Shigenaga JK, Grunfeld C, Feingold KR. The acute phase response stimulates the expression of angiopoietin like protein 4. Biochem Biophys Res Commun. 2010;391:1737–41.
Baranowski T, Kralisch S, Bachmann A, Lössner U, Kratzsch J, Blüher M, Stumvoll M, Fasshauer M. Serum levels of the adipokine fasting-induced adipose factor/angiopoietin-like protein 4 depend on renal function. Horm Metab Res. 2011;43:117–20.
Yang LY, Yu CG, Wang XH, Yuan SS, Zhang LJ, Lang JN, Zhao D, Feng YM. Angiopoietin-like protein 4 is a high-density lipoprotein (HDL) component for HDL metabolism and function in nondiabetic participants and Type-2 diabetic patients. J Am Heart Assoc. 2017;23;6. pii: e005973. https://doi.org/10.1161/JAHA.117.005973.
Tan KC, Shiu SW, Wong Y, Tam S. Plasma phospholipid transfer protein activity and subclinical inflammation in type 2 diabetes mellitus. Atherosclerosis. 2005;178:365–70.
Cheung MC, Brown BG, Marino Larsen EK, Frutkin AD, O'Brien KD, Albers JJ. Phospholipid transfer protein activity is associated with inflammatory markers in patients with cardiovascular disease. Biochim Biophys Acta. 1762;2006:131–7.
Dullaart RP, de Vries R, Dallinga-Thie GM, Sluiter WJ, van Tol A. Phospholipid transfer protein activity is determined by type 2 diabetes mellitus and metabolic syndrome, and is positively associated with serum transaminases. Clin Endocrinol. 2008;68:375–81.
Levels JH, Pajkrt D, Schultz M, Hoek FJ, van Tol A, Meijers JC, van Deventer SJ. Alterations in lipoprotein homeostasis during human experimental endotoxemia and clinical sepsis. Biochim Biophys Acta. 1771;2007:1429–38.
Speijer H, Groener JE, van Ramshorst E, van Tol A. Different locations of cholesteryl ester transfer protein and phospholipid transfer protein activities in plasma. Atherosclerosis. 1991;90:159–68.
Riemens S, van Tol A, Sluiter W, Dullaart R. Elevated plasma cholesteryl ester transfer in NIDDM: relationships with apolipoprotein B-containing lipoproteins and phospholipid transfer protein. Atherosclerosis. 1998;140:71–9.
de Vries R, Dallinga-Thie GM, Smit AJ, Wolffenbuttel BH, van Tol A, Dullaart RP. Elevated plasma phospholipid transfer protein activity is a determinant of carotid intima-media thickness in type 2 diabetes mellitus. Diabetologia. 2006;49:398–404.
Alex S, Lange K, Amolo T, Grinstead JS, Haakonsson AK, Szalowska E, Koppen A, Mudde K, Haenen D, Al-Lahham S, Roelofsen H, Houtman R, van der Burg B, Mandrup S, Bonvin AM, Kalkhoven E, Müller M, Hooiveld GJ, Kersten S. Short-chain fatty acids stimulate angiopoietin-like 4 synthesis in human colon adenocarcinoma cells by activating peroxisome proliferator-activated receptor γ. Mol Cell Biol. 2013;33:1303–16.
Al Majali K, Cooper MB, Staels B, Luc G, Taskinen MR, Betteridge DJ. The effect of sensitisation to insulin with pioglitazone on fasting and postprandial lipid metabolism, lipoprotein modification by lipases, and lipid transfer activities in type 2 diabetic patients. Diabetologia. 2006;49:527–37.
Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–25.
Gautier T, Lagrost L. Plasma PLTP (phospholipid-transfer protein): an emerging role in 'reverse lipopolysaccharide transport' and innate immunity. Biochem Soc Trans. 2011;39:984–8.
Schlitt A, Liu J, Yan D, Mondragon-Escorpizo M, Norin AJ, Jiang XC. Anti-inflammatory effects of phospholipid transfer protein (PLTP) deficiency in mice. Biochim Biophys Acta. 2005;1733:187–91.
Myocardial Infarction Genetics and CARDIoGRAM Exome Consortia Investigators, Stitziel NO, Stirrups KE, Masca NG, Erdmann J, Ferrario PG, König IR, Weeke PE, Webb TR, Auer PL, Schick UM, Lu Y, Zhang H, Dube MP, Goel A, Farrall M, Peloso GM, Won HH, Do R, van Iperen E, Kanoni S, Kruppa J, Mahajan A, Scott RA, Willenberg C, Braund PS, van Capelleveen JC, Doney AS, Donnelly LA, Asselta R, Merlini PA, Duga S, Marziliano N, Denny JC, Shaffer CM, El-Mokhtari NE, Franke A, Gottesman O, Heilmann S, Hengstenberg C, Hoffman P, Holmen OL, Hveem K, Jansson JH, Jöckel KH, Kessler T, Kriebel J, Laugwitz KL, Marouli E, Martinelli N, MI MC, Van Zuydam NR, Meisinger C, Esko T, Mihailov E, Escher SA, Alver M, Moebus S, Morris AD, Müller-Nurasyid M, Nikpay M, Olivieri O, Lemieux Perreault LP, AlQarawi A, Robertson NR, Akinsanya KO, Reilly DF, Vogt TF, Yin W, Asselbergs FW, Kooperberg C, Jackson RD, Stahl E, Strauch K, Varga TV, Waldenberger M, Zeng L, Kraja AT, Liu C, Ehret GB, Newton-Cheh C, Chasman DI, Chowdhury R, Ferrario M, Ford I, Jukema JW, Kee F, Kuulasmaa K, Nordestgaard BG, Perola M, Saleheen D, Sattar N, Surendran P, Tregouet D, Young R, Howson JM, Butterworth AS, Danesh J, Ardissino D, Bottinger EP, Erbel R, Franks PW, Girelli D, Hall AS, Hovingh GK, Kastrati A, Lieb W, Meitinger T, Kraus WE, Shah SH, McPherson R, Orho-Melander M, Melander O, Metspalu A, Palmer CN, Peters A, Rader D, Reilly MP, Loos RJ, Reiner AP, Roden DM, Tardif JC, Thompson JR, Wareham NJ, Watkins H, Willer CJ, Kathiresan S, Deloukas P, Samani NJ, Schunkert H. Coding variation in ANGPTL4, LPL, and SVEP1 and the risk of coronary disease. N Engl J Med. 2016;374:1134–44.
Vergeer M, Boekholdt SM, Sandhu MS, Ricketts SL, Wareham NJ, Brown MJ, de Faire U, Leander K, Gigante B, Kavousi M, Hofman A, Uitterlinden AG, van Duijn CM, Witteman JC, Jukema JW, Schadt EE, van der Schoot E, Kastelein JJ, Khaw KT, Dullaart RP, van Tol A, Trip MD, Dallinga-Thie GM. Genetic variation at the phospholipid transfer protein locus affects its activity and high-density lipoprotein size and is a novel marker of cardiovascular disease susceptibility. Circulation. 2010;122:470–7.
Huuskonen J, Ekström M, Tahvanainen E, Vainio A, Metso J, Pussinen P, Ehnholm C, Olkkonen VM, Jauhiainen M. Quantification of human plasma phospholipid transfer protein (PLTP): relationship between PLTP mass and phospholipid transfer activity. Atherosclerosis. 2000;151:451–61.
Oka T, Kujiraoka T, Ito M, Egashira T, Takahashi S, Nanjee MN, Miller NE, Metso J, Olkkonen VM, Ehnholm C, Jauhiainen M, Hattori H. Distribution of phospholipid transfer protein in human plasma: presence of two forms of phospholipid transfer protein, one catalytically active and the other inactive. J Lipid Res. 2000;41:1651–7.
Murdoch SJ, Wolfbauer G, Kennedy H, Marcovina SM, Carr MC, Albers JJ. Differences in reactivity of antibodies to active versus inactive PLTP significantly impacts PLTP measurement. J Lipid Res. 2002;43:281–9.
Acknowledgements
Plasma lipid and apolipoprotein measurement was carried out in the laboratory of Dr. L.D. Dikkeschei, Ph.D., Department of Clinical Chemistry, Isala Clinics Zwolle, The Netherlands. PLTP activity was measured by dr. G.M. Dallinga-Thie, Ph.D., Laboratory of Vascular Medicine, University of Amsterdam, the Netherlands.
Funding
Not applicable.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
EGG and RPFD analyzed the data. All authors discussed the results and contributed to the final manuscript. All authors approved the final version of this article.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the medical ethics committee of the University Medical Center Groningen, and written informed consent had been obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Gruppen, E.G., Kersten, S. & Dullaart, R.P.F. Plasma angiopoietin-like 4 is related to phospholipid transfer protein activity in diabetic and non-diabetic subjects: role of enhanced low grade inflammation. Lipids Health Dis 17, 60 (2018). https://doi.org/10.1186/s12944-018-0717-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-018-0717-5