Abstract
Background
Consumption of ≤10% total energy from fat as saturated fatty acids (SFA) is recommended for cardiovascular disease risk reduction in the UK; however there is no clear guidance on the optimum replacement nutrient. Lipid-associated single-nucleotide polymorphisms (SNPs) have been shown to modify the lipid responses to dietary fat interventions. Hence, we performed a retrospective analysis in 120 participants from the Dietary Intervention and VAScular function (DIVAS) study to investigate whether lipoprotein lipase (LPL) and apolipoprotein E (APOE) SNPs modify the fasting lipid response to replacement of SFA with monounsaturated (MUFA) or n-6 polyunsaturated (PUFA) fatty acids.
Methods
The DIVAS study was a randomized, single-blinded, parallel dietary intervention study performed in adults with a moderate cardiovascular risk who received one of three isoenergetic diets rich in SFA, MUFA or n-6 PUFA for 16 weeks.
Results
After the 16-week intervention, a significant diet-gene interaction was observed for changes in fasting total cholesterol (P = 0.001). For the APOE SNP rs1064725, only TT homozygotes showed a significant reduction in total cholesterol after the MUFA diet (n = 33; −0.71 ± 1.88 mmol/l) compared to the SFA (n = 38; 0.34 ± 0.55 mmol/l) or n-6 PUFA diets (n = 37; −0.08 ± 0.73 mmol/l) (P = 0.004). None of the interactions were statistically significant for the other SNPs.
Conclusions
In summary, our findings have demonstrated a greater sensitivity of the APOE SNP rs1064725 to dietary fat composition, with a total cholesterol lowering effect observed following substitution of SFA with MUFA but not n-6 PUFA. Further large intervention studies incorporating prospective genotyping are required to confirm or refute our findings.
Trial registration
The trial was registered at www.clinicaltrials.gov as NCT01478958.
Similar content being viewed by others
Background
A high consumption of saturated fatty acids (SFA) has been linked to increased circulating concentrations of low-density lipoprotein cholesterol (LDL-C) [1], and is consequently associated with an increased cardiovascular disease (CVD) risk [2]. Therefore, dietary guidelines have focused on reducing intakes of SFA by ≤10% of total energy (TE) for CVD risk reduction [3]. It is important to consider the nutrients that replace SFAs and previous findings have suggested substitution of SFA with unsaturated fatty acids may provide a greater reduction in CVD risk than refined carbohydrates [4, 5]. In particular, replacement with cis-monounsaturated fatty acids (MUFA) or polyunsaturated fatty acids (PUFA) has been shown to significantly lower fasting total and LDL-C [6, 7]. However, the inter-individual variability in fasting plasma lipid responses to dietary fat intake is high; evidence supports that this is influenced by lipid-associated single-nucleotide polymorphisms (SNPs) such as apolipoprotein E (APOE) and lipoprotein lipase (LPL) genotypes [8,9,10].
Several genes are involved in the regulation of lipid transport and metabolism [11]. Among these, the most commonly studied genes with central roles in lipid metabolism are LPL and APOE [12,13,14]. The LPL SNPs, rs320 (HindIII) and rs328 (S447X), have been proposed as important genetic determinants of the inter-individual variability in fasting and postprandial triacylglycerol (TAG) concentrations and high-density lipoprotein cholesterol (HDL-C) [15,16,17]. Increased activity of the LPL enzyme in minor allele carriers of LPL SNP rs328 has been shown to be associated with lower plasma TAG and higher HDL-C levels [18]. To date, there has only been one study reporting an interaction between LPL rs328 and n-6 PUFA intake on fasting TAG concentrations [19]. The effect of genetic variations of APOE on lipid concentrations (i.e. LDL-C) [20,21,22,23] and the effect of the APOE polymorphisms on the circulating lipid response to dietary fat (i.e. SFA and MUFA) have been previously demonstrated; however, the findings have been inconsistent [24,25,26]. In addition, investigations into other SNPs of the APOE gene are limited.
In the Dietary Intervention and VAScular function (DIVAS) study, the isoenergetic replacement of 9.5–9.6% TE from SFA with cis MUFA or n–6 PUFA for 16 weeks in 195 adults at moderate CVD risk resulted in significant reductions of 8.4% and 9.2%, respectively, in total cholesterol, and 11.3% and 13.6%, in LDL-C, in the fasted state [6]. To investigate whether genetic polymorphisms contributed to the observed reductions in total and LDL-C, a retrospective post hoc analysis of the DIVAS study was performed. We examined whether the two LPL and seven tagging SNPs (TagSNPs) in the APOE gene modified the response of the fasting lipid profile to substitution of SFA with MUFA or n-6 PUFA in this study population at moderate CVD risk.
Participants and methods
Study participants
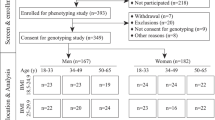
A detailed description of the DIVAS study design and methods has been reported elsewhere [6, 27]. Briefly, participants were recruited from Reading, UK and the surrounding area in three cohorts between November 2009 and July 2012. Participants were aged between 21 and 60 years and were all non-smoking men and women with a moderate risk of CVD. A scoring tool [27] was used to determine CVD risk based on the presence of single or multiple risk factors, including elevated fasting total cholesterol or fasting glucose, raised blood pressure, low HDL-C, being overweight or obese, and/or having a family history of premature myocardial infarction or type 2 diabetes. Eligible participants had a risk score of ≥ 2 combined points, reflecting a moderate CVD risk (≥50% above the population mean). Other criteria for exclusion were the presence of abnormal fasting blood biochemistry, taking dietary supplements or the use of medications that affect lipid metabolism or hypertension, and having inflammatory disorders. The West Berkshire Local Research ethics committee (09/ H0505/56) and the University of Reading Research Ethics Committee (09/40) gave a favourable ethical opinion for conduct. The trial was registered at www.clinicaltrials.gov as NCT01478958. All participants provided written informed consent before participating. In our retrospective analysis, 120 of the 195 participants who completed the DIVAS study consented to genetic analysis, and were included in the present study.
Study design and diets
The DIVAS study was a randomized, single-blinded, parallel design. The participants completed 16 weeks of dietary intervention, receiving one of three isoenergetic diets based on a minimization program that matched for age, sex, body mass index (BMI), and total CVD risk score. The three intervention diets (%TE derived from SFA:MUFA:n-6 PUFA) were either rich in SFAs (17:11:4), MUFAs (9:19:4), or n-6 PUFAs (9:13:10). Given that dietary guidelines recommend limiting n-6 PUFA intake to ≤10% TE [28], SFA were replaced with 6% TE n-6 PUFA and 2% TE MUFA in the n-6-PUFA-rich diet. The total fat content of all three intervention diets was 36% TE, and intakes of protein, carbohydrates, and n-3 PUFA were unchanged. A greater SFA exchange than the target 8% TE was achieved: SFA vs MUFA was 9.5% TE and SFA vs n-6 PUFA was 9.6% TE [27].
Further details of the dietary intervention procedure and measures of compliance have been published previously [27]. In summary, these interventions were based on a flexible food-exchange model to achieve the target fatty acid intakes in free-living individuals for 16 weeks. Participants, who were randomly assigned to one of three intervention diets, replaced routinely consumed sources of exchangeable fats with study foods. The study foods included spreads, oils, dairy products, and commercially available snacks of a specific fatty acid composition. Specially formulated spreads (80% total fat) and oils (Unilever Research and Development) were used for the MUFA-rich diet (refined olive oil and olive oil/rapeseed oil blended spread) and n–6 PUFA-rich diet (safflower oil and spread). Butter (Wyke Farm) was used as both a spread and oil replacement in the SFA-rich diet.
Anthropometric measurements and biochemical parameters
Clinical visits took place at the Hugh Sinclair Unit of Human Nutrition, University of Reading, during weeks 0 (baseline; V1) and 16 (after intervention; V2) as described elsewhere [6]. Alcohol and aerobic exercise were avoided 24 h before visits. Participants consumed a provided low-fat meal the evening before visits and fasted for 12 h, only drinking low-nitrate water during this time. Height and weight was recorded at the study visits at weeks 0 and 16 in order to calculate BMI. Height was recorded to the nearest 0.5 cm using a wall-mounted stadiometer and weight was measured using a digital scale (Tanita Europe) using standard settings (normal body type and 1 kg for clothing).
At weeks 0 and 16, fasting blood samples collected into a serum separator vacutainer and a K3EDTA-containing vacutainer (week 0 only) were used for the measurement of the fasting lipid profile and isolation of the buffy coat, respectively. The K3EDTA-containing vacutainer was kept on ice for 30 min before the blood tubes were centrifuged at 1700 g for 15 min at 20 °C (for serum) and 4 °C (for plasma). The buffy coat was stored at −20 °C and serum samples stored at −80 °C prior to analysis of total cholesterol, TAG, and HDL-C, and glucose (baseline only) concentrations using an autoanalyzer (reagents and analyzer: Werfen UK Ltd). Fasting LDL-C was estimated using the Friedewald formula [29]. With the use of A/A grade automated oscillometric ambulatory blood pressure (ABP) monitors (A&D Instruments Ltd.), baseline 24 h ABP was measured every 30 min from 07:00 to 21:59 and every 60 min from 22:00 to 06:59, approximately 48 h before the clinical visits.
SNP selection and genetic analysis
The APOE gene is located on chromosome 19q13.32 and comprises of four exons, which are transcribed into the 1180 nucleotides long APOE mRNA. The seven tagSNPs for the APOE gene were chosen based on International HapMap Phase II collected in individuals of Northern and Western European ancestry (CEU) (HapMap Data release 27 Phase 2 + 3, Feb 09, NCBI B36 assembly, dbSNP b126). The Haploview software V3.3 (https://www.broadinstitute.org/haploview/haploview) was used to assess the linkage disequilibrium structure between SNPs. Tagger software was used to select tag SNPs with the ‘pairwise tagging only’ option. Two criteria were used to filter the SNPs included in the analysis - minor allele frequency ≥ 5% and Hardy–Weinberg equilibrium P-value >0.01. Seven tagSNPs (rs405509 (G > T) [30, 31], rs1160985 (C > T) [32], rs769450 (G > A) [33], rs439401 (C > T) [34], rs445925 (G > A) [35], rs405697 (G > A) [36], and rs1064725 (T > G)) representing the entire common genetic variations across the APOE gene were selected for the study. In addition, the two commonly studied LPL SNPs, rs320 and rs328, were chosen. In total, nine common SNPs were examined in the present study.
DNA was extracted from the buffy coat using a QIAamp DNA blood kit (QIAGEN) and stored at −20 °C. The genotyping of the LPL and APOE SNPs was outsourced to LGC Genomics (http://www.lgcgroup.com/services/genotyping), which employs the competitive allele-specific PCR-KASP® assay.
Statistical analysis
The data are presented as mean ± standard deviation (SD) in the tables and text, and as standard error in the figure. The normal distribution was tested for variables, and none of the variables skewed the distribution. The minor allele frequency was calculated by counting. The dominant models were a better fit for SNPs rs320, rs328, rs769450, rs439401, rs445925, rs405697, and rs1064725; thus, homozygosity for the common allele was compared with carriers of the minor allele (heterozygous and homozygous for the minor allele) in the analysis. The additive model was applied for SNPs rs405509 and rs1160985 (major allele homozygotes vs. heterozygotes vs. minor allele homozygotes). The genotype distributions of the nine SNPs at the LPL and APOE genes were in Hardy-Weinberg equilibrium (P > 0.05). Independent t-tests were used to compare means between men and women at baseline. The baseline and over 16 weeks’ associations of the selected SNPs with continuous phenotypes were evaluated by the general linear model (GLM). Moreover, potential interactions between genotype and dietary intervention on 16-week changes of lipids were analyzed by using GLM, where an interaction term was included in the model. Potential confounders associated with the outcomes were adjusted in all GLM analyses (i.e. age, sex, BMI, and ethnicity). When a significant diet x genotype interaction was found, data were split by genotype group and analyzed further by using GLM. A Bonferroni correction was applied and the significant P value was 0.0013 (0.05/9 SNPs*4 lipid outcomes). For all analyses, the statistical package SPSS version 22.0 (SPSS, Chicago, IL, USA) was used.
Results
In this retrospective analysis, 120 participants (mean age, 47 ± 9 years; BMI, 26.4 ± 4.0 kg/m2) were included. Table 1 illustrates the main characteristics of the study participants stratified according to sex at baseline. Women had significantly lower levels of fasting TAG (P < 0.0001), LDL-C (P = 0.01), glucose (P = 0.02), blood pressures (P ≤ 0.03), and higher levels of fasting HDL-C (P < 0.0001) compared to men.
The genotype distributions of both LPL and APOE polymorphisms are shown in Table 2. The participants’ characteristics at the beginning of the dietary interventions (week 0) are presented in Table 3 according to LPL and APOE genotypes. None of the variables (including fasting TAG, total cholesterol, LDL-C, and HDL-C) were associated with the LPL and APOE SNPs at baseline. After 16 weeks of intervention, there was also no significant association of the LPL and APOE SNPs with changes in the lipid outcomes after Bonferroni correction (Tables 4, 5 and Additional file 1: Tables S1-S3).
At 16 weeks, after adjustment for age, sex, ethnicity and baseline BMI, a significant interaction between the APOE SNP rs1064725 and dietary intervention (SFA vs. MUFA vs. n-6 PUFA) on changes in fasting total cholesterol (Pinteraction = 0.001) was observed (Fig. 1). The ‘TT’ homozygotes (n = 108) of SNP rs1064725 had significantly lower total cholesterol concentrations after the MUFA (n = 33; −0.71 ± 1.88 mmol/l) compared with the SFA (n = 38; 0.34 ± 0.55 mmol/l; P = 0.003) and n-6 PUFA-rich diets (n = 37; −0.08 ± 0.73 mmol/l; P = 0.15) (Passociation = 0.004) (Fig. 1).
Mean (±SE) of changes in total cholesterol concentrations following three intervention diets [rich in either saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), and n-6 polyunsaturated fatty acids (PUFA)] according to the APOE SNP rs1064725 genotype (Pinteraction = 0.001). A general linear model analysis was performed with adjustments for age, sex, body mass index, and ethnicity. Individuals carrying the ‘TT’ genotype had lower total cholesterol levels after consuming the MUFA diet compared to the SFA or n-6 PUFA diets (Passociation = 0.004)
In addition, we also observed an interaction between LPL SNP rs320 and the dietary fat intervention (SFA vs. MUFA vs n-6 PUFA) on changes in LDL-C concentrations after 16 weeks (Pinteraction = 0.005) (Table 5). In the n-6 PUFA diet group, the G allele carriers (n = 19) of the LPL SNP showed a reduction in LDL-C levels (−1.0 ± 2.51 mmol/l) compared to the TT genotype (n = 24; 0.91 ± 2.23 mmol/l) (Passociation = 0.007). However, this interaction was not statistically significant after correction for multiple testing. None of the other SNPs showed a significant interaction on changes in lipid concentrations after the 16-week dietary intervention (Additional file 1: Tables S1-S3).
Discussion
To our knowledge, this is the first study to investigate the effects of SNPs in both LPL and APOE genes on fasting serum lipid response after substituting SFA with MUFA or n-6 PUFA. Our findings from this retrospective analysis of the DIVAS study showed that ‘TT’ homozygotes (90% of study population) at APOE SNP rs1064725 had significantly lower total cholesterol concentrations after the 16-week replacement of SFA with MUFA in adults at moderate risk of CVD. Our findings indicate a greater sensitivity of this genotype group to dietary fat composition, particularly with respect to replacement of SFA with MUFA, which may have important public health implications.
Findings from cross-sectional studies are not adequate to prove the beneficial impact of a dietary component on disease prevention; therefore, data from chronic dietary intervention studies are preferable to detect changes in disease biomarkers over a period of time [37]. A dietary intervention study has shown a reduction of 51% in fasting total cholesterol in non-diabetic adults with mild abdominal obesity after two weeks of following a MUFA-rich diet (20% TE) compared to a SFA-rich diet (19% TE) [38]. In support of the beneficial effect of MUFA-rich olive oil, a Mediterranean diet supplemented with extra-virgin olive oil for 4.8 years in older adults has also been shown to reduce the incidence of major CVD events [39], which suggests the potential role of MUFA and/or nutraceuticals such as polyphenols found in extra-virgin olive oil in the prevention of CVD-related outcomes [40]. Our retrospective data analysis has demonstrated a significant interaction between APOE SNP rs1064725 and a MUFA-rich diet on total cholesterol levels in adults at moderate CVD risk, where the MUFA-rich diet reduced fasting total cholesterol in ‘TT’ homozygotes compared to the SFA- and n-6 PUFA-rich diets. Our finding is in line with a previous study that also showed APOE genotypes to modulate changes in plasma total cholesterol and LDL-C in healthy individuals after consuming MUFA- (22% TE, virgin olive oil), SFA- (20% TE), and carbohydrate- (55% TE) rich diets for 4 weeks, where levels were higher in the E4/E3 carriers, intermediate in E3/E3 carriers, and lower in E3/E2 carriers [24]. Another study showed that a MUFA-rich dietary intervention (mainly olive oil) for 12 months increased the secretion of TAG-rich lipoproteins (TRL) containing apoE and decreased the secretion of those without apoE. As a result, a MUFA-rich diet shortened the residence time of very low density lipoprotein (VLDL) particles in the circulation and increased the direct clearance of TRL from the circulation (due to the enrichment of TRLs with apoE, a ligand for receptor mediated uptake), decreasing their conversion to LDLs [41]. Hence, it can be hypothesised that a MUFA-rich diet is likely to regulate the clearance rate of TRL among ‘TT’ genotype carriers of the APOE SNP rs1064725 via effects on TRL particle apolipoprotein composition. However, the underlying mechanism of how the ‘TT’ genotype acts differently from the ‘G’ allele on TRL metabolism in response to a MUFA-rich diet remains unclear and requires further investigation.
In our study, the common LPL SNP rs320 was found to modify the association between the n-6 PUFA-rich diet with changes in LDL-C levels, where the ‘G’ allele carriers had a tendency for a greater reduction in LDL-C concentrations compared to TT homozygotes. As far as the authors are aware, there are currently no studies to compare our findings with, except for one which showed that minor allele (‘G’) carriers of LPL SNP rs328 had lower fasting TAG concentrations when the participants had n-6 PUFA intake below 35.48% of total fat (below 35.48% of total fat median intake of LIPGENE study population) [19]. Besides LPL, evidence also suggests that the genetic effect of SNPs in APOA5 and TNFA on lipid metabolism is modulated by n-6 PUFA [42, 43]. In mice, n-6 PUFA intake has been shown to play a role in the upregulation of genes encoding proteins involved in adipogenesis [44]. Thus, dietary n-6 PUFA may upregulate LPL gene expression and/or activity, leading to lower circulating lipid concentrations [19, 45]. In addition to the role of LPL in hydrolysing TRL, LPL plays a role in binding TRL (i.e. VLDL) to hepatic LDL receptors, which help to mediate the clearance of these particles [46]. This leads to a reduced conversion of VLDL to LDL, resulting in lower plasma LDL-C levels [47]. Even though the interaction between the LPL SNP rs320 and n-6 PUFA-rich diet on LDL-C concentrations in the current data analysis was not statistically significant after Bonferroni correction, which could be due to the small sample size, further large studies are required to explore this gene-diet interaction.
Statistically significant interactions were demonstrated in this study, however there are some limitations. The sample size was relatively small for some of the genotype groups as the genotyping was performed retrospectively, and investigation of the lipid response according to APOE and LPL SNPs was not the main objective of the DIVAS study. Compared with cross-sectional studies, randomized clinical trials are conducted with smaller sample sizes. In our study, only 120 participants out of 195 consented to genetic analysis and hence this resulted in a small sample size for the analysis. However, we were able to identify significant gene-diet interactions on total cholesterol even after Bonferroni correction. Thus, this hypothesis testing analysis has identified the need for suitably-powered dietary intervention trials using prospective genotyping to investigate the impact of dietary fat composition on plasma lipid responses according to APOE genotypes. A selection bias may also have existed because the participants were multi-ethnic (Asian 7% and Black 7%). However, to reduce this potential confounding effect, the analyses were adjusted for ethnicity. Furthermore, the interaction between SNP rs1064725 at APOE and the intervention diets on total cholesterol was still significant (P = 0.003) even after excluding other ethnic groups from the analysis (data not shown). One of the main strengths of our study was that it examined the effects of three types of dietary fat (isoenergetically) consumed for a long duration (16 weeks) on lipid phenotypes in a robust randomised controlled intervention study, which addressed current dietary fat recommendations. Furthermore, we used a tagSNP approach whereby all the genetic variations in the APOE gene have been investigated in this study.
Conclusion
In conclusion, our study shows an interaction between APOE SNP rs1064725 and dietary fat intake on fasting total cholesterol concentrations, suggesting a greater sensitivity of the ‘TT’ homozygotes (90%) to dietary fat composition, with a total cholesterol lowering effect observed following substitution of SFA with MUFA but not with n-6 PUFA. However, given that the present study was conducted in a relatively small group of individuals, further large studies using prospective genotyping are required to confirm our findings.
Abbreviations
- APOE :
-
Apolipoprotein E
- BMI:
-
Body mass index
- CVD:
-
Cardiovascular disease
- DIVAS:
-
Dietary Intervention and VAScular function study
- GLM:
-
General linear model
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- LPL :
-
Lipoprotein lipase
- MUFA:
-
Monounsaturated fatty acids
- PUFA:
-
n-6 Polyunsaturated fatty acids
- SD:
-
Standard deviation
- SFA:
-
Saturated fatty acids
- SNPs:
-
Single-nucleotide polymorphisms
- TAG:
-
triacylglycerol
- TagSNPs:
-
Tagging SNPs
- TE:
-
Total energy
- TRL:
-
TAG-rich lipoproteins
- VLDL:
-
Very low density lipoprotein
References
Mensink RP. Effects of saturated fatty acids on serum lipids and lipoproteins: a systematic review and regression analysis. Geneva: World Health Organization; 2016. p 63.
Daida H, et al. The relationship between low-density lipoprotein cholesterol levels and the incidence of cardiovascular disease in high-risk patients treated with pravastatin: main results of the APPROACH-J study. Int Heart J. 2014;55(1):39–47.
Levy L, Tedstone A, Dietary Policy UK. For the prevention of cardiovascular disease. Healthcare (Basel). 2017;5(1).
Vannice G, Rasmussen H. Position of the academy of nutrition and dietetics: dietary fatty acids for healthy adults. J Acad Nutr Diet. 2014;114(1):136–53.
Szostak-Wegierek D, et al. The role of dietary fats for preventing cardiovascular disease. A review. Rocz Panstw Zakl Hig. 2013;64(4):263–9.
Vafeiadou K, et al. Replacement of saturated with unsaturated fats had no impact on vascular function but beneficial effects on lipid biomarkers, E-selectin, and blood pressure: results from the randomized, controlled dietary intervention and VAScular function (DIVAS) study. Am J Clin Nutr. 2015;102(1):40–8.
Livingstone KM, Lovegrove JA, Givens DI. The impact of substituting SFA in dairy products with MUFA or PUFA on CVD risk: evidence from human intervention studies. Nutr Res Rev. 2012;25(2):193–206.
Carvalho-Wells AL, et al. APOE genotype influences triglyceride and C-reactive protein responses to altered dietary fat intake in UK adults. Am J Clin Nutr. 2012;96(6):1447–53.
Caslake MJ, et al. Effect of sex and genotype on cardiovascular biomarker response to fish oils: the FINGEN study. Am J Clin Nutr. 2008;88(3):618–29.
Corella D, et al. MicroRNA-410 regulated lipoprotein lipase variant rs13702 is associated with stroke incidence and modulated by diet in the randomized controlled PREDIMED trial. Am J Clin Nutr. 2014;100(2):719–31.
Corella D, Ordovas JM. Single nucleotide polymorphisms that influence lipid metabolism: Interaction with dietary factors. Annu Rev Nutr. 2005;25:341–90.
Mead JR, Irvine SA, Ramji DP. Lipoprotein lipase: structure, function, regulation, and role in disease. J Mol Med (Berl). 2002;80(12):753–69.
Merkel M, Eckel RH, Goldberg IJ. Lipoprotein lipase: genetics, lipid uptake, and regulation. J Lipid Res. 2002;43(12):1997–2006.
Song Y, Stampfer MJ, Liu S. Meta-analysis: apolipoprotein E genotypes and risk for coronary heart disease. Ann Intern Med. 2004;141(2):137–47.
Tang W, et al. Associations of lipoprotein lipase gene polymorphisms with longitudinal plasma lipid trends in young adults: the coronary artery risk development in young adults (CARDIA) study. Circ Cardiovasc Genet. 2010;3(2):179–86.
Ariza MJ, et al. Additive effects of LPL, APOA5 and APOE variant combinations on triglyceride levels and hypertriglyceridemia: results of the ICARIA genetic sub-study. BMC Med Genet. 2010;11:66.
Shatwan IM, et al. Impact of lipoprotein lipase gene polymorphism, S447X, on postprandial triacylglycerol and glucose response to sequential meal ingestion. Int J Mol Sci. 2016;17(3).
Rip J, et al. Lipoprotein lipase S447X: a naturally occurring gain-of-function mutation. Arterioscler Thromb Vasc Biol. 2006;26(6):1236–45.
Garcia-Rios A, et al. Genetic variations at the lipoprotein lipase gene influence plasma lipid concentrations and interact with plasma n-6 polyunsaturated fatty acids to modulate lipid metabolism. Atherosclerosis. 2011;218(2):416–22.
Radwan ZH, et al. Comprehensive evaluation of the association of APOE genetic variation with plasma lipoprotein traits in U.S. whites and African blacks. PLoS One. 2014;9(12):e114618.
Ferreira CN, et al. Comparative study of apolipoprotein-E polymorphism and plasma lipid levels in dyslipidemic and asymptomatic subjects, and their implication in cardio/cerebro-vascular disorders. Neurochem Int. 2010;56(1):177–82.
Hanh NT, et al. Association of apolipoprotein E polymorphism with plasma lipid disorders, independent of obesity-related traits in Vietnamese children. Lipids Health Dis. 2016;15(1):176.
Hubacek JA, et al. Polygenic hypercholesterolemia: examples of GWAS results and their replication in the Czech-Slavonic population. Physiol Res. 2017;66(Supplementum 1):S101–s111.
Moreno JA, et al. The effect of dietary fat on LDL size is influenced by apolipoprotein E genotype in healthy subjects. J Nutr. 2004;134(10):2517–22.
Sarkkinen E, et al. Effect of apolipoprotein E polymorphism on serum lipid response to the separate modification of dietary fat and dietary cholesterol. Am J Clin Nutr. 1998;68(6):1215–22.
Couture P, et al. Influences of apolipoprotein E polymorphism on the response of plasma lipids to the ad libitum consumption of a high-carbohydrate diet compared with a high-monounsaturated fatty acid diet. Metabolism. 2003;52(11):1454–9.
Weech M, et al. Development of a food-exchange model to replace saturated fat with MUFAs and n-6 PUFAs in adults at moderate cardiovascular risk. J Nutr. 2014;144(6):846–55.
Dietary reference values for food energy and nutrients for the United Kingdom. Report of the panel on dietary reference values of the committee on medical aspects of food policy. Rep Health Soc Subj (Lond). 1991;41:1–210.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502.
Komurcu-Bayrak E, et al. The APOE -219G/T and +113G/C polymorphisms affect insulin resistance among Turks. Metabolism. 2011;60(5):655–63.
Viiri LE, et al. Interactions of functional apolipoprotein E gene promoter polymorphisms with smoking on aortic atherosclerosis. Circ Cardiovasc Genet. 2008;1(2):107–16.
Zhou L, et al. A genome wide association study identifies common variants associated with lipid levels in the Chinese population. PLoS One. 2013;8(12):e82420.
Son KY, et al. Genetic association of APOA5 and APOE with metabolic syndrome and their interaction with health-related behavior in Korean men. Lipids Health Dis. 2015;14:105.
Kring SI, et al. Impact of psychological stress on the associations between apolipoprotein E variants and metabolic traits: findings in an American sample of caregivers and controls. Psychosom Med. 2010;72(5):427–33.
Trompet S, et al. Replication of LDL GWAs hits in PROSPER/PHASE as validation for future (pharmaco)genetic analyses. BMC Med Genet. 2011;12:131.
Zhang Z, et al. Association of genetic loci with blood lipids in the Chinese population. PLoS One. 2011;6(11):e27305.
Ferguson JF, et al. NOS3 gene polymorphisms are associated with risk markers of cardiovascular disease, and interact with omega-3 polyunsaturated fatty acids. Atherosclerosis. 2010;211(2):539–44.
Bos MB, et al. Effect of a high monounsaturated fatty acids diet and a Mediterranean diet on serum lipids and insulin sensitivity in adults with mild abdominal obesity. Nutr Metab Cardiovasc Dis. 2010;20(8):591–8.
Estruch R, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279–90.
Scicchitano P, et al. Nutraceuticals and dyslipidaemia: beyond the common therapeutics. J Funct Foods. 2014;6(Supplement C):11–32.
Zheng C, et al. Dietary monounsaturated fat activates metabolic pathways for triglyceride-rich lipoproteins that involve apolipoproteins E and C-III. Am J Clin Nutr. 2008;88(2):272–81.
Lai CQ, et al. Dietary intake of n-6 fatty acids modulates effect of apolipoprotein A5 gene on plasma fasting triglycerides, remnant lipoprotein concentrations, and lipoprotein particle size: the Framingham heart study. Circulation. 2006;113(17):2062–70.
Fontaine-Bisson B, El-Sohemy A. Genetic polymorphisms of tumor necrosis factor-alpha modify the association between dietary polyunsaturated fatty acids and plasma high-density lipoprotein-cholesterol concentrations in a population of young adults. J Nutrigenet Nutrigenomics. 2008;1(5):215–23.
Madsen L, et al. cAMP-dependent signaling regulates the adipogenic effect of n-6 polyunsaturated fatty acids. J Biol Chem. 2008;283(11):7196–205.
Jump DB, et al. Dietary polyunsaturated fatty acid regulation of gene transcription. Prog Lipid Res. 1996;35(3):227–41.
Medh JD, et al. Lipoprotein lipase binds to low density lipoprotein receptors and induces receptor-mediated catabolism of very low density lipoproteins in vitro. J Biol Chem. 1996;271(29):17073–80.
Zheng C, et al. Rapid turnover of apolipoprotein C-III-containing triglyceride-rich lipoproteins contributing to the formation of LDL subfractions. J Lipid Res. 2007;48(5):1190–203.
Acknowledgements
We would like to thank Dr. Katerina Vafeiadou and Rada Mihaylova for their help conducting the DIVAS study, and Marinela Hasaj for her assistance with the DNA extraction. The Saudi government funded genotyping analysis of selected genetic variants. The study’s funders had no influence on the design of the study, analysis and interpretation of the data, writing, review, approval or submission of the manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
IMS and KSV performed the data analysis and wrote the manuscript. MW conducted the DIVAS study, analyzed the serum samples and critically reviewed the manuscript. KGJ and JAL designed the intervention study and critically reviewed the manuscript. KSV conceived the genetic research. All authors approved the final draft of this article prior to submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The West Berkshire Local Research ethics committee (09/ H0505/56) and the University of Reading Research Ethics Committee (09/40) gave a favourable ethical opinion for conduct. The trial was registered at www.clinicaltrials.gov as NCT01478958. All participants provided written informed consent before participating.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1: Table S1.
Changes in lipid levels after intervention with one of three diets over 16 weeks according to LPL rs328 genotypes. Table S2. Changes in lipid levels after intervention with one of three diets over 16 weeks according to APOE rs405509 and rs1160985 genotypes. Table S3. Changes in lipid levels after intervention with one of three diets over 16 weeks according to APOE rs769450, rs439401, rs445925 and rs405697 genotypes. (DOCX 26 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Shatwan, I.M., Weech, M., Jackson, K.G. et al. Apolipoprotein E gene polymorphism modifies fasting total cholesterol concentrations in response to replacement of dietary saturated with monounsaturated fatty acids in adults at moderate cardiovascular disease risk. Lipids Health Dis 16, 222 (2017). https://doi.org/10.1186/s12944-017-0606-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-017-0606-3