Abstract
Background
Modeling neo-aortic valve for arterial switch surgical planning to simulate the neo-aortic valve closure performance.
Methods
We created five geometrical models of neo-aortic valve, namely model A, model B, model C, model D and model E with different size of sinotubular junction or sinus. The nodes at the ends of aorta and left ventricle duct fixed all the degrees of freedom. Transvalvular pressure of normal diastolic blood pressure of 54 mmHg was applied on the neo-aortic valve cusps. The neo-aortic valve closure performance was investigated by the parameters, such as stress of neo-aortic root, variation of neo-aortic valve ring as well as aortic valve cusps contact force in the cardiac diastole.
Results
The maximum stress of the five neo-aortic valves were 96.29, 98.34, 96.28, 98.26, and 90.60 kPa, respectively. Compared among five neo-aortic valve, aortic valve cusps contact forces were changed by 43.33, −10.00% enlarging or narrowing the sinotubular junction by 20% respectively based on the reference model A. The cusps contact forces were changed by 6.67, −23.33% with sinus diameter varying 1.2 times and 0.8 times respectively.
Conclusions
Comparing with stress of healthy adult subjects, the neo-aortic valve of infant creates lower stress. It is evident that enlarging or narrowing the sinotubular junction within a range of 20% can increase or decrease the maximum stress and aortic valve cusps contact force of neo-aortic valve.
Similar content being viewed by others
Background
The arterial switch operation (ASO) is now preferred surgical approach to treat complete transposition of the great arteries (TGA) presenting in the neonatal period [1]. Although this surgery is thought to be an improvement compared with the earlier procedures, late cardiac complications have been reported in children, including pulmonary artery stenosis, neo-aortic valve insufficiency, and coronary obstruction [1–3]. Neo-aortic valve insufficiencies are approximate 15% after a 75 month follow-up [4]. At least moderate neo-aortic regurgitation is present in 3.4% [5].
Arterial switch operation involves four steps generally, such as closure of intracardiac defects, coronary transfer, aortic reconstruction and pulmonary artery reconstruction [6]. For coronary transfer, the dimension of excised aortic wall can control the sinus diameter (SD). Additionally, for aortic reconstruction, the diameter of pulmonary artery is larger than that of aorta on aortic anastomosis site. The surgical technique includes pulmonary artery constriction or patch enlargement of ascending aorta [7–9]. How to choose the size of STJ and SD of neo-aortic valve during the intraoperative period and profitable is still an open problem.
A structural model of neo-aortic valve for ASO was developed for finite element analysis so as to simulate closure performance of neo-aortic valve with the different size of sinotubular junction (DSTJ) and SD. Different geometric models with various diameter of DSTJ and SD were investigated by the parameters, such as stress of neo-aortic root, change of the neo-aortic valve ring and neo-aortic valve cusps contact force during cardiac diastole.
Methods
Modeling neo-aortic root with ASO was accomplished by using a 3-dimensional (3D) tool of computer aided design. We created five neo-aortic valve geometric models with the different size (summarized in Table 1) of DSTJ and SD suggested by Labrosse [10–12], Haj-Ali [13] and Marino [14], namely model A, model B, model C, model D and model E (Fig. 1). Stress of neo-aortic root, diameter of neo-aortic valve ring and cusps contact force were simulated with a finite element model for structural mechanics.
Geometry, mesh, tissue properties and boundary conditions of neo-aortic valve
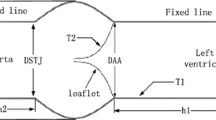
The five 3D neo-aortic valves were created by SolidWorks (SolidWorks, Concord, MA). The parametric dimensions (DSTJ; SD; valve height, hL; sinus height, hS; h1, h2) were scaled with the size of neo-aortic valve ring (9.70 mm) [15]. A constant thickness of neo-aortic wall and the three cusps was 0.6 and 0.3 mm, respectively [13]. We took no account of twist and tilt of ascending aorta in geometric models. Rigid cylindrical parts (5 mm) on both sides of neo-aortic valve mimic the aorta and left ventricle duct, so as to apply the fixity and boundary conditions. The geometries were meshed with shell elements in HyperMesh (Altair Engineering, Troy MI). Three leaflets were meshed with triangular elements, and other parts of aortic root were meshed with quadrilateral element (Fig. 2). All models of neo-aortic valve steered automatic time stepping (ATS) manually. ATS can be used to vary the time step while no convergence is obtained in the original time step. The solver subdivides the time steps, and attempts to solve again. We conducted mesh-dependence trials with three sizes of mesh density (Table 2) for structural model of neo-aortic valve. Mesh2 and mesh3 increased the calculation steps than mesh1. So mesh1 has more element number but processes faster than mesh2 and mesh3. Mesh1 achieved satisfactory results in less solution time. We chose the mesh density which is the same as the mesh 1 and generated five meshes of neo-aortic valve (Table 3).
We concentrated on closure performance of the neo-aortic valve during cardiac diastolic phase with normal diastolic blood pressure of 54 mmHg at six-month after birth [16, 17]. The valve model was then studied by applying known pressure load, as described by Zinner et al. [16]. The calculation models loaded with the peak pressure on the internal surface of neo-aortic, cusps surfaces and ventricular pressure to left ventricle inner wall [16]. In the neo-aortic valve models, the value of Young’s modulus and density were 1 and 2 MPa, 1100 and 2000 kg/m3 for cusps, ascending aorta and left ventricular duct [18, 19].
Solution of the five neo-aortic valve models
The structural solver used a dynamics implicit method. To eliminate the numerical oscillation of the neo-aortic valve cusps, the Rayleigh damping factor β = 0.15 was adopted for all elements at every time step [18]. We adopted the constraint function algorithm to simulate the interaction among cusps of neo-aortic valve. Coulomb friction coefficient was 0.013 among the cusps. the five models of neo-aortic valves were simulated and post-processed by finite element code of ADINA 8.9 (ADINA R&D, Watertown, MA) used 4 cores on Xeon 8 3.60 GHz HP Z420 workstation with 16.0 GB RAM. Both the software version and computer are the same as our previous publication [20].
Results
The closure performance of neo-aortic valve was investigated by the parameters, such as stress of neo-aortic root, variation of neo-aortic valve ring and cusps contact force during cardiac diastole.
Stress of neo-aortic root
The approach described above successfully computed the closing phase of the neo-aortic root. The closure performance of neo-aortic valve was described from the calculated data. The quality of the closure can be seen from the maximum stress, because excessive stress values can damage the valve and reduce its durability [19]. The stresses of neo-aortic root in Fig. 3 depict that the highest stresses occur always at the top of commissures attachments. The locations of all structure model maximum stress agree well with simulated data by Labrosse [11]. The neo-aortic root model from A to E show the maximum stresses of 96.29, 98.34, 96.28, 98.26 and 90.60 kPa, respectively. Enlarging or narrowing the DSTJ and SD by 20% increase or decrease maximum stress for neo-aortic valve. Several research groups reported maximum stresses of healthy adult subjects in previous studies (range in 300–600 kPa) [10]. Comparing with the maximum stress of healthy adult subjects, the infant creates lower stress.
Stress of neo-aortic root during diastole for all models. The neo-aortic root models from a to e show the maximum stresses of 96.29, 98.34, 96.28, 98.26 and 90.60 kPa, respectively. Increasing the DSTJ and SD within a range of 20% can increase the maximum stress for neo-aortic root, and vice versa. a Model A: DSTJ = 9.70 mm, SD = 12.30 mm; b Model B: DSTJ = 11.60 mm, SD = 12.30 mm; c Model C: DSTJ = 7.76 mm, SD = 12.30 mm; d Model D: DSTJ = 9.70 mm, SD = 14.76 mm; e Model E DSTJ = 9.70 mm, SD = 9.84 mm
Diameter of neo-aortic valve ring
We calculated the diameters of neo-aortic valve ring in the cardiac diastole period (Table 4). Diameters of neo-aortic valve ring were changed by 15.46, −24.74% enlarging or narrowing DSTJ by 20%. Diameters of neo-aortic valve ring were decreased by 14.43, 54.38% enlarging or narrowing SD by 20%. It is evident that increasing the DSTJ can decrease the diameter of the neo-aortic valve ring. Enlarging or narrowing SD can decrease the diameter of neo-aortic valve ring. Marom found that decreasing the aortic annulus diameter increased the coaptation height and area [19].
Contact force among neo-aortic valve cusps
We calculated the five neo-aortic valve models in the cardiac diastole to investigate closure performance with structural finite element method. Summation of nodes contact pressure was calculated to get the contact force among neo-aortic valve cusps, while enlarging or narrowing the DSTJ and SD. Contact force among neo-aortic valve cusps represents closure performance [19]. Contact forces (Table 5) among neo-aortic valve cusps are changed by 43.33, −10.00% enlarging or narrowing the DSTJ respectively by 20% compared. Contact forces among the neo-aortic valve cusps are changed by 6.67, −23.33% with SD varying 1.2× and 0.8× respectively. It is evident that enlarging and narrowing the DSTJ increase and decrease the contact force among the neo-aortic valve cusps respectively. Either enlarging or narrowing SD rise contact force among neo-aortic valve cusps.
Discussions
Detailed working process of aortic valve has two phases. Several studies focused on the opening phase of the valve. Some metrics are used to evaluate the opening performance in terms of opening area, blood flow velocity, transvalvular pressure gradient, shear stress, maximum stress values [21, 22]. Several studies concentrated on cardiac diastole period. Some metrics are used to evaluate the closure performance, such as aortic valve cusps contact pressure, cusps coaptation and regurgitation [18, 19, 23, 24]. In this paper, we concentrated on closure performance of neo-aortic valve in the cardiac diastolic period.
Labrosse listed the dynamic analysis results in the literature, and showed that the maximum stress is within the range of 300–600 kPa which come from five research groups [18]. In the literature reported by Marom research group, the maximum stress is 350 kPa during the aortic valve closure [10]. In conclusion, the infant creates lower maximum stress than healthy adult subjects.
When the DSTJ and SD increase within a range of 20%, the increment leads to increasing the surface area of sinus inner wall and leaflet. If the aortic valve could close normally, it needs to generate more contact force among three leaflets. So enlarging or narrowing the DSTJ or SD will lead to neo-aortic valve regurgitation after a long period of time after the ASO to the patient with complete TGA. However, from hemodynamic perspective, in further studies, FSI method are necessary to simulate the parameters such as blood flow resistance, transvalvular pressure gradients, and energy loss, which are currently used for the hemodynamic evaluation of native heart valves. The parameters could increase with decreasing DSTJ and SD [25].
Previously we have investigated the effects of DSTJ and Maximum SD on Aortic Valve when the DSTJ of reference model A is 26 mm. It is evident that enlarging or narrowing the DSTJ and SD by 20% increases or decreases the neo-aortic valve cusps contact force respectively [26]. However, When the DSTJ of the reference model is 9.7 mm, it is evident that increasing or decreasing SD can decrease the change of the aortic annulus diameter and increase neo-aortic valve cusps contact force. As to the effect of different age groups on dynamic behavior of aortic root, some further considerations are necessary.
In this paper, we focused on the effects of geometric factors and ignored the effects of the material property on aortic root for the moment. For further study based on patient specific model, it is strongly needed to consider the effects of material property on calculation results. In physiological condition, the pressure load is non-uniform distribution on the leaflets and other part of neo-aortic root [27, 28]. Coronary orifices cause that pressure on the sinus inner wall drops in systole period of cardiac cycle. Additional studies should be performed with FSI method that could simulate the biomechanical performance of blood flow, aortic cusps and other parts simultaneously. So we could investigate closure performance with more metrics such as geometric orifice area, coaptation area, stroke volume, and regurgitation flow. Besides, we are trying to study on the aortic valve based on patient specific model. For example, we are studying surgical planning of aortic valve orifice direction for ASO. We are continuing to collect and analyze new cases with aortic valve disease before and after the operation. In further study, we will consider increasing both DSTJ and SD based on patient specific model. The structural finite element model descripted in this paper could use to investigate the closure performance and explore the stress, variation of neo-aortic valve ring and cusps contact force [29].
Conclusion
We investigated the influence of varying the DSTJ and SD on the closure performance of neo-aortic valve after the ASO by structural finite element models. It is evident that enlarging or narrowing the DSTJ within a range of 20% can increase or decrease the maximum stress and the neo-aortic valve cusps contact force. Enlarging or narrowing the SD can decrease the change of the neo-aortic valve ring and increase the cusps contact force. It was a hint that varying the DSTJ and SD will lead to neo-aortic valve regurgitation after a long period of time after the ASO to the patient with complete TGA.
References
Tobler D, Williams WG, Jegatheeswaran A, Van Arsdell GS, McCrindle BW, Greutmann M, Oechslin EN, Silversides CK. Cardiac outcomes in young adult survivors of the arterial switch operation for transposition of the great arteries. J Am Coll Cardiol. 2010;56:58–64.
Schwartz ML, Gauvreau K, del Nido P, Mayer JE, Colan SD. Long-term predictors of aortic root dilation and aortic regurgitation after arterial switch operation. Circulation. 2004;110:128–32.
Legendre A, Losay J, Touchot-Koné A, Serraf A, Belli E, Piot JD, Lambert V, Capderou A, Planche C. Coronary events after arterial switch operation for transposition of the great arteries. Circulation. 2003;108:186–90.
Losay J, Touchot A, Capderou A, Piot JD, Belli E, Planché C, Serraf A. Aortic valve regurgitation after arterial switch operation for transposition of the great arteries incidence, risk factors, and outcome. J Am Coll Cardiol. 2006;47:2057–62.
Khairy P, Clair M, Fernandes SM, Blume ED, Powell AJ, Newburger JW, Landzberg MJ, Mayer JE Jr. Cardiovascular outcomes after the arterial switch operation for D-transposition of the great arteries. Circulation. 2013;127:331–9.
Talwar S, Reddy AV, Rajashekar P, Choudhary SK, Airan B. A simple modification to fix the commissural pillar during right ventricular outflow tract reconstruction during the arterial switch operation. Heart Lung Circ. 2014;23:383–4.
Nianguo D, Zongquan S, Wei S. Key issues and surgical strategies for one-stage arterial switch operation for transposition of the great arteries. Acta Medicinae Universitatis Scientiae et Technologiae Huazhong. 2008;37:514–6.
Nianguo D, Zongquan S, Wei S. One-stage arterial switch operation on transposition of the great arteries. J Clinical Cardiology. 2009;25:122–4.
Lacour-Gayet F. Arterial switch operation with ventricular septal defect repair and aortic arch reconstruction. Semin Thorac Cardiovasc Surg. 2007;19:245–8.
Labrosse MR, Lobo K, Beller CJ. Structural analysis of the natural aortic valve in dynamics: from unpressurized to physiologically loaded. J Biomech. 2010;43:1916–22.
Labrosse MR, Boodhwani M, Sohmer B, Beller CJ. Modeling leaflet correction techniques in aortic valve repair: a finite element study. J Biomech. 2011;44:2292–8.
Labrosse MR, Beller CJ, Robicsek F, Thubrikar MJ. Geometric modeling of functional trileaflet aortic valves: development and clinical applications. J Biomech. 2006;39:2665–72.
Haj-Ali R, Marom G, Ben Zekry S, Rosenfeld M, Raanani E. A general three-dimensional parametric geometry of the native aortic valve and root for biomechanical modeling. J Biomech. 2012;45:2392–7.
Marino BS, Wernovsky G, McElhinney DB, Jawad A, Kreb DL, Mantel SF, van der Woerd WL, Robbers-Visser D, Novello R, Gaynor JW, Spray TL, Cohen MS. Neo-aortic valvar function after the arterial switch. Cardiol Young. 2006;16:481–9.
Cohen MS, Marino BS, McElhinney DB, Robbers-Visser D, van der Woerd W, Gaynor JW, Spray TL, Wernovsky G. Neo-aortic root dilation and valve regurgitation up to 21 years after staged reconstruction for hypoplastic left heart syndrome. J Am Coll Cardiol. 2003;42:533–40.
Zinner SH, Rosner B, Oh W, Kass EH. Significance of blood pressure in infancy. Familial aggregation and predictive effect on later blood pressure. Hypertension. 1985;7:411–6.
Weltert L, de Tullio MD, Afferrante L, Salica A, Scaffa R, Maselli D, Verzicco R, De Paulis R. Annular dilatation and loss of sino-tubular junction in aneurysmatic aorta: implications on leaflet quality at the time of surgery. A finite element study. Interact CardioVasc Thorac Surg. 2013;17:8–12.
Marom G, Haj-Ali R, Raanani E, Schäfers HJ, Rosenfeld M. A fluid–structure interaction model of the aortic valve with coaptation and compliant aortic root. Med Biol Eng Comput. 2012;50:173–82.
Marom G, Haj-Ali R, Rosenfeld M, Schäfers HJ, Raanani E. Aortic root numeric model: annulus diameter prediction of effective height and coaptation in post-aortic valve repair. J Thorac Cardiovasc Surg. 2013;145:406–11.
Youlian P, Aike Q, Nianguo D. Fluid-structure interaction simulation of aortic valve closure with various sinotubular junction and sinus diameters. Ann Biomed Eng. 2015;43:1363–9.
Marom G, Peleg M, Halevi R, Rosenfeld M, Raanani E, Hamdan A, Haj-Ali R. Fluid-structure interaction model of aortic valve with porcine-specific collagen fiber alignment in the cusps. J Biomech Eng. 2013;135:101001–6.
Weinberg EJ, Mack PJ, Schoen FJ, García-Cardeña G, Kaazempur Mofrad MR. Hemodynamic environments from opposing sides of human aortic valve leaflets evoke distinct endothelial phenotypes in vitro. Cardiovasc Eng. 2010;10:5–11.
Griffith BE, Luo X, McQueen DM, Peskin CS. Simulating the fluid dynamics of natural and prosthetic heart valves using the immersed boundary method. Int J Appl Mech. 2009;1:137–77.
Hammer PE, Chen PC, del Nido PJ, Howe RD. Computational model of aortic valve surgical repair using grafted pericardium. J Biomech. 2012;45:1199–204.
Garcia D, Pibarot P, Dumesnil JG, Sakr F, Durand LG. Assessment of aortic valve stenosis severity a new index based on the energy loss concept. Circulation. 2000;101:765–71.
Qiao A, Pan Y, Dong N. Modeling study of aortic root for ross procedure: a structural finite element analysis. J Heart Valve Dis. 2014;23:683–7.
Carmody CJ, Burriesci G, Howard IC, Patterson EA. An approach to the simulation of fluid–structure interaction in the aortic valve. J Biomech. 2006;39:158–69.
Sturla F, Votta E, Stevanella M, Conti CA, Redaelli A. Impact of modeling fluid–structure interaction in the computational analysis of aortic root biomechanics. Med Eng Phys. 2013;35:1721–30.
Marom G, Halevi R, Haj-Ali R, Rosenfeld M, Schäfers HJ, Raanani E. Numerical model of the aortic root and valve: optimization of graft size and sinotubular junction to annulus ratio. J Thorac Cardiovasc Surg. 2013;146:1227–31.
Declarations
Authors’ contributions
AQ proposed the research direction of closure performance for neo-aortic valve and overall investigation. ZG and YP created the neo-aortic valve models, conducted simulation and postprocessing and wrote this manuscript. XH, ND, XL, YL and DS explained the clinical knowledge and evaluated the potential application. All authors read and approved the final manuscript.
Competing interests
All authors declare that they have no competing interests.
About this supplement
This article has been published as part of BioMedical Engineering OnLine Volume 15 Supplement 2, 2016. Computational and experimental methods for biological research: cardiovascular diseases and beyond. The full contents of the supplement are available online http://biomedical-engineering-online.biomedcentral.com/articles/supplements/volume-15-supplement-2.
Availability of data and materials
All data and materials in this article are available without restriction.
Funding
Publication charges for this article have been funded by National Natural Science Foundation of China (11472023, 81400290).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Gu, Z., Pan, Y., Qiao, A. et al. Numerical simulation of closure performance for neo-aortic valve for arterial switch operation. BioMed Eng OnLine 15 (Suppl 2), 150 (2016). https://doi.org/10.1186/s12938-016-0264-0
Published:
DOI: https://doi.org/10.1186/s12938-016-0264-0