Abstract
Background
Between 2011 and 2018, an estimated 134.8 million pyrethroid-treated long-lasting insecticidal nets (LLINs) were distributed nationwide in the Democratic Republic of Congo (DRC) for malaria control. Pyrethroid resistance has developed in DRC in recent years, but the intensity of resistance and impact on LLIN efficacy was not known. Therefore, the intensity of resistance of Anopheles gambiae sensu lato (s.l.) to permethrin and deltamethrin was monitored before and after a mass distribution of LLINs in Kinshasa in December 2016, and in 6 other sites across the country in 2017 and 11 sites in 2018.
Methods
In Kinshasa, CDC bottle bioassays using 1, 2, 5, and 10 times the diagnostic dose of permethrin and deltamethrin were conducted using An. gambiae s.l. collected as larvae and reared to adults. Bioassays were conducted in four sites in Kinshasa province 6 months before a mass distribution of deltamethrin-treated LLINs and then two, six, and 10 months after the distribution. One site in neighbouring Kongo Central province was used as a control (no mass campaign of LLIN distribution during the study). Nationwide intensity assays were conducted in six sites in 2017 using CDC bottle bioassays and in 11 sites in 2018 using WHO intensity assays. A sub-sample of An. gambiae s.l. was tested by PCR to determine species composition and frequency of kdr-1014F and 1014S alleles.
Results
In June 2016, before LLIN distribution, permethrin resistance intensity was high in Kinshasa; the mean mortality rate was 43% at the 5× concentration and 73% at the 10× concentration. Bioassays at 3 time points after LLIN distribution showed considerable variation by site and time and there was no consistent evidence for an increase in pyrethroid resistance intensity compared to the neighbouring control site. Tests of An. gambiae s.l. in 6 sites across the country in 2017 and 11 sites in 2018 showed all populations were resistant to the diagnostic doses of 3 pyrethroids. In 2018, the intensity of resistance varied by site, but was generally moderate for all three pyrethroids, with survivors at ×5 the diagnostic dose. Anopheles gambiae sensu stricto (s.s.) was the most common species identified across 11 sites in DRC, but in Kinshasa, An. gambiae s.s. (91%) and Anopheles coluzzii (8%) were sympatric.
Conclusions
Moderate or high intensity pyrethroid resistance was detected nationwide in DRC and is a serious threat to sustained malaria control with pyrethroid LLINs. Next generation nets (PBO nets or bi-treated nets) should be considered for mass distribution.
Similar content being viewed by others
Background
Malaria remains the leading cause of consultation, hospitalization, and death in the Democratic Republic of Congo (DRC), with on average more than 5000 malaria deaths per month [1]. The National Malaria Control Programme (NMCP) has a strategic goal of protecting at least 80% of the population at risk with preventative measures by 2020 [2]. The primary vector control method used to protect people in DRC is the free distribution of long-lasting insecticidal nets (LLINs). LLINs have been distributed on a provincial level, with rolling mass distributions scheduled for provinces approximately every 3 years, and routine distribution being done through ante-natal clinic (ANC) visits, expanded programme of immunization (EPI) visits, and in some provinces, school-based distribution.
Pyrethroids have been the insecticide class of choice for mosquito nets for more than 30 years and are still an important component of every LLIN that currently has prequalified status by World Health Organization (WHO) [3]. These compounds are fast-acting, safe for human contact, and have shown impressive community-level effects when deployed in areas where malaria vectors are susceptible [4, 5]. Pyrethroid resistance was first reported in malaria vectors in West Africa in the 1980s and 1990s [6, 7] and has since become widespread across most of sub-Saharan Africa [8]. The implications for malaria vector control are not clear, but resistance is a serious concern to the WHO [9] and the NMCP of DRC [10], with fears that pyrethroid resistance may compromise the efficacy of pyrethroid LLINs. Despite this, a number of studies in sub-Saharan Africa have shown that LLINs continued to help reduce malaria cases even with the presence of pyrethroid resistant malaria vectors [11,12,13,14,15].
In DRC, studies from 2013 have shown no significant difference in the odds of malaria infection between people owning a permethrin LLIN and those without a net, while those with deltamethrin- and alpha-cypermethrin-treated nets had significantly reduced odds of malaria infection [16, 17]. These results corresponded with the higher frequency of permethrin resistance than resistance to deltamethrin noted in susceptibility tests conducted in 2013 [18] (MPSMRM, 2014). Molecular analysis of pyrethroid resistant Anopheles malaria vectors in several locations in DRC has shown the upregulation of genes related to metabolic resistance that were associated with high rates of Plasmodium infection and loss of LLIN efficacy [19, 20].
Previous published susceptibility data from DRC has focused on the use of a diagnostic concentration of insecticide to determine whether a mosquito population is susceptible or resistant [9, 18, 21]. However, it is thought that the intensity of pyrethroid resistance may be an important indicator of potential pyrethroid LLIN control failure [22, 23]. For this reason, annual insecticide resistance intensity testing has been scaled-up in DRC [18, 21]. While agricultural use of pyrethroids has been associated with initial development of resistance in some studies [24, 25], other studies have found that mass distribution of LLINs was associated with increasing resistance [26,27,28]. These studies have mostly looked at mosquito populations retrospectively and little is known about how rapidly these changes occur following a LLIN distribution campaign. Therefore, part of this study was to monitor pyrethroid resistance intensity in suburbs of Kinshasa before and after mass LLIN distribution in December 2016. Additionally, intensity of pyrethroid resistance was monitored nationwide in six other sites in 2017 and 11 sites in 2018.
Methods
Study sites
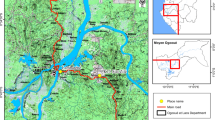
The first part of the study was conducted in Kinshasa Province in 2016 and 2017. Four sites were selected for mosquito larval collection to monitor changes in Anopheles gambiae sensu lato (s.l.) pyrethroid resistance intensity following mass LLIN (DawaPlus 2.0 coated with deltamethrin at a target dose of 80 mg/m2) distribution in December 2016 (Fig. 1). A fifth site, Kasangulu, in neighbouring Kongo Central province was selected to provide a “control” site, which would not be included in the Kinshasa Province LLIN distribution campaign in 2016. However, a limitation is that PermaNet 2.0 LLINs (formulation of deltamethrin at a target dose of 55 mg/m2)) were distributed in a mass campaign in Kongo Central Province in 2014. More details of the study sites and previous mass LLIN distributions in each region are presented in Additional file 1: Table S1.
The second part of the study involved nationwide testing of pyrethroid resistance intensity. Deltamethrin and permethrin resistance intensity tests were conducted in six sites in 2017 using Centers for Disease Control and Prevention (CDC) bottle bioassays. In 2018, testing was expanded to eleven sites, with resistance intensity to permethrin, alpha-cypermethrin and deltamethrin monitored using WHO tube tests for intensity (Fig. 2). More site details are included in Additional file 1: Table S1.
Sites where pyrethroid intensity assays were conducted throughout DRC in 2017 and 2018. Red stars indicate sites where bio-assays were conducted in 2017 and 2018; blue stars indicate sites where bio-assays were conducted in 2018 only. Note that the 2017 Kingasani results are presented with the Kinshasa results
Insecticide susceptibility tests
Mass LLIN distribution took place in December 2016 in Kinshasa Province. CDC intensity assays were conducted once before the mass distribution of LLINs (June 2016) and two, six, and ten months after the distribution (February 2017, June 2017, and October 2017). For each round of bioassays, An. gambiae s.l. larvae were collected from the five sites (Fig. 1) and returned to the laboratory at the Institute National de Recherche Biomédicale (INRB) in Kinshasa city, where they were reared to adults. Adult mosquitoes were kept in cages and provided with 10% sugar solution ad libitum until the time of testing at the age of 2–5 days.
The intensity assays conducted nationwide followed the same protocol, but tests were conducted once per year (all tests between January and August in 2017 and 2018) and mosquitoes were reared and tested in field insectaries.
CDC bottle bioassays
CDC bottle bioassays were conducted to determine the intensity of pyrethroid resistance, following standard guidelines [29, 30]. Pre-measured vials of technical grade active ingredient were supplied by CDC and made into stock solutions for each insecticide dose by diluting with acetone. Stock solutions were stored in the refrigerator (4 °C) in light-proof bottles for future use. Glass Wheaton bottles (250 ml) were washed with warm soapy water and rinsed thoroughly with water at least three times and left to dry overnight. A disposable pipette was used to transfer 1 ml of acetone into the negative control bottle and 1 ml of each stock solution into the respective treatment bottle. Bottles were swirled so that the glass bottom and inside cap were coated before being placed on their side and rotated while rocking so that the sides were evenly coated with insecticide. The bottles were protected from sunlight, and caps removed before being left to dry overnight.
An aspirator was used to gently add twenty-five mosquitoes into each bottle per replicate. Four replicates of each dose were done to reach approximately 100 mosquitoes tested. Mosquitoes were exposed for the diagnostic time of 30 min, with knock-down being recorded at the end of exposure. A knocked-down mosquito was defined as not being able to stand. Deltamethrin was tested at 1× (12.5 μg/bottle), 2× (25 μg/bottle), 5× (62.5 µg/bottle), and 10× (125 µg/bottle) the diagnostic dose for Anopheles. Permethrin was also tested at 1× (21.5 μg/bottle), 2× (43 μg/bottle), 5× (107.5 µg/bottle), and 10 times (215 µg/bottle) the diagnostic dose for Anopheles.
WHO susceptibility tests
In 2018, insecticide susceptibility and resistance intensity testing were conducted in 11 sentinel sites (Fig. 1) using the WHO tube test. The insecticides tested in 2018 were: deltamethrin 1×, 5×, 10× (0.05%, 0.25%, 0.5%); permethrin 1×, 5×, 10× (0.75%, 3.75%, 7.5%) and alpha-cypermethrin 1×, 5×, 10× (0.05%, 0.25%, 0.5%). In all sites, susceptibility testing was conducted with adult An. gambiae s.l., following WHO protocols [22]. INRB entomologists traveled to each site to collect larvae and pupae, which were reared to female adult mosquitoes aged 2–5 days and exposed for 1 hour to insecticide-treated filter papers provided by the WHO (prepared by Universiti Sains Malaysia). All tests were accompanied by negative control tests where mosquitoes were exposed to filter papers impregnated with oil or solvent. Testing was done according to WHO protocols, with mortality read 24 h after exposure. Four replicates of 25 An. gambiae s.l. were exposed to each concentration.
Identification of species and target site mutations
A subset of An. gambiae s.l. that were collected from the four sites in Kinshasa (Bu, Kimpoko, Kingasani and Kinkole) and 1 ‘control’ site in Kongo Central (Kasungulu) in October 2017 and tested in CDC control bottles, were sent to CDC, Atlanta, USA for molecular analysis. In addition, 100 mosquitoes used for WHO bioassays in each of the eleven nationwide sites in 2018 were used for molecular analysis at INRB, Kinshasa, DRC. PCR was used to determine the species of mosquitoes from the An. gambiae complex and to determine the frequency of the voltage-gated sodium channel mutation (VGSC) 1014S (formerly known as kdr-east) and VGSC-1014F (formerly known as kdr-west).
Genomic DNA was extracted from whole mosquitoes at CDC using ExtractaTM DNA Prep for PCR-Tissue kits (QuantaBio, USA) and at INRB using the CTAB method [31]. Species identification was performed according to the protocol of Wilkins et al. [32] at CDC and using the protocol of Santolamazza et al. [33] at INRB. The VGSC-1014S and 1014F alleles were detected using adapted protocols for allele-specific PCR (AS-PCR) [34,35,36]. Anopheles coluzzii AKDR and An. gambiae sensu stricto (s.s.) RSP-ST strains from the Malaria Research and Reference Reagent Resource Center (MR4), were used as positive controls, alongside negative (no template) controls.
Analysis
Scoring of bottle bioassays using the diagnostic dose followed WHO and CDC criteria, with mortality of 98–100% indicating susceptibility, 90–97% indicating possible resistance that should be confirmed, and less than 90% indicating resistance [22, 29]. Mortality of 98–100% at the 5× concentration (but < 90% at 1×) indicates low resistance intensity. Mortality < 98% at the 5 × concentration but 98–100% at the 10× concentration indicates moderate resistance intensity. Mortality < 98% at the 10× concentration indicates high resistance intensity [22].
The comparison of bioassay results prior to the LLIN mass distribution and after distribution in Kinshasa were made using a logistic regression model, taking into account the dose, site, time period, and an interaction between dose and site as fixed effects and bottle as a random effect. Analysis was done using the glmm function in R (version 3.2.3). Pearson’s Chi squared test was used to determine deviations from Hardy–Weinberg equilibrium for VGSC-1014F allele frequencies.
Results
Intensity of resistance in Kinshasa Province before and after LLIN mass distribution using CDC bottle bioassay
Over the four periods of testing, a total of 15,200 An. gambiae s.l. were used for resistance intensity bioassays in Kinshasa Province. Resistance to permethrin and deltamethrin was found in all sites (Fig. 3). In June 2016, before LLIN distribution mean results for Kinshasa (mean of Kingasani, Kinkole, Kimpoko, Bu) showed that permethrin resistance intensity was high and the mean mortality rate was 43% at the 5× concentration and 73% at the 10× concentration. After the mass distribution of LLINs in December 2016 (mean results for February, June and October tests) the mean mortality rate in Kinshasa was 32% for 5× and 60% with permethrin at the 10× concentration. The mean resistance intensity to deltamethrin was also high before LLIN distribution (75% at 5× and 94% mortality at 10× concentration) but decreased after LLIN distribution to a mean of 95% and 99% mortality at 5 and 10× concentrations, respectively. In general, levels of resistance were lower for deltamethrin, compared to permethrin. However, there was considerable variation in the results by site (Table 1). The hypothesis was that resistance intensity to permethrin and deltamethrin would increase in Kinshasa following LLIN distribution, compared to the control site of Kasangulu. In the control site of Kasangulu, mortality in permethrin intensity tests decreased significantly in 2017 (indicating an increase in resistance).
Resistance intensity was greater in Kinkole for permethrin (OR 11.49, p-value < 0.001) and deltamethrin (OR 22.00, p-value < 0.001) compared to Kasangulu post-LLIN distribution and also in Kimpoko for deltamethrin (OR 2.57, p-value < 0.001). In Kingasani, the opposite trend was recorded with a significantly lower resistance intensity following LLIN distribution for permethrin (OR 0.11, p-value < 0.001) and deltamethrin (OR 0.21, p-value < 0.001) compared to the control site, while in Bu there was no significant change post-distribution in resistance intensity for either insecticide.
Intensity of permethrin and deltamethrin resistance in six sites in DRC in 2017 using CDC bottle bioassay
Nationwide bioassay testing showed that permethrin resistance was present in all 6 sites, with less than 10% mortality at the diagnostic dose. Mortality rates increased slightly with increased concentration, but high intensity permethrin resistance was present in all sites, with considerably less than 98% mortality at 10× the diagnostic concentration of permethrin (Fig. 4).
Anopheles gambiae s.l. populations were less intensely resistant to deltamethrin, although all populations tested were resistant at 1× and 2× the diagnostic dose. The resistance intensity was low (> 98% mortality at 5× dose) in Kabondo and Inongo, moderate in Kalemie and Katana (> 98% mortality at 10× dose) and high (< 98% mortality at 10× dose) in Mikalayi and Kapolowe (Fig. 5).
Intensity of permethrin, deltamethrin and alpha-cypermethrin resistance in eleven sites in 2018 in DRC using the WHO tube test
In 2018, nationwide WHO insecticide susceptibility and resistance intensity tests were completed with An. gambiae s.l. populations in 11 sites. The data is presented in Figs. 6, 7, and 8 for permethrin, deltamethrin and alpha-cypermethrin, respectively. In Kabondo, testing with alpha-cypermethrin 5× and 10× was not completed as mortality was > 20% in the control and the field team was unable to find sufficient larvae for repeat tests. Resistance to permethrin (< 90% mortality) was observed in all sites at the diagnostic dose (1×), except Katana, where there was possible resistance (90–98% mortality). Resistance intensity was low in Katana, Inongo, and Kapolowe; moderate (> 98% mortality at 5× dose) in Karawa, Kimpese, Mikalayi, and Pawa; and high (< 98% mortality at 10× dose) in Kingasani, Lodja, and Kalemie (Fig. 6).
Resistance to deltamethrin was recorded in all sites, except Katana (possible resistance), Lodja and Inongo (susceptible). The intensity of resistance was low in Kapolowe and Kabondo, moderate in Mikalayi, and high in Kingasani, Karawa, Kimpese, Kalemie, and Pawa (Fig. 6).
Resistance to alpha-cypermethrin was also observed in all sites. The intensity was low in Kalemie and Kapolowe, high in Katana, Mikalayi, and Lodja, and moderate in the remaining five sites (Fig. 7).
Species identification within the An. gambiae complex and frequency of VGSC-1014F and 1014S resistance alleles
Kinshasa resistance intensity study post-LLIN distribution (2017)
A total of 217 An. gambiae s.l. were tested for species identification and of those samples, 189 were analysed for VGSC-1014F and 1014S frequency. The primary species found was An. gambiae s.s. (91%). Anopheles coluzzii were only identified in Kimpoko (9%) and Kinkole (32%). Hybrid An. gambiae s.s./An. coluzzii were only found in Kinkole (2/44) (Table 2). One percent of samples did not amplify.
Overall, both An. gambiae s.s. and An. coluzzii carried VGSC-1014F and 1014S resistance alleles, albeit at different frequencies. The mean frequency of resistance alleles determined across all sites for An. gambiae s.s. was 83% for homozygote VGSC-1014F, 3% for homozygote 1014S and 14% for heterozygous 1014F/1014S (Table 3). All VGSC-1014F frequencies were over 70% for An. gambiae s.s. and the highest was observed in Kingasani and Kinkole (both 91%). The trend was different for An. coluzzii, with 94% (16/17) being homozygous for the VGSC-1014S allele.
Resistance intensity survey covering 11 sites nationwide (2018)
A total of 998/1100 (91%) An. gambiae s.l. were successfully amplified for species identification and 862/1100 (78%) for L1014F. Overall, An. gambiae s.s. (98.5%) was the primary species in all 11 sites. Anopheles coluzzii were only found in Kingasani (1%) and Mikalayi (1%) and 1% of hybrid An. gambiae s.s./An. coluzzii were found in Kingasani (Table 4). The VGSC-1014F frequency for An. gambiae s.l. recorded over eleven sites in 2018 varied between 0.85 (Kingasani) and 1.0 (Pawa and Karawa). The mean VGSC-1014F was close to fixation at 0.98 (Table 5). Evidence for significant deviations from Hardy–Weinberg equilibrium were observed for VGSC-1014F in Kingasani, Kalemie, Kabondo and Katana (Table 5).
Discussion
Insecticide-treated nets are believed to be an important source of selection pressure for pyrethroid resistance genes in African malaria vectors [27, 37]. In addition to LLINs, other environmental factors such as agricultural pesticide run off into mosquito larval sites, may exert additional selection pressure on malaria vectors [24, 38]. Between 2011 and 2018, an estimated 134.8 million LLINs were distributed nationwide in DRC through mass campaigns and through routine distribution in schools and during ANC and EPI visits [39]. National Demographic and Health Surveys (DHS) have documented a substantial increase in net ownership, from just 9% of households nationwide owning at least one LLIN in 2007 [40], compared with 51% in 2010 [41] and 70% in 2013/14 [42]. This scale up of LLINs in DRC has coincided with the gradual spread of pyrethroid resistance and more recent increase in resistance intensity. Following a mass LLIN distribution campaign in Kinshasa in 2016, this study produced no consistent evidence for an increase in pyrethroid resistance intensity compared to the neighbouring control site of Kasungulu, where there was no mass LLIN campaign in 2016. There was a great deal of variation over time by insecticide and site. It is difficult to design a study to effectively measure the contribution of mosquito nets to selection pressure of mosquitoes, since LLINs are already widely distributed in DRC and pyrethroid resistance is prevalent in all malaria eco-epidemiological settings. It is also difficult to measure the impact of household and agricultural use of pyrethroids. Mass LLIN campaigns had previously been conducted in Kinshasa in 2008 and 2013, and in Kongo Central Province (where Kasungulu is situated) in 2012 and 2014 (Additional file 1: Table S1). Pyrethroid selection pressure had been ongoing for many years before the 2016 distribution in Kinshasa, which may explain the lack of difference between sites following the 2016 LLIN campaign in Kinshasa.
Nationwide tests of malaria vector populations in 6 sites in 2017 and 11 sites in 2018 showed all populations were resistant to diagnostic doses of type I (permethrin) and type II (deltamethrin and alpha-cypermethrin) pyrethroids. Regular monitoring of vector resistance has shown that pyrethroid resistance in An. gambiae s.l. became widespread in DRC relatively recently. Permethrin susceptible An. gambiae s.l. were present in Kinshasa in 2009 [21], while deltamethrin susceptibility was recorded in Lodja (Sankuru Province), Kalemie (Tanganyika Province), Kapolowe (Haut Katanga), Katana (Sud Kivu) and Kinshasa in 2016 [18]. Resistance to permethrin, deltamethrin and alpha-cypermethrin now appears to be present nationwide.
Anopheles gambiae s.s. was the most common vector species identified among the An. gambiae complex analysed across 11 sites in DRC. However, in Kinshasa, An. gambiae s.s. (91%) and An. coluzzii (8%) were sympatric and there was a small proportion of hybrid An. gambiae s.s./An. coluzzii (5%) in Kinkole. Though the frequency of hybrids in the Anopheles population from Kinkole is low, mating seems to be occurring between the two species. Both species are commonly sympatric in Central Africa, but hybrids of An. gambiae s.s./An. coluzzii are usually very uncommon [43, 44]. Populations of An. gambiae and An. coluzzii have previously been shown to be sympatric in several geographical areas in DRC, including Lodja, Mikalayi, Kalemie, Katana, Kinshasa, Kimpese and Inongo [18].
Both An. gambiae s.s. and An. coluzzii carried VGSC-1014F and 1014S alleles. However, An. coluzzii in Kinshasa had a high frequency of the 1014S allele, while An. gambiae s.s. had a high frequency of the 1014F allele; the latter observation may partially explain the higher levels of local permethrin resistance, despite deltamethrin-treated LLINs predominating in the most recent mass distribution campaigns. VGSC-1014F and L1014S are suspected to play a larger contributing role in resistance to type I (permethrin) versus type II (deltamethrin and alpha-cypermethrin) pyrethroids [45]. Interestingly, a proportion of heterozygous An. gambiae s.s. from Kinshasa harboured both VGSC-1014F and 1014S alleles. The phenomenon of these mutations co-occurring in individual mosquitoes has previously been reported in Senegal [46] and Uganda [47] and in Nord Ubangi, DRC [48]; however, the biological implications of possessing both resistance genotypes remain unknown and warrant further investigation. A limitation of this study is that only target site mutations for resistance were investigated. Mixed function oxidases (MFO) are implicated in pyrethroid resistance in several sites in DRC [49]. In addition, bioassays in 2016 showed increased mortality in permethrin resistant populations in DRC after pre-exposure to synergist piperonyl butoxide (PBO) [18]. The genetic basis conferring resistance to pyrethroids in malaria vectors An. gambiae s.s. and An. coluzzii needs to be investigated at the national level to improve malaria control decision-making, particular with regard to choice of LLINs for mass distribution campaigns.
Widespread pyrethroid resistance, particularly high intensity resistance, is of great importance for the NMCP for the implementation of evidence-based resistance management strategies and deployment of efficacious malaria vector control tools. Resistance intensity assays showed that neither 1, 5 or 10 times the diagnostic concentrations of permethrin, deltamethrin and alpha-cypermethrin were sufficient to provide adequate mortality of An. gambiae s.l. collected from 6 nationwide sites in 2017 tested using CDC bottle bioassays and 11 sites in 2018 using WHO tube tests. The WHO states that “when resistance is confirmed at the 5× and especially at the 10× concentrations, operational failure is likely” [22]. Pyrethroid LLINs should continue to offer some protection from malaria even in locations with high intensity resistance, through a combination of physical barrier, reduced survival of malaria vectors and malaria parasites [50,51,52]. However, next generation LLINs either impregnated with pyrethroids and the synergist PBO or containing chlorfenapyr (Interceptor G2®) are potential alternatives for the improved efficacy of LLINs and for resistance management. Several experimental hut studies have shown improved efficacy of PBO and chlorfenapyr LLINs in controlling pyrethroid resistant malaria vectors compared to conventional pyrethroid LLINs [53,54,55,56,57]. LLINs containing PBO or novel insecticide classes should be considered by the NMCP of DR Congo for future LLIN distribution campaigns in areas of moderate to high intensity of pyrethroid resistance, although the costs of these nets would also need to be considered.
Conclusion
The widespread presence of moderate to high intensity pyrethroid resistance across all sentinel sites in DRC is a great concern. There was a great deal of variation in resistance over time by insecticide and no consistent evidence for an increase in pyrethroid resistance intensity was observed following the mass LLIN campaign. The difficulties in defining resistance and understanding its complexities don’t change the fact that it is a great concern and next generations nets should be considered in DRC to sustain effective malaria control.
Availability of data and materials
All data generated or analysed during this study are included in this article and are available from the corresponding author.
Abbreviations
- DRC:
-
Democratic Republic of Congo
- LLIN:
-
Long lasting insecticidal net
- PCR:
-
Polymerase chain reaction
- WHO:
-
World Health Organization
- CDC:
-
Center for Disease Control
- Kdr:
-
Knock down resistance
- NMCP:
-
National Malaria Control Program
- ANC:
-
Ante-natal clinic
- EPI:
-
Expanded program of immunization
- VGSC:
-
Voltage-gated sodium channel
- INRB:
-
Institut National de Recherche Biomédicale
- MFO:
-
Mixed function oxidases
- PBO:
-
Piperonyl butoxide
References
Ecole de Santé Publique de l’Université de Kinshasa (ESPK). République Démocratique du Congo: evaluation des Prestations des Services de soins de Santé 2017–2018. Kinshasa; 2019.
Programme National de Lutte contre le Paludisme, Republique Democratique du Congo. Plan strategique national de lutte contre le paludisme: 2016–2020. Kinshasa; 2016.
List of WHO Prequalified Vector Control Products (http://www.who.int/pq-vector-control/prequalified-lists/PQT_VC_17July2018.pdf?ua=1).
Gimnig JE, Kolczak MS, Hightower AW, Vulule JM, Schoute E, Kamau L, et al. Effect of permethrin-treated bed nets on the spatial distribution of malaria vectors in western Kenya. Am J Trop Med Hyg. 2003;68:115–20.
Lyimo EO, Msuya FH, Rwegoshora RT, Nicholson EA, Mnzava AE, Lines JD, et al. Trial of pyrethroid impregnated bednets in an area of Tanzania holoendemic for malaria. Part 3. Effects on the prevalence of malaria parasitaemia and fever. Acta Trop. 1991;49:157–63.
Malcolm CA. Current status of pyrethroid resistance in anophelines. Parasitol Today. 1988;4:S13–5.
Elissa N, Mouchet J, Rivière F, Meunier JY, Yao K. Resistance of Anopheles gambiae s.s. to pyrethroids in Côte d’Ivoire. Ann Soc Belg Med Trop. 1993;73:291–4.
Ranson H, Lissenden N. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016;32:187–96.
WHO. The Global Plan for Insecticide Resistance Management in Malaria Vectors (GPIRM). Geneva: World Health Organization, 2012. (http://www.who.int/malaria/publications/atoz/gpirm/en/).
National Malaria Control Programme Democratic Republic of Congo. Plan National de gestion de la resistance des vecteurs du paludisme aux insecticides en Republique Democratique du Congo, 2017–2020.
Briet OJ, Penny MA, Hardy D, Awolola TS, Van Bortel W, Corbel V, et al. Effects of pyrethroid resistance on the cost effectiveness of a mass distribution of long-lasting insecticidal nets: a modelling study. Malar J. 2013;12:77.
Strode C, Donegan S, Garner P, Enayati AA, Hemingway J. The impact of pyrethroid resistance on the efficacy of insecticide-treated bed nets against African anopheline mosquitoes: systematic review and meta-analysis. PLoS Med. 2014;11:e1001619.
Bradley J, Ogouyemi-Hounto A, Cornelie S, Fassinou J, de Tove YSS, Adeothy AA, et al. Insecticide-treated nets provide protection against malaria to children in an area of insecticide resistance in Southern Benin. Malar J. 2017;16:225.
Tokponnon FT, Sissinto Y, Ogouyemi AH, Adeothy AA, Adechoubou A, Houansou T, et al. Implications of insecticide resistance for malaria vector control with long-lasting insecticidal nets: evidence from health facility data from Benin. Malar J. 2019;18:37.
World Health Organization: WHO-coordinated multi-country evaluation. Implications of insecticide resistance for malaria vector control 2016; WHO/HTM/GMP/2016.8.rev.
Janko MM, Irish SR, Reich BJ, Peterson M, Doctor SM, Mwandagalirwa MK, et al. The links between agriculture, Anopheles mosquitoes, and malaria risk in children younger than 5 years in the Democratic Republic of the Congo: a population-based, cross-sectional, spatial study. Lancet Planet Health. 2018;2:e74–82.
Levitz L, Janko M, Mwandagalirwa K, Thwai KL, Likwela JL, Tshefu AK, et al. Effect of individual and community-level bed net usage on malaria prevalence among under-fives in the Democratic Republic of Congo. Malar J. 2018;17:39.
Wat’senga F, Manzambi EZ, Lunkula A, Mulumbu R, Mampangulu T, Lobo N, et al. Nationwide insecticide resistance status and biting behaviour of malaria vector species in the Democratic Republic of Congo. Malar J. 2018;17:129.
Nardini L, Hunt RH, Dahan-Moss YL, Christie N, Christian RN, Coetzee M, et al. Malaria vectors in the Democratic Republic of the Congo: the mechanisms that confer insecticide resistance in Anopheles gambiae and Anopheles funestus. Malar J. 2017;16:448.
Riveron JM, Watsenga F, Irving H, Irish SR, Wondji CS. High Plasmodium Infection rate and reduced bed net efficacy in multiple insecticide-resistant malaria vectors in Kinshasa, Democratic Republic of Congo. J Infect Dis. 2018;217:320–8.
Basilua Kanza JP, El Fahime E, Alaoui S, el Essassi M, Brooke B, Nkebolo Malafu A, et al. Pyrethroid, DDT and malathion resistance in the malaria vector Anopheles gambiae from the Democratic Republic of Congo. Trans R Soc Trop Med Hyg. 2013;107:8–14.
WHO. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes, second edition. Geneva: World Health Organization; 2016. (https://apps.who.int/iris/bitstream/handle/10665/250677/9789241511575-eng.pdf?sequence=1).
Agossa FR, Gnanguenon V, Anagonou R, Azondekon R, Aizoun N, Sovi A, et al. Impact of insecticide resistance on the effectiveness of pyrethroid-based malaria vectors control tools in Benin: decreased toxicity and repellent effect. PLoS ONE. 2015;10:e0145207.
Diabate A, Baldet T, Chandre F, Akogbeto M, Guiguemde TR, Darriet F, et al. The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. Am J Trop Med Hyg. 2002;67:617–22.
Lines JD. Do agricultural insecticides select for insecticide resistance in mosquitoes? a look at the evidence. Parasitol Today. 1988;4:S17–20.
Stump AD, Atieli FK, Vulule JM, Besansky NJ. Dynamics of the pyrethroid knockdown resistance allele in western Kenyan populations of Anopheles gambiae in response to insecticide-treated bed net trials. Am J Trop Med Hyg. 2004;70:591–6.
Czeher C, Labbo R, Arzika I, Duchemin JB. Evidence of increasing Leu-Phe knockdown resistance mutation in Anopheles gambiae from Niger following a nationwide long-lasting insecticide-treated nets implementation. Malar J. 2008;7:189.
Reddy MR, Godoy A, Dion K, Matias A, Callender K, Kiszewski AE, et al. Insecticide resistance allele frequencies in Anopheles gambiae before and after anti-vector interventions in continental Equatorial Guinea. Am J Trop Med Hyg. 2013;88:897–907.
Brogdon WG, Chan A. Guideline for evaluating insecticide resistance in vectors using the CDC bottle bioassay. CDC, 2010.
Brogdon WG, Chan A. Insert 2—enhanced surveillance protocol for the CDC intensity bottle bioassay. guideline for evaluating insecticide resistance in vectors using the CDC bottle bioassay. CDC. 2013.
Myriam C. Extraction d’ADN au CTAB, sur moustique entier. Manuel de laboratoire de biologie moleculaire et biochimie, Version 5, 10/115, 2003.
Wilkins EE, Howell PI, Benedict MQ. IMP PCR primers detect single nucleotide polymorphisms for Anopheles gambiae species identification, Mopti and Savanna rDNA types, and resistance to dieldrin in Anopheles arabiensis. Malar J. 2006;5:125.
Santolamazza F, Mancini E, Simard F, Qi Y, Tu Z, Torre DA. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar J. 2008;7:163.
Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, Devonshire AL, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae ss. Insect Mol Biol. 1998;7:179–84.
Ranson H, Jensen B, Vulule JM, Wang X, Hemingway J, Collins FH. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol. 2000;9:491–7.
Huynh L, Sandve S, Hannan L, Van Ert M, Gimnig J. Fitness costs of pyrethroid insecticide resistance in Anopheles gambiae., Annual meeting of the society for the study of evolutionChristchurch: New Zealand; 2007.
Protopopoff N, Verhaeghen K, Van Bortel W, Roelants P, Marcotty T, Baza D, et al. A significant increase in kdr in Anopheles gambiae is associated with an intensive vector control intervention in Burundi highlands. Trop Med Int Health. 2008;13:1479–87.
Nkya TE, Akhouayri I, Kisinza W, David JP. Impact of environment on mosquito response to pyrethroid insecticides: facts, evidences and prospects. Insect Biochem Mol Biol. 2013;43:407–16.
Tracking the number and location of LLINs in malaria-endemic countries. http://netmappingproject.allianceformalariaprevention.com/.
Ministère du Plan, Macro Int. Enquête Démographique et de Santé, République Démocratique du Congo 2007. Kinshasa, RDC, Calverton; 2008.
Ministry of Planning, National Institute of Statistics, (UNICEF). Democratic Republic of Congo Multiple Indicator Cluster Survey MICS-2010. Kinshasa, RDC, 2011.
Ministère du Plan et Suivi de la Mise en oeuvre de la Révolution de la Modernité (MPSMRM), Ministère de la Santé Publique (MSP), Macro Int. Enquête Démographique et de Santé en République Démocratique du Congo 2013–2014. Kinshasa, RDC, Rockville, 2014.
Lehmann T, Licht M, Elissa N, Maega BT, Chimumbwa JM, Watsenga FT, Wondji CS, et al. Population structure of Anopheles gambiae in Africa. J Hered. 2003;94:133–47.
Wondji C, Simard F, Fontenille D. Evidence for genetic differentiation between the molecular forms M and S within the Forest chromosomal form of Anopheles gambiae in an area of sympatry. Insect Mol Biol. 2002;11:11–9.
Reimer L, Fondjo E, Patchoke S, Diallo B, Lee Y, Ng A, et al. Relationship between kdr mutation and resistance to pyrethroid and DDT insecticides in natural populations of Anopheles gambiae. J Med Entomol. 2008;45:260–6.
Thiaw O, Doucoure S, Sougoufara S, Bouganali C, Konate L, Diagne N, et al. Investigating insecticide resistance and knock-down resistance (kdr) mutation in Dielmo, Senegal, an area under long lasting insecticidal-treated nets universal coverage for 10 years. Malar J. 2018;17:123.
Verhaeghen K, Van Bortel W, Roelants P, Backeljau T, Coosemans M. Detection of the East and West African kdr mutation in Anopheles gambiae and Anopheles arabiensis from Uganda using a new assay based on FRET/Melt Curve analysis. Malar J. 2006;5:16.
Lynd A, Oruni A, Van’t Hof AE, Morgan JC, Naego LB, Pipini D, et al. Insecticide resistance in Anopheles gambiae from the northern Democratic Republic of Congo, with extreme knockdown resistance (kdr) mutation frequencies revealed by a new diagnostic assay. Malar J. 2018;17:412.
Riveron JM, Ibrahim SS, Mulamba C, Djouaka R, Irving H, Wondji MJ, Ishak IH, et al. Genome-wide transcription and functional analyses reveal heterogeneous molecular mechanisms driving pyrethroids resistance in the major malaria vector Anopheles funestus across Africa. G3 (Bethesda). 2017;7:1819–32.
Kristan M, Lines J, Nuwa A, Ntege C, Meek SR, Abeku TA. Exposure to deltamethrin affects development of Plasmodium falciparum inside wild pyrethroid resistant Anopheles gambiae s.s. mosquitoes in Uganda. Parasit Vectors. 2016;9:100.
Jones CM, Sanou A, Guelbeogo WM, Sagnon N, Johnson PC, Ranson H. Aging partially restores the efficacy of malaria vector control in insecticide-resistant populations of Anopheles gambiae s.l. from Burkina Faso. Malar J. 2012;11:24.
Port GR, Boreham PFL. The effect of bed nets on feeding by Anopheles gambiae Giles (Diptera:Culicidae). Bull Ent Res. 1982;72:483–8.
Toe KH, Muller P, Badolo A, Traore A, Sagnon N, Dabire RK, et al. Do bednets including piperonyl butoxide offer additional protection against populations of Anopheles gambiae s.l. that are highly resistant to pyrethroids? an experimental hut evaluation in Burkina Faso. Med Vet Entomol. 2018;32:407–16.
Ketoh GK, Ahadji-Dabla KM, Chabi J, Amoudji AD, Apetogbo GY, Awokou F, et al. Efficacy of two PBO long lasting insecticidal nets against natural populations of Anopheles gambiae s.l. in experimental huts, Kolokope, Togo. PLoS One. 2018;13:e0192492.
N’Guessan R, Ngufor C, Kudom AA, Boko P, Odjo A, Malone D, et al. Mosquito nets treated with a mixture of chlorfenapyr and alphacypermethrin control pyrethroid resistant Anopheles gambiae and Culex quinquefasciatus mosquitoes in West Africa. PLoS ONE. 2014;9:e87710.
Bayili K, N’do S, Namountougou M, Sanou R, Ouattara A, Dabire RK, et al. Evaluation of efficacy of Interceptor® G2, a long-lasting insecticide net coated with a mixture of chlorfenapyr and alpha-cypermethrin, against pyrethroid resistant Anopheles gambiae s.l. in Burkina Faso. Malar J. 2017;16:190.
N’Guessan R, Odjo A, Ngufor C, Malone D, Rowland M. A Chlorfenapyr mixture net Interceptor® G2 shows high efficacy and wash durability against resistant mosquitoes in West Africa. PLoS ONE. 2016;1:e0165925.
Acknowledgements
The authors thank entomology technicians who conducted larval collections in all sites and the communities for their co-operation. Barb Marston is thanked for her careful revision of the manuscript.
Funding
This study has been financially supported by U.S. President’s Malaria Initiative.
Author information
Authors and Affiliations
Contributions
FW, FA, TC, FN, AS, MN, TM, RMO and SRI designed the study. FW, FA, TM, GI, and EZM carried out the field WHO and CDC susceptibility and intensity tests and laboratory analysis. YL and SI performed the molecular analysis. MP, RMO, FA, FW and SRI analysed the data. FA, FW, RMO, YL and SRI drafted the manuscript. LAM, and CF critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The work described in this manuscript was determined to be non-human subjects’ research by the CDC Center for Global Health (CDC 2016-242, 2016-082). The Kinshasa intensity study was approved by the Ethics Committee of the School of Public Health (University of Kinshasa), and the nationwide testing work was conducted within the remit of the Institut National de Recherche Biomédicale.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
Sites and periods where intensity assays were conducted and the history of LLIN distribution at provincial level.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wat’senga, F., Agossa, F., Manzambi, E.Z. et al. Intensity of pyrethroid resistance in Anopheles gambiae before and after a mass distribution of insecticide-treated nets in Kinshasa and in 11 provinces of the Democratic Republic of Congo. Malar J 19, 169 (2020). https://doi.org/10.1186/s12936-020-03240-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-020-03240-6