Abstract
Background
Duffy blood group antigens serve as receptors for Plasmodium vivax invasion into erythrocytes, and they are determined by polymorphisms of the Duffy antigen receptor for chemokines (DARC), also known as Fy glycoprotein (FY). Duffy negativity, i.e., absence of the antigens, protects against P. vivax infection and is rare among non-African populations. However, data on DARC polymorphisms and their impact on Plasmodium infection in India are scarce.
Methods
In a case–control study among 909 malaria patients and 909 healthy community controls in Mangaluru, southwestern India, DARC polymorphisms T-33C (rs2814778), G125A (rs12075), C265T (rs34599082), and G298A (rs13962) were genotyped. Associations of the polymorphisms with the odds of malaria, parasite species and manifestation were assessed.
Results
Among patients, vivax malaria (70%) predominated over falciparum malaria (9%) and mixed species infections (21%). DARC T-33C was absent and C265T was rare (1%). FYB carriage (deduced from DARC G125A) was not associated with the risk of malaria per se but it protected against severe falciparum malaria (P = 0.03), and hospitalization (P = 0.006) due to falciparum malaria. Vice versa, carriage of DARC 298A was associated with increased odds of malaria (aOR, 1.46 (1.07–1.99), P = 0.015) and vivax malaria (aOR, 1.60 (1.14–2.22), P = 0.006) and with several reported symptoms and findings of the patients.
Conclusion
This report from southern India is the first to show an independent effect of the DARC 298A polymorphism on the risk of malaria. Functional studies are required to understand the underlying mechanism. Moreover, FYB carriage appears to protect against severe falciparum malaria in southern India.
Similar content being viewed by others
Background
Malaria is considered a major driving force in shaping the human genome [1]. “Classical” erythrocyte variants such as the sickle-cell trait offer relative resistance against malaria and are thus subject to evolutionary selection in endemic regions. In addition, various further host genetic polymorphisms influence susceptibility to the disease and/or its manifestation [2, 3]. This includes the Duffy antigen receptor for chemokines (DARC, or Duffy antigen), which is a glycosylated erythrocyte membrane protein. The encoding DARC gene is located on chromosome 1. A common DARC polymorphism, G125A (rs12075), generates the FYA (G125) and FYB (125A) alleles. The resulting genotypes include the wildtype FYA/FYA, which correspond to the phenotype Fy (a+, b−), FYA/FYB (Fy (a+, b+)) and FYB/FYB (Fy (a−, b+)). An additional T-33C mutation silences antigen expression giving rise to Duffy blood group negativity (Fy (a−, b−)). Further single nucleotide polymorphisms (SNPs), C265T and G298A, are together responsible for weakening the expression of the FYB allele, whereas G298A alone is not able induce this effect [4].
In line with its function as a multi-specific receptor for a wide range of chemokines [5, 6], the absence of DARC on the erythrocyte cell surface (Duffy blood group negativity) has been associated with diverse conditions including inflammation, HIV infection, and malignancies [7, 8]. With respect to malaria, Duffy blood group negativity is predominant among Africans and renders erythrocytes resistant to invasion by Plasmodium vivax and Plasmodium knowlesi [5, 9,10,11,12]. Moreover, binding of DARC to platelet factor 4 (PF4) is essential for platelet-mediated killing of Plasmodium falciparum parasites [13, 14]. Associations of DARC genotypes with vivax malaria are reportedly conflicting. For instance in Brazil, FYA/FYA conferred reduced odds of vivax malaria [15]. However, in another Brazilian study, FYA/FYA was significantly more frequent in vivax malaria patients as compared to healthy blood donors without a history of malaria [11]. In India, the FYA allele has been associated with a reduced incidence of vivax malaria and the FYB allele with an increased one [16]. However, the few available individual studies from India did not show a link between DARC genotypes and vivax malaria [17,18,19].
In India, FYA/FYA is the predominant genotype and Duffy negativity occurs only in a few tribal populations [16, 19]. At the same time, India contributes to nearly half of the global Plasmodium vivax cases, and P. vivax and P. falciparum are responsible for 37% and 63% of malaria cases, respectively [20]. This provides the opportunity to study the effect of DARC genotypes on the risk of malaria per se, and of vivax and falciparum malaria separately. Of note, the manifestation of Plasmodium infection is not only caused by the infecting parasite but also by pro-inflammatory host responses, which potentially contribute to pathophysiology [21]. In this regard, the function of DARC as a receptor for diverse chemokines [5, 6] might possibly influence the clinical manifestation. Against this background, the present study aimed at describing the DARC genotype distribution pattern in Mangaluru and as a next step at examining the association of DARC genotypes with (i) malaria, (ii) malaria as caused by the various Plasmodium spp., and (iii) clinical presentation.
Methods
A total of 909 malaria out-patients were recruited at Wenlock Hospital, Mangaluru, Karnataka, India between June to December 2015. Wenlock Hospital (900 beds) is the largest governmental hospital in Mangaluru offering treatment particularly for the economically-deprived part of the population. In parallel, an average of 40 (26–53) healthy community controls were randomly recruited in each of the 60 census wards of Mangaluru yielding a total number of 2478 individuals. The study protocol was approved by the Institutional Ethics Committee of Kasturba Medical College, Mangalore, Manipal University, and permission to conduct the study was granted by the Directorate of Health and Family Welfare Services, Government of Karnataka. Informed written consent was obtained from all individuals enrolled in this study.
Details of the patient recruitment process as well as socio-economic, clinical and laboratory data have been reported elsewhere [22]. Briefly, socio-economic data were collected by trained interviewers from patients (cases) and controls. Venous blood was collected into EDTA from malaria patients and by finger prick blood on Whatman™ 3MM paper from controls. Malaria parasites were counted per 200 white blood cells (WBCs) on Giemsa-stained thick blood films, and parasite species was defined based on thin-film microscopy. Following DNA extraction (QIAamp DNA Blood Mini kit, Qiagen, Hilden, Germany), Plasmodium species was ascertained by semi-nested polymerase chain reaction (PCR) assays [23]. Out of 2383 Plasmodium-negative controls, 909 were randomly selected for this case–control study. DARC SNP genotyping including T-33C, G125A, C265T and G298A was achieved by melting curve analysis on the Light Cycler 480 instrument (Roche, Basel, Switzerland) using commercial primers and probes; reagent concentrations and PCR conditions are available with the manufacturer (TIB MOLBIOL, Berlin, Germany).
Data analysis was performed using RStudio 3.5.1 (2018) (Integrated Development for R. RStudio, Inc., Boston, USA) and SPSS 25 (IBM Corp., Armonk, USA). The distribution of DARC genotypes between case and controls were compared by χ2 test or Fisher’s exact test as appropriate, and odds ratios (ORs) and 95% confidence intervals (95% CI) were calculated. Binomial logistic regression was used to calculate the adjusted odds ratios (aORs) of individuals with variant genotypes for malaria per se and for malaria separated by species with probable confounders: age, sex and migration to Mangaluru. Continuous parameters were compared using Student’s t test, Mann–Whitney U test, or Kruskal–Wallis test as applicable. A P value < 0.05 was considered statistically significant.
Results
Essential characteristics of malaria patients and controls are displayed in Table 1. More than 90% of malaria patients were male adults. Their median age was 26 years, and more than three in four had migrated to Mangaluru a median period of 6 months before presentation (range 1–600 days). Their overall socio-economic status including educational background was low, and more than half of the patients were either construction workers or daily labourers [22]. In comparison, among control individuals, the proportion of males was lower, age was higher, and only a minority had migrated to Mangaluru city (each, P < 0.0005). Among patients, vivax malaria (70%) predominated over falciparum malaria (9%), and mixed P. vivax–P. falciparum infections (21%). The geometric mean parasite density (GMPD) was 3412/µl (95% CI 3081–3779).
The DARC SNP-33 T>C was absent in 570 random samples genotyped, and the SNP 265 C>T was rare (1% (6/564) heterozygous). These polymorphisms were thus omitted from analysis. Genotyping of the DARC SNPs G125A and G298A was successful in all patients and controls. DARC 298A occurred exclusively when also 125A was present, i.e., on an FYB background (P < 0.0001). In the study sample and based on DARC G125A, FYA/FYB (43.9%) and FYA/FYA (43.5%) were the most common Duffy genotypes (FYB/FYB, 12.5%). Of note, these genotypes did not differ between cases and controls, and thus were not associated with the odds of malaria (Table 1), irrespective of stratification by parasite species (Table 2).
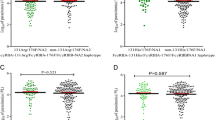
In contrast, carriage of DARC 298A (GA or AA), i.e., genotypes involved in but not solely responsible for a weakened expression of the FYB allele [4], appeared to be more common in malaria patients (16.7%, 152/909; P = 0.19) and in vivax malaria patients in particular (17.7%, 112/633; P = 0.09) as compared to controls (14.5%, 132/909) (Table 2; Fig. 1). Adjusting for the observed differences in age, sex, and migration, carriage of DARC 298A was associated with increased odds of malaria (aOR, 1.46 (1.07–1.99), P = 0.015) and of vivax malaria in particular (aOR, 1.60 (1.14–2.22), P = 0.006) (Fig. 1). No significant association with falciparum or mixed species malaria was observed.
DARC 298A carriage is associated with increased odds of malaria and vivax malaria. OR, odds ratio; 95% CI, 95% confidence interval; unadjusted, crude odds ratio; adjusted, odds ratio adjusted for age, sex, and migration status; *P < 0.05 as compared to DARC G298 wildtype individuals. Forest plots display the odds of malaria according to DARC G298A genotypes. χ2 test or Fisher’s exact test was applied to calculate unadjusted odds ratios (95% CIs). Adjusted odds ratios were derived from logistic regression models adjusting for age, sex and migration status. As compared to control individuals (14.5%, 132/909), DARC 298A carriage was more common in malaria patients [16.7%, 152/909; aOR, 1.46 (1.07–1.99)] and in vivax malaria patients in particular [17.7%, 112/633; aOR, 1.60 (1.14–2.22)]
In a next step of analysis among malaria patients, the proportions of hospitalization and of severe malaria were compared between DARC genotypes. For that, FYA/FYA and wildtype DARC G298, respectively, were set as reference groups. Among the patients, 3.5% (32/909) and 3.8% (35/909) of individuals were hospitalized and had severe malaria, respectively. The proportion of patients who were admitted to ward was highest in individuals with FYA/FYA (5.0%, 20/399), lower in FYA/FYB (3.5%, 14/401, P = 0.29) and lowest in FYB/FYB (0.9%, 1/109, P = 0.06). This was due to the absence of hospital admissions in patients with falciparum malaria carrying the FYB allele (P = 0.006, Table 3). Severe malaria due to any parasite species occurred at similar proportions in patients with the different FY genotypes, but severe falciparum malaria was absent in individuals carrying FYB (P = 0.03, Table 3). DARC 298A carriage did neither affect the proportion of hospitalized patients nor that of severe malaria. Also, it did not substantially change the associations of FYB carriage with the odds of hospitalization or severe malaria (Table 3).
Lastly, signs and symptoms as well as laboratory parameters were analysed with respect to DARC genotypes. These did not differ significantly with the three FY genotypes. However, DARC 298A carriage was associated with increased proportions of patients reporting a history of muscle pain, back pain, fatigue, and at borderline, diarrhoea. Basically, the same findings were seen for vivax malaria, whereas in falciparum malaria DARC 298A carriage was associated with a history of sweats (P = 0.05) and of vomiting (Table 4). Clinically, the proportions of splenomegaly and of elevated bilirubin concentration were increased in patients with DARC 298A carriage as was axillary temperature (P = 0.05), specifically in mixed species infections (P = 0.01).
Discussion
The present results indicate that FYB carriage in an Indian population does not influence the risk of malaria per se, but, in case of P. falciparum infection, it is associated with protection from hospitalization and severe malaria. Vice versa, DARC 298A carriage appeared to increase the risk of malaria, and of vivax malaria in particular, and to affect the occurrence of several symptoms.
Despite its sample size the study has several limitations which need to be considered when interpreting the results: subgroups, e.g., patients with falciparum malaria, were relatively small affecting the power of analyses. Patients and controls differed in essential parameters such as age, gender, and migration status, because of which risk estimates had to be adjusted accordingly. The DARC polymorphisms T-33C and C265T were too rare to deduce meaningful findings. No interaction in terms of associations with malaria or signs or symptoms was seen for the FYA or FYB alleles and DARC G298A. Therefore, data were presented separately.
In the present study, the FYA/FYA and FYA/FYB genotypes occurred in each approximately 44%. Among more than 3000 blood donors in New Delhi, FYA/FYA and FYA/FYB were observed in 32.5% and 48.9%, respectively. Duffy blood group negativity, absent in the present study, was observed in 0.3% [24]. Of note, the proportion of Duffy blood group genotypes differs across India but findings from the South of the country closely match with the prevalence data of the present study [16]. In comparison to other ethnic groups, the predominant FYA/FYB genotype in the present study is slightly more common in Caucasians (49%) but rare in sub-Saharan Africans (1.0%) and Chinese (8.9%). This is due to a higher FYA allele frequency among Indians than in Caucasians and sub-Saharan Africans. Duffy blood group negativity is absent or very rare in all populations except for sub-Saharan Africans (68%) [24]. Carriage of DARC 298A was found in 15.6% of the current study participants, corresponding to an allele frequency of 0.08. Based on the 1000 Genomes Project, this matches the respective figure of 0.09 among South Asians, but it is lower than the allele frequency of 0.18 among Caucasians and higher than the value of 0.005 in Africans [4].
Duffy blood group antigens are known to play an important role in P. vivax malaria [5, 6, 25] and to be essential in platelet-mediated killing of P. falciparum [13]. However, actual findings in various populations including Indians have been ambiguous [11, 15,16,17,18,19]. In the present study FYA or FYB did not affect the odds of malaria, irrespective of parasite species.
This contrasts with recent report on protective effects of FYA/FYA against vivax malaria in Brazil [15]. Likewise, in one study from India, FYA was found to be associated with a reduced 5 years average incidence of vivax malaria [16]. In Brazil, no association with falciparum malaria was observed [15], in India, falciparum malaria was not analysed [16]. In another study from Brazil, FYA/FYA was associated with increased susceptibility to vivax malaria [11], and in older work from India, no impact of the Duffy blood group genotypes on vivax or falciparum malaria was observed [17,18,19]. The reason for these conflicting results may be related to variable proportions of P. vivax and P. falciparum among the patients included, partially low sample sizes and genetic variation among the diverse populations, including the Indian one. Of note, in the present study, falciparum malaria patients with FYA/FYA showed the highest rate of hospitalization and severe malaria, which was unexpected considering the protective effects against vivax malaria mentioned above [15, 16]. In-vitro, binding of Duffy antigens to platelet factor 4 (PF4) is crucial for platelet-mediated killing of P. falciparum [13, 14] even though the role of FY variation in that is unknown. One explanation for the finding of reduced odds of severe malaria in patients with FYB carriage could be that it affects binding affinity towards PF4 and thereby the capacity of platelet mediated killing. On the other hand, parasite densities and other severity markers of infection were not reduced in patients with FYB carriage. Consequently, further work is required to explain the observed association of FYB carriage with hospitalization and severe falciparum malaria.
A novel finding is that carriage of the DARC 298A variant increased the odds of malaria by roughly 50%. Moreover, this polymorphism was associated with increased proportions of patients reporting several signs and symptoms. This SNP has not been observed to be independently associated with malaria. One previous study from Brazil did not observe an association with malaria susceptibility when combining DARC C265T and G298A as a condition weakening the expression of Duffy antigens (FYX) [11]. DARC C265T was absent in the present study population. DARC 298 G>A results in an amino acid substitution in the first intracellular loop of the Duffy glycoprotein. It has been linked with reduced FYB expression only in the presence of C265T [4, 26]. On the other hand, DARC acts as a multi-specific receptor for chemokines. These include the melanoma growth stimulatory activity, interleukin-8, regulated upon activation normal T-expressed, monocyte chemotactic protein-1, neutrophil activating protein 2 and 3, epithelial neutrophil activating peptide-78, angiogenesis-related platelet factor 1, and growth-related gene alpha [5, 6]. In line with this wide-range receptor function, DARC per se has been associated with several inflammatory and infectious diseases including increased rates of prostate cancer and asthma as well as an increased risk of HIV infection in its absence [7, 27]. DARC also influences inflammation in terms of chemokine levels and leukocyte trafficking and malignancy [8]. Monocytes and neutrophils phagocytize infected red blood cells, and they are important sources of cytokines, which act as signaling molecules in activating immune responses against malaria [28]. Increased phagocytic activity via neutrophils is observed in vivax malaria [29]. A possible explanation in support of the present study findings could be the involvement of variant DARC 298A in altering the chemoattractant properties of the Duffy glycoprotein, leading to a modified activation of the pro-inflammatory signal cascade. Functional studies are needed to verify this hypothesis.
Conclusion
This study from southern India is the first to show an independent effect of DARC 298A in Plasmodium infection. DARC 298A carriage appears to be associated with increased susceptibility to malaria and to vivax malaria in particular, and to worsen several signs and symptoms. Functional studies on the role of this polymorphism are required to disentangle the underlying mechanisms. The same applies to the role of FYB genotype carriage protecting against severe falciparum malaria. Considering Duffy blood group antigens being studied as vaccine candidates against vivax malaria [30] and the present clinico-epidemiological findings, unravelling the molecular mechanisms of Duffy blood group antigens influencing malaria susceptibility and resistance is urgently needed.
Availability of data and materials
The dataset generated and/or analysed in this study is not publicly available due to issues of confidentiality and ongoing analyses, but are available from the corresponding author on reasonable request.
Abbreviations
- DARC:
-
Duffy antigen receptor for chemokines
- FY:
-
Fy glycoprotein
- P. vivax :
-
Plasmodium vivax
- P. falciparum :
-
Plasmodium falciparum
- WBCs:
-
white blood cells
- PF4:
-
platelet factor 4
- SNP:
-
single nucleotide polymorphism
- PCR:
-
polymerase chain reaction
- ORs:
-
odds ratios
- CI:
-
confidence interval
- GMPD:
-
geometric mean parasite density
- BMI:
-
body mass index
References
Kwiatkowski DP. How malaria has affected the human genome and what human genetics can teach us about malaria. Am J Hum Genet. 2005;77:171–92.
Driss A, Hibbert JM, Wilson NO, Iqbal SA, Adamkiewicz TV, Stiles JK. Genetic polymorphisms linked to susceptibility to malaria. Malar J. 2011;10:271.
da Silva Santos S, Clark TG, Campino S, Suarez-Mutis MC, Rockett KA, Kwiatkowski DP, et al. Investigation of host candidate malaria-associated risk/protective SNPs in a Brazilian Amazonian population. PLoS One. 2012;7:e36692.
Höher G, Fiegenbaum M, Almeida S. Molecular basis of the Duffy blood group system. Blood Transfus. 2018;16:93–100.
Horuk R, Chitnis CE, Darbonne WC, Colby TJ, Rybicki A, Hadley TJ, et al. A receptor for the malarial parasite Plasmodium vivax: the erythrocyte chemokine receptor. Science. 1993;261:1182–4.
Pogo AO, Chaudhuri A. The Duffy protein: a malarial and chemokine receptor. Semin Hematol. 2000;37:122–9.
He W, Neil S, Kulkarni H, Wright E, Agan BK, Marconi VC, et al. Duffy Antigen Receptor for Chemokines mediates trans-infection of HIV-1 from red blood cells to target cells and affects HIV-AIDS susceptibility. Cell Host Microbe. 2008;17(4):52–62.
Horne K, Woolley IJ. Shedding light on DARC: the role of the Duffy antigen/receptor for chemokines in inflammation, infection and malignancy. Inflamm Res. 2009;58:431–5.
Miller LH, Mason SJ, Dvorak JA, Mcginniss MH, Rothman IK. Erythrocyte receptors for (Plasmodium knowlesi) malaria: duffy blood group determinants. Science. 1975;15(189):561–3.
Howes RE, Patil AP, Piel FB, Nyangiri OA, Kabaria CW, Gething PW, et al. The global distribution of the Duffy blood group. Nat Commun. 2011;2:266.
Albuquerque SRL, De Oliveira Cavalcante F, Sanguino EC, Tezza L, Castilho F, Lilian C, et al. FY polymorphisms and vivax malaria in inhabitants of Amazonas State, Brazil. Parasitol Res. 2010;106:1049–53.
Iwamoto S, Li J, Sugimoto N, Okuda H, Kajii E. Characterization of the Duffy gene promotor: evidence for tissue-specific abolishment of expression in Fy(a-b-) of black individuals. Biochem Biophys Res Commun. 1996;222:852–9.
McMorran BJ, Wieczorski L, Drysdale KE, Chan JA, Huang HM, Smith C, et al. Platelet factor 4 and duffy antigen required for platelet killing of Plasmodium falciparum. Science. 2012;338:1348–51.
McMorran BJ, Burgio G, Foote SJ. New insights into the protective power of platelets in malaria infection. Commun Integr Biol. 2013;6:e23653.
King CL, Adams JH, Xianli J, Grimberg BT, McHenry AM, Greenberg LJ, et al. Fya/Fyb antigen polymorphism in human erythrocyte Duffy antigen affects susceptibility to Plasmodium vivax malaria. Proc Natl Acad Sci USA. 2011;108:20113–8.
Chittoria A, Mohanty S, Jaiswal YK, Das A. Natural selection mediated association of the Duffy (FY) gene polymorphisms with Plasmodium vivax malaria in India. PLoS One. 2012;7:e45219.
Kar S, Seth S, Seth PK. Duffy blood groups and malaria in the ao nagas in Nagaland, India. Hum Hered. 1991;41:231–5.
Singh IP, Walter H, Bhasin MK, Bhardwaj V, Sudhakar K. Genetic markers and malaria. Observations in Gujarat, India. Hum Hered. 1986;36:31–6.
Verma IC, Thakur A. Duffy blood group determinants and malaria in India. J Genet. 1993;72:15–9.
WHO. World malaria report. Geneva: World Health Organization; 2018.
Malaguarnera L, Musumeci S. The immune response to Plasmodium falciparum malaria. Lancet Infect Dis. 2002;2:472–8.
Gai PP, Mockenhaupt FP, Siegert K, Wedam J, Boloor A, Kulkarni SS, et al. Manifestation of malaria in Mangaluru, southern India. Malar J. 2018;17:313.
Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol. 1993;58:283–92.
Makroo RN, Bhatia A, Gupta R, Phillip J. Prevalence of Rh, Duffy, Kell, Kidd & MNSs blood group antigens in the Indian blood donor population. Indian J Med Res. 2013;137:521–6.
Miller LH, Mason SJ, Clyde DF, McGinniss MH. The resistance factor to Plasmodium vivax in blacks. N Engl J Med. 1976;295:302–4.
Yazdanbakhsh K, Rios M, Storry JR, Kosower N, Parasol N, Chaudhuri A, et al. Molecular mechanisms that lead to reduced expression of Duffy antigens. Transfusion. 2000;40:310–20.
Lentsch AB. The Duffy antigen/receptor for chemokines (DARC) and prostate cancer. A role as clear as black and white? FASEB J. 2002;16:1093–5.
Aitken EH, Alemu A, Rogerson SJ. Neutrophils and malaria. Front Immunol. 2018;9:3005.
de Leoratti FMS, Trevelin SC, Cunha FQ, Rocha BC, Costa PAC, Gravina HD, et al. Neutrophil paralysis in Plasmodium vivax malaria. PLoS Negl Trop Dis. 2012;6:e1710.
Singh K, Mukherjee P, Shakri AR, Singh A, Pandey G, Bakshi M, et al. Malaria vaccine candidate based on Duffy-binding protein elicits strain transcending functional antibodies in a Phase I trial. NPJ Vaccines. 2018;3:48.
Acknowledgements
We thank the patients, staff as well as doctors and administration at Wenlock Hospital and Kasturba Medical College, Mangalore. We also thank the control individuals from Mangaluru, staff as well as field workers of the Mangaluru City Corporation and the District Vector Borne Disease Control Programme Office. This work forms part of the doctoral thesis of PPG, WVL, KS and JW.
Funding
This study was supported by grants from the German Research Foundation (GRK 1673 to PPG and GRK 2046 to WVL) and from the Sonnenfeld-Foundation to PPG. The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
PPG, FPM, PG, AJ, RD and DS designed the study. PPG, KS, JW, AB, AK, SB, RD, and DS were responsible for patient recruitment, clinical and laboratory examinations. PPG, KS, RR and SK did the PCR analyses. PPG, WVL and FPM did the statistical analyses and wrote the paper with major contributions of the other authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was reviewed and approved by the Institutional Ethics Committee of Kasturba Medical College, Mangalore, Manipal University (IEC KMC MLR 05-1598), and informed written consent was obtained by all study patients. Permission to conduct the study was granted by the Directorate of Health and Family Welfare Services, Government of Karnataka.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Gai, P.P., van Loon, W., Siegert, K. et al. Duffy antigen receptor for chemokines gene polymorphisms and malaria in Mangaluru, India. Malar J 18, 328 (2019). https://doi.org/10.1186/s12936-019-2966-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-019-2966-9